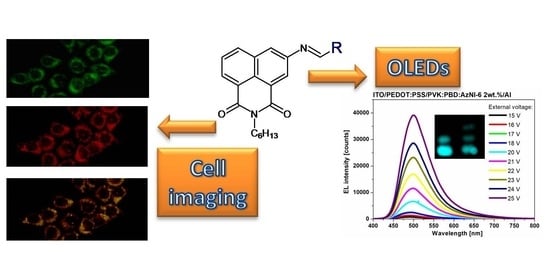
Luminescence and Electrochemical Activity of New Unsymmetrical 3-Imino-1,8-naphthalimide Derivatives
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Characterization Methods
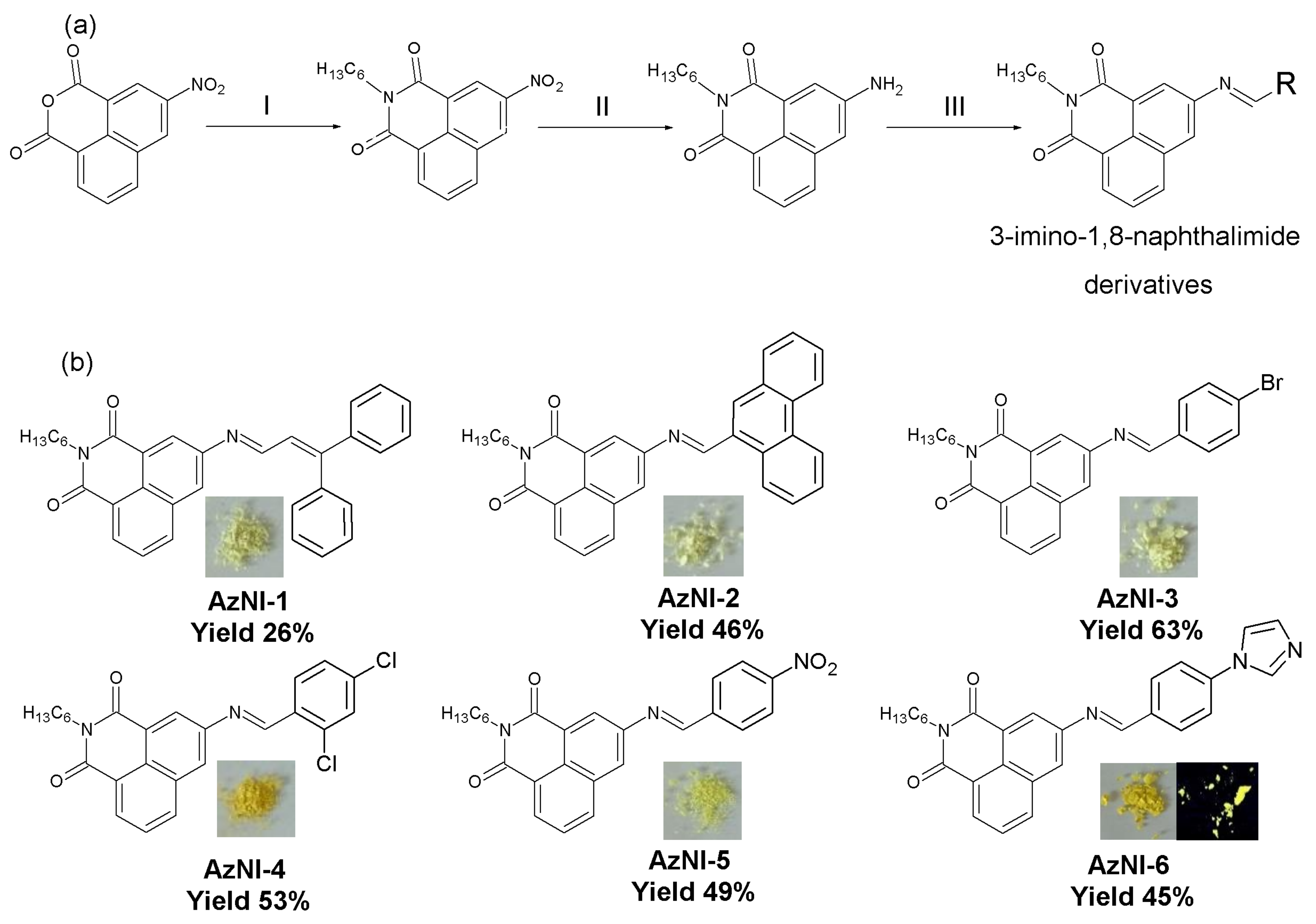
2.2. Synthesis of N-Hexyl-1,8-naphthalimides Derivatives
2.3. Concentration Study
2.4. Study of Spectroscopic Properties Related to Aggregation
2.5. Spectroscopic Studies of the Imine Bond Protonation by Trifluoroacetic Acid (TFA)
2.6. Cell Lines and Culture Conditions
2.7. Cytotoxicity Studies
2.8. The Cellular Staining
2.9. Co-Localization Studies
3. Result and Discussion
3.1. Structural and Thermal Characterization
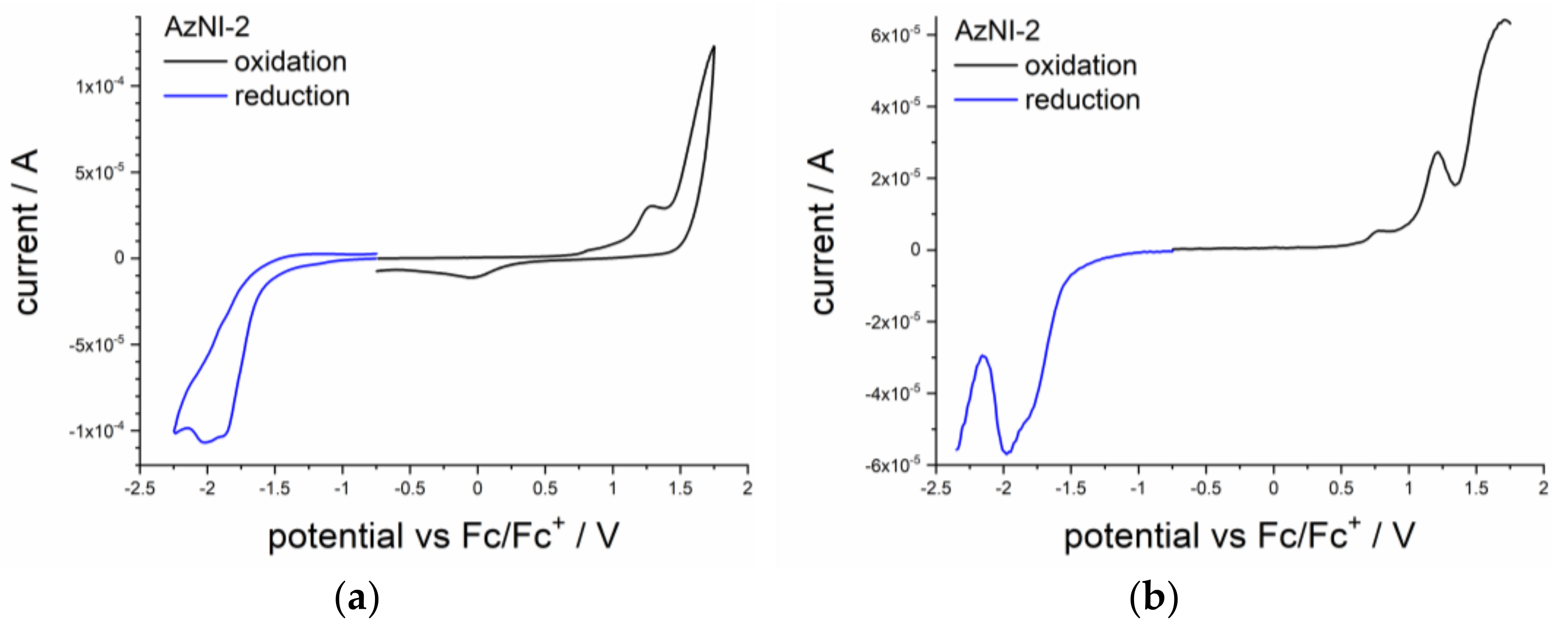
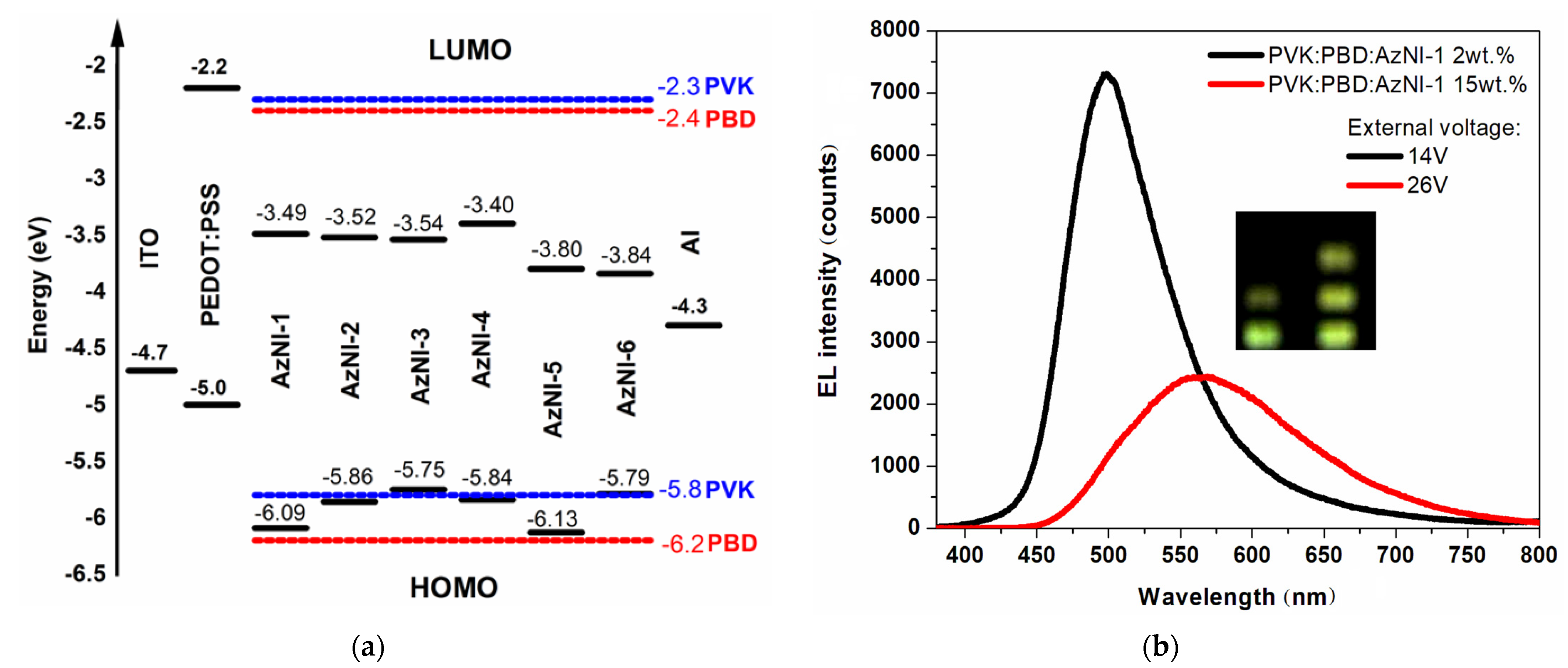
3.2. Electrochemical Study
3.3. Luminescence Investigations
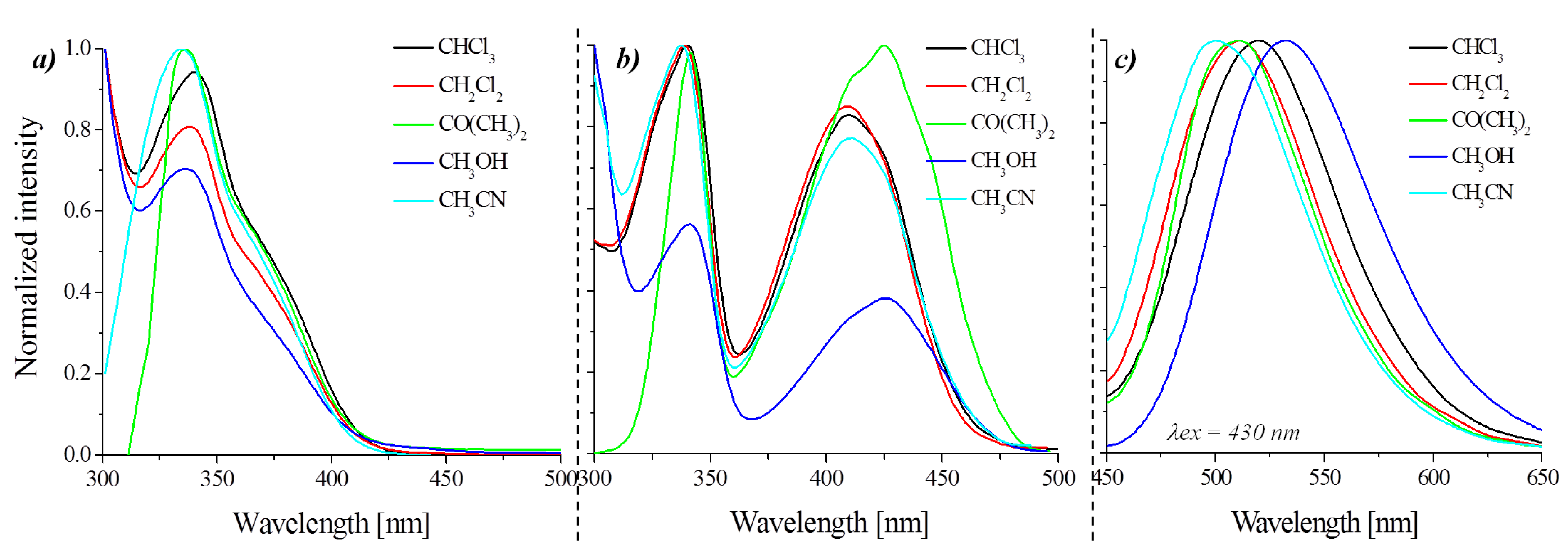
3.3.1. Photoluminescence in Aggregated State
3.3.2. Photoinduced Electron Transport Inhibition Process
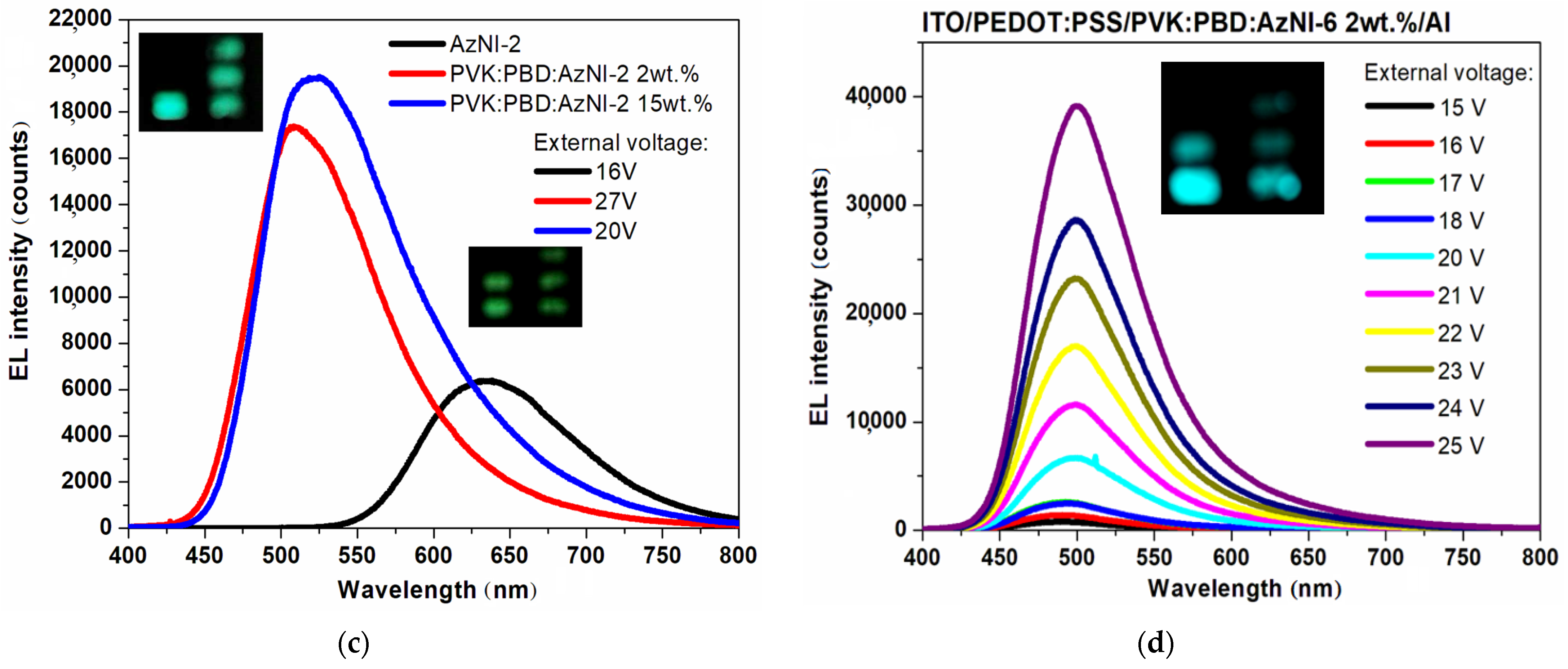
3.3.3. Luminescence Induced by External Voltage
3.4. Biological Studies
Cell Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishna, P.; Meher, N.; Iyer, P.K. Functional 1,8-Naphthalimide AIE/AIEEgens: Recent Advances and Prospects. ACS Appl. Mater. Interfaces 2017, 10, 12081–12111. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.D.; Walczak, K.Z. l,8-Naphthalimide based DNA intercalators and anticancer agents. A systematic re-view from 2007 to 2017. Eur. J. Med. Chem. 2018, 159, 393–422. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Hayashi, N.; Nakahata, M.; Sakaic, S.; Hiraia, T. Naphthalimide–coumarin conjugate: Ratiometric fluo-rescent receptor for self-calibrating quantification of cyanide anions in cells. RSC Adv. 2017, 7, 32304–32309. [Google Scholar] [CrossRef]
- Dong, H.-Q.; Wei, T.-B.; Ma, X.-Q.; Yang, Q.-Y.; Zhang, Y.-F.; Sun, Y.-J.; Shi, B.-B.; Yao, H.; Zhang, Y.-M.; Lin, Q. 1,8-Naphthalimide-based fluorescent chemosensors: Recent advances and perspectives. J. Mater. Chem. C 2020, 8, 13501–13529. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, I.; Goel, R.; Paul, K.; Luxami, V. Dual-channel ratiometric recognition of Al3+ and F− ions through an ESIPT-ESICT signalling mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 247, 119112. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.L.; Yiu, C.G.; Fu, Y.; Ye, F. A naked-eye visible colorimetric and ratiometric chemosensor based on Schiff base for fluoride anion detection. J. Mol. Struct. 2021, 1236, 130343. [Google Scholar] [CrossRef]
- Gudeika, D. A review of investigation on 4-substituted 1,8-naphthalimide derivatives. Synth. Met. 2020, 262, 116328. [Google Scholar] [CrossRef]
- Luo, S.; Lin, J.; Zhou, J.; Wang, Y.; Liu, X.; Huang, Y.; Lu, Z.; Hu, C. Novel 1,8-naphthalimide derivatives for standard-red organic light-emitting device applications. J. Mater. Chem. C 2015, 3, 5259–5267. [Google Scholar] [CrossRef]
- Gan, J.-A.; Song, Q.L.; Hou, X.Y.; Chen, K.; Tian, H. 1,8-Naphthalimides for non-doping OLEDs: The tunable emission color from blue, green to red. J. Photochem. Photobiol. A Chem. 2004, 162, 399–406. [Google Scholar] [CrossRef][Green Version]
- Bezvikonnyi, O.; Gudeika, D.; Volyniuk, D.; Grazulevicius, J.V.; Bagdziunas, G. Pyrenyl substituted 1,8-naphthalimide as a new material for weak efficiency-roll-off red OLEDs: A theoretical and experimental study. New J. Chem. 2018, 42, 12492–12502. [Google Scholar] [CrossRef]
- Martínez-Calvo, M.; Bright, S.A.; Veale, E.B.; Henwood, A.F.; Williams, D.C.; Gunnlaugsson, T. 4-Amino-1,8-naphthalimide based fluorescent photoinduced electron transfer (PET) pH sensors as liposomal cellu-lar imaging agents: The effect of substituent patterns on PET directional quenching. Front. Chem. Sci. Eng. 2020, 14, 61–75. [Google Scholar] [CrossRef]
- Xu, T.; Huang, J.; Fang, M.; Sui, M.; Zhu, Y.; Shentu, Y.; Li, C.; Zhu, W. A novel “turn-on” fluorescent probe based on naphthalimide for the tracking of lysosomal Cu2+ in living cells. New J. Chem. 2020, 44, 21167–21175. [Google Scholar] [CrossRef]
- Misra, S.; Singh, P.; Das, A.; Brandão, P.; Sahoo, P.; Sepay, N.; Bhattacharjee, G.; Datta, P.; Mahapatra, A.K.; Satpati, B.; et al. Supramolecular assemblies of a 1,8-naphthalimide conjugate and its aggregation-induced emission property. Mater. Adv. 2020, 1, 3532–3538. [Google Scholar] [CrossRef]
- Yang, K.; Leslie, K.G.; Kim, S.Y.; Kalionis, B.; Chrzanowski, W.; Jolliffe, K.A.; New, E.J. Tailoring the proper-ties of a hypoxia-responsive 1,8-naphthalimide for imaging applications. Org. Biomol. Chem. 2018, 16, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, Y.; Terakado, K.; Nampo, T.; Mori, M.; Inoue, H. 4-Bromo-1,8-naphthalimide derivatives as fluoro-genic substrates for live cell imaging of glutathione S-transferase (GST) activity. Talanta 2019, 1, 633–640. [Google Scholar] [CrossRef]
- Liu, D.-Y.; Qi, J.; Liu, X.-Y.; He, H.-R.; Chen, J.-T.; Yang, G.-M. 4-Amino-1,8-naphthalimide-based fluorescent sensor with high selectivity and sensitivity for Zn2+ imaging in living cells. Inorg. Chem. Commun. 2014, 43, 173–178. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Y.; Shi, J.; Zhu, H.; Zhang, T.; Qi, P.; Chen, J.; Yang, G.; He, H. A highly selective and sensitive 1,8-naphthalimide-based fluorescent sensor for Zn2+ imaging in living cells. Bioorg. Med. Chem. Lett. 2019, 29, 2646–2649. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mi, Q.L.; Wang, G.K.; Chen, J.H.; Zhang, J.F.; Zhao, Q.H.; Zhou, Y. 1,8-Naphthalimide-based ‘turn-on’ fluorescent sensor for the detection of zinc ion in aqueous media and its applications for bioimag-ing. Tetrahedron Lett. 2013, 54, 3353–3358. [Google Scholar] [CrossRef]
- Gao, Y.G.; Liu, F.L.; Patil, S.; Li, D.J.; Qadi, A.; Lin, X.; Tian, Y.; Li, Y.; Qian, A.R. 1,8-Naphthalimide-Based Multifunc-tional Compounds as Cu2+ Probes, Lysosome Staining Agents, and Non-viral Vectors. Front. Chem. 2019, 7, 616. [Google Scholar] [CrossRef]
- Shen, R.; Yang, J.; Luo, H.; Wang, B.; Jiang, Y. A sensitive fluorescent probe for cysteine and Cu2+ based on 1,8-naphthalimide derivatives and its application in living cells imaging. Tetrahedron 2017, 73, 373–377. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Ali, R.; Singh, M.; Gupta, T.; Kar, A.K.; Prakash, V.; Sadasivam, A.; Patnaik, S.; Misra, A. A simple naph-thalimide based PET probe for Fe3+ and selective detection of pyrophosphate through displacement approach: Cell imaging studies and logic interpretation. J. Photochem. Photobiol. A Chem. 2020, 403, 112854. [Google Scholar] [CrossRef]
- Li, M.; Ge, H.; Mirabello, V.; Arrowsmith, R.L.; Kociok-Köhn, G.; Botchway, S.W.; Zhu, W.; Pascu, S.I.; James, T.D. Lysosomal tracking with a cationic naphthalimide using multiphoton fluorescence lifetime imaging microscopy. Chem. Commun. 2017, 53, 11161–11164. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, B.; Das, D. Aggregation-Induced Emission or Hydrolysis by Water? The Case of Schiff Bases in Aqueous Organic Solvents. J. Phys. Chem. C 2018, 122, 3655–3661. [Google Scholar] [CrossRef]
- Korzec, M.; Senkała, S.; Rzycka-Korzec, R.; Kotowicz, S.; Schab-Balcerzak, E.; Polański, J. A highly selective and sensi-tive sensor with imine and phenyl-ethynyl-phenyl units for the visual and fluorescent detection of copper in water. J. Photochem. Photobiol. A Chem. 2019, 382, 111893. [Google Scholar] [CrossRef]
- Senkała, S.; Małecki, J.G.; Vasylieva, M.; Łabuz, A.; Nosek, K.; Piwowarczyk, K.; Czyż, J.; Schab-Balcerzak, E.; Janec-zek, H.; Korzec, M. Hydrolysis of Schiff bases with phenyl-ethynyl-phenyl system: The importance for biological and physicochemical studies. J. Photochem. Photobiol. B Biol. 2020, 212, 112020. [Google Scholar] [CrossRef]
- Lin, W.; Yuan, L.; Feng, J.; Cao, X. A Fluorescence-Enhanced Chemodosimeter for Fe3+ Based on Hydrolysis of Bis(coumarinyl) Schiff Base. Eur. J. Org. Chem. 2008, 2008, 2689–2692. [Google Scholar] [CrossRef]
- Korzec, M.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Rzycka-Korzec, R.; Schab-Balcerzak, E.; Polański, J. Live cell imaging by 3-imino-(2-phenol)-1,8-naphthalimides: The effect of ex-vivo hydrolysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118442. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Huang, K.F.; Yen, Y.P. A new selective colorimetric and fluorescent chemodosimeter for HSO4- based on hydrolysis of Schiff base. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 552–558. [Google Scholar] [CrossRef]
- Pandey, A.; Asthana, S.K.; Prakash, A.; Roy, J.K.; Tiwari, I.; Upadhyay, K.K. A selective hydrolytic and re-structuring approach through a Schiff base design on a coumarin platform for “turn-on” fluorogenic sens-ing of Zn2+. Dalton Trans. 2019, 48, 2068–2076. [Google Scholar] [CrossRef]
- Dey, S.K.; Janiak, C. The curious case of salicylidene-based fluoride sensors: Chemosensors or chemo-dosimeters or none of them. RSC Adv. 2020, 10, 14689–14693. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Yin, Y.; Luo, J.; Kong, L. Coumarin-naphthol conjugated Schiff base as a “turn-on” fluo-rescentprobe for Cu2+via selective hydrolysis of imine and its application inlive cell imaging. J. Photochem. Photobiol. A Chem. 2017, 333, 213–219. [Google Scholar] [CrossRef]
- Kotowicz, S.; Korzec, M.; Siwy, M.; Golba, S.; Malecki, J.G.; Janeczek, H.; Mackowski, S.; Bednarczyk, K.; Libera, M.; Schab-Balcerzak, E. Novel 1,8-naphthalimides substituted at 3-C position: Synthesis and evaluation of thermal, electrochemical and luminescent properties. Dye. Pigment. 2018, 158, 65–78. [Google Scholar] [CrossRef]
- Korzec, M.; Kotowicz, S.; Rzycka-Korzec, R.; Schab-Balcerzak, E.; Małecki, J.G.; Czichy, M.; Łapkowski, M. Novel β-ketoenamines versus azomethines for organic electronics: Characterization of optical and electro-chemical properties supported by theoretical studies. J. Mater. Sci. 2020, 55, 3812–3832. [Google Scholar] [CrossRef]
- Korzec, M.; Kotowicz, S.; Gawecki, R.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Siwy, M.; Schab-Balcerzak, E.; Grzelak, J.; Maćkowski, S. 1,8-Naphthalimides 3-substituted with imine or β-ketoenamine unit evaluated as compounds for organic electronics and cell imaging. Dye. Pigment. 2021, 193, 109508. [Google Scholar] [CrossRef]
- Kotowicz, S.; Korzec, M.; Pająk, A.K.; Golba, S.; Małecki, J.G.; Siwy, M.; Grzelak, J.; Maćkowski, S.; Schab-Balcerzak, E. New Acceptor–Donor–Acceptor Systems Based on Bis-(Imino-1,8-Naphthalimide). Materials 2021, 14, 2714. [Google Scholar] [CrossRef] [PubMed]
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895–8999. [Google Scholar] [CrossRef]
- Chen, W.; Nakano, M.; Kim, J.-H.; Takimiya, K.; Zhang, Q. Naphtho[2,3-b]thiophene diimide (NTI): A mono-functionalisable core-extended naphthalene diimide for electron-deficient architectures. J. Mater. Chem. C 2016, 4, 8879–8883. [Google Scholar] [CrossRef]
- Gautam, P.; Sharma, R.; Misra, R.; Keshtov, M.; Kuklin, S.A.; Sharma, G.D. Donor–acceptor–acceptor (D–A–A) type 1,8-naphthalimides as non-fullerene small molecule acceptors for bulk heterojunction solar cells. Chem. Sci. 2016, 8, 2017–2024. [Google Scholar] [CrossRef]
- Sharma, H.; Tan, N.K.; Trinh, N.; Yeo, J.H.; New, E.J.; Pfeffer, F.M. A fluorescent naphthalimide NADH mimic for continuous and reversible sensing of cellular redox state. Chem. Commun. 2020, 56, 2240–2243. [Google Scholar] [CrossRef]
- Alonso-Navarro, M.J.; Harbuzaru, A.; de Echegaray, P.; Arrechea-Marcos, I.; Harillo-Bañs, A.; de la Peña, A.; Ramos, M.M.; Navarrete, J.T.L.; Campoy-Quiles, M.; Ortiz, R.P.; et al. Effective in-terplay of donor and acceptor groups for tuning optoelectronic properties in oligothiophene–naphthalimide assemblies. J. Mater. Chem. C 2020, 8, 15277–15289. [Google Scholar] [CrossRef]
- Martῐn, E.; Weigand, R. A correlation between redox potentials and photophysical behaviour of compounds with intramolecular charge transfer: Application to N-substituted 1,8-naphthalimide derivatives. Chem. Phys. Lett. 1998, 288, 52–58. [Google Scholar] [CrossRef]
- Udhayakumari, D. Various Sensing Mechanisms for the Design of Naphthalimide based Chemosensors Emerging in Recent Years. Recent Innov. Chem. Eng. Former. Recent Patents Chem. Eng. 2020, 13, 262–289. [Google Scholar] [CrossRef]
- Hoche, J.; Schmitt, H.-C.; Humeniuk, A.; Fischer, I.; Mitrić, R.; Rohr, M.I. The mechanism of excimer formation: An experimental and theoretical study on the pyrene dimer. Phys. Chem. Chem. Phys. 2017, 19, 25002–25015. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, H.; Zhang, S.; Gu, Q.; Shen, Y.; Ge, Y.; Yang, B. Excimer formation and evaluation of excited state properties in discrete dimeric stacking of an anthracene derivative: A computational investigation. Phys. Chem. Chem. Phys. 2018, 20, 12129–12137. [Google Scholar] [CrossRef]
- Glowacki, I.; Szamel, Z. The nature of trapping sites and recombination centres in PVK and PVK–PBD elec-troluminescent matrices seen by spectrally resolved thermoluminescence. Phys. D Appl. Phys. 2010, 43, 295101. [Google Scholar] [CrossRef]
- Peng, Z.; Ji, Y.C.; Huang, Z.; Tong, B.; Shi, J.; Dong, Y. A strategy for the molecular design of aggregation-induced emission units further modified by substituents. Mater. Chem. Front. 2018, 2, 1175–1183. [Google Scholar] [CrossRef]
- Mao, H.T.; Yang, Y.; Zhao, K.Y.; Duan, Y.C.; Song, W.L.; Shan, G.G. Fine-tuning emission color of aggrega-tion-induced emission-active Ir(III) phosphors through simple ligand modification. Dye. Pigment. 2021, 192, 109439. [Google Scholar] [CrossRef]
- Galán, L.A.; Cordes, D.B.; Slawin, A.M.Z.; Jacquemin, D.; Ogden, M.I.; Massi, M.; Zysman-Colman, E. Analyzing the Relation between Structure and Aggregation Induced Emission (AIE) Properties of Iridi-um(III) Complexes through Modification of Non-Chromophoric Ancillary Ligands. Eur. J. Inorg. Chem. 2019, 19, 152–163. [Google Scholar] [CrossRef]
- Lee, J.; Park, Y.; Jung, J.; Han, W.-S. Blue-shifted aggregation-induced emission of siloles by simple structural modification and their application as nitro explosive chemosensors. Photochem. Photobiol. Sci. 2017, 16, 1495–1501. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Krasteva, P.V.; Bojinov, V.B. A ratiometric 4-amido-1,8-naphthalimide fluorescent probe based on ex-cimer-monomer emission for determination of pH and water content in organic solvents. J. Lumin. 2019, 212, 271–278. [Google Scholar] [CrossRef]
- Idrees, M.; Bibi, R.; Khan, M.N. Phenanthrene Fluorescence Quenching in Aqueous Sodium Dodecyl Sulphate (SDS) and Determination of Important Metal Ions. J. Fluoresc. 2018, 28, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-F.; Zhang, X.-Q.; Sun, R.; Xu, Y.-J.; Ge, J.-F. Fluorescent probes based 1,8-naphthalimide-nitrogen het-erocyclic for monitoring the fluctuation of mitochondrial viscosity. Dye. Pigment. 2021, 194, 109559. [Google Scholar] [CrossRef]
- Colston, J.; Horobin, R.; Rashid-Doubell, F.; Pediani, J.; Johal, K. Why fluorescent probes for endoplasmic re-ticulum are selective: An experimental and QSAR-modelling study. Biotech. Histochem. 2013, 78, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; de Stefani, D.; Bononi, A.; Rizzuto, R.; Pinton, P. Structural and functional link between the mito-chondrial network and the endoplasmic reticulum. Int. J. Biochem. Cell Biol. 2009, 41, 1817–1827. [Google Scholar] [CrossRef]
- Zych, D.; Slodek, A.; Krompiec, S.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Musiol, R. 4′-Phenyl-2,2′:6′,2′′-terpyridine Derivatives Containing 1-Substituted-2,3-Triazole Ring: Synthesis, Charac-terization and Anticancer Activity. Chem. Sel. 2018, 3, 7009–7017. [Google Scholar] [CrossRef]
- Wang, S.-S.; Du, S.-Y.; He, X.; Qi, Y.-M.; Li, X.-L.; Rong, R.-X.; Cao, Z.-R.; Wang, K.-R. Nucleus-targeting imaging and enhanced cytotoxicity based on naphthalimide derivatives. Bioorg. Chem. 2021, 115, 105188. [Google Scholar] [CrossRef]
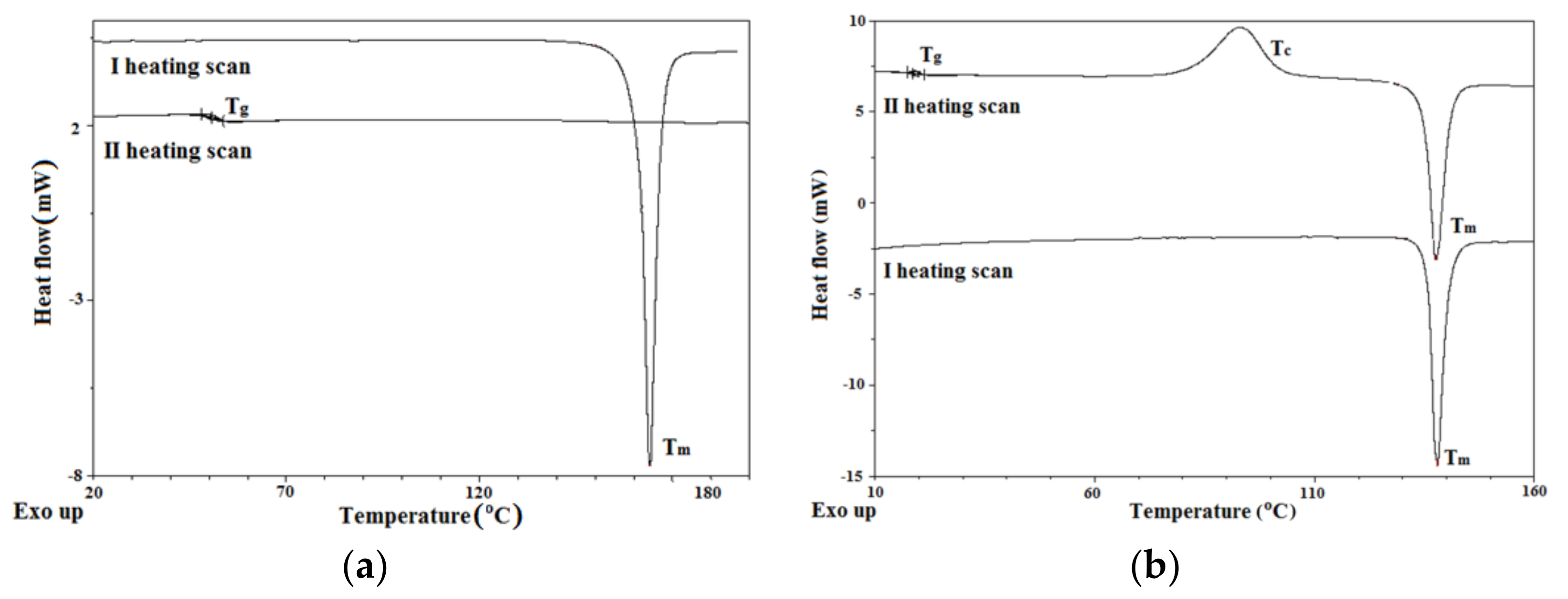





| Molecules | TGA | DSC | |||||
|---|---|---|---|---|---|---|---|
| Scan I | Scan II | ||||||
| T5 a | T10 a | Tmax b | Tm c | Tg e | Tc d | Tm c | |
| (°C) | (°C) | (°C) | (°C) | (°C) | (°C) | (°C) | |
| AzNI-1 | 337 | 354 | 388 | 143 | 35 | nd | nd |
| AzNI-2 | 365 | 387 | 460 | 164 | 51 | nd | nd |
| AzNI-3 | 291 | 312 | 365 | 120 | 18 | 78 | 120 |
| AzNI-4 | 297 | 322 | 376 | 138 | 18 | 93 | 138 |
| AzNI-5 | 309 | 326 | 374 | 153 | 34 | 72 | 152 |
| AzNI-6 | 288 | 327 | 397 | 154 | 53 | nd | nd |
| Molecule | Method | Ered a | Ered(onset) a | Eox a | Eox(onset) a | ELUMO | EHOMO | Eg |
|---|---|---|---|---|---|---|---|---|
| (V) | (V) | (V) | (V) | (eV) | (eV) | (eV) | ||
| AzNI-1 | CV b | −1.91 | −1.61 | 1.33 | 0.99 | −3.49 | −6.09 | 2.60 |
| DPV c | −1.88 | −1.55 | 1.12 | 0.96 | −3.55 | −6.06 | 2.52 | |
| AzNI-2 | CV | −1.87 | −1.58 | 0.83 | 0.76 | −3.52 | −5.86 | 2.34 |
| DPV | −1.80 | −1.52 | 0.77 | 0.68 | −3.58 | −5.78 | 2.20 | |
| AzNI-3 | CV | −1.86 | −1.54 | 0.88 | 0.65 | −3.56 | −5.75 | 2.21 |
| DPV | −1.85 | −1.53 | 0.87 | 0.55 | −3.57 | −5.65 | 2.23 | |
| AzNI-4 | CV | −1.94 | −1.70 | 0.82 | 0.74 | −3.45 | −5.84 | 2.44 |
| DPV | −2.03 | −1.67 | 0.72 | 0.52 | −3.46 | −5.62 | 2.19 | |
| AzNI-5 | CV | −1.57 | −1.27 | broad wave | 1.03 | −3.83 | −6.08 | 2.33 |
| DPV | −1.36 | −1.14 | 0.77 | 0.55 | −3.96 | −5.62 | 1.69 | |
| AzNI-6 | CV | −1.58 | −1.36 | 0.92 | 0.69 | −3.74 | −5.79 | 1.95 |
| DPV | −1.44 | −1.21 | 0.86 | 0.65 | −3.89 | −5.75 | 1.86 |
| Molecules | Medium | UV-Vis λmax (nm) (ε∙104) a | λex (nm) | λem (nm) | Φ (%) | τ (ns) |
|---|---|---|---|---|---|---|
| AzNI-1 | CHCl3 | 340 (4.02), 388 sh | 337, 410 | 519 | 0.6 | 12.03 |
| CH2Cl2 | 338 (4.59), 386 sh | 334, 412 | 500, 524 sh | - | - | |
| CO(CH3)2 | 338 (4.15), 380 sh | 326, 380 | 495, 515 sh | - | - | |
| CH3OH | 338 (4.46), 383 sh | 341, 430 | 541 | - | - | |
| CH3CN | 336 (4.20), 381 sh | 336, 422 | 500, 513 | - | - | |
| Blend PVK:PBD b | 310 sh, 344 sh | - | 378, 476 | 3.6, 2.1 | - | |
| Blend PVK:PBD c | 310 sh, 344 sh | - | 380, 486 | 2.9, 1.4 | - | |
| AzNI-2 | CHCl3 | 346 (3.87), 385 sh | 329 sh, 341, 410, 427 sh | 519 | 3.5 | 14.0 |
| CH2Cl2 | 345 (3.95), 380 sh | 329 sh, 340, 408, 427 sh | 510 | - | - | |
| CO(CH3)2 | 342 (3.93), 380 sh | 341, 410 sh, 425 | 507 | - | - | |
| CH3OH | 342 (3.48), 378 sh | 326 sh, 340, 407 sh, 425 | 532 | - | - | |
| CH3CN | 340 (3.67), 378 sh | 322 sh, 340, 409, 423 sh | 495 | - | - | |
| Film | 342 | - | 525 | 3.5 | - | |
| Blend PVK:PBD b | 310 sh, 344 sh | - | 379, 477 | 4.6, 3.2 | - | |
| Blend PVK:PBD c | 310 sh, 344 sh | - | 379, 493 | 5.9, 2.5 | - | |
| AzNI-3 | CHCl3 | 328 (2.86), 346 (0.82) | 325 sh, 342, 408, 425 sh | 520 | 1.2 | 12.0 |
| CH2Cl2 | 342 sh, 382 sh, 428 (0.18) | 326 sh, 340, 409, 423 sh | 510 | - | - | |
| CO(CH3)2 | 336 (2.23), 380 sh, 434 (0.13) | 341, 410 sh, 425 | 510 | - | - | |
| CH3OH | 347 sh, 379 sh, 440 (0.23) | 342, 408 sh, 426 | 540 | - | - | |
| CH3CN | 338 sh, 378 sh, 426 (0.14) | 326 sh, 340, 408 sh, 425 | 510 | - | - | |
| Film | 325 | - | 390 | 1.1 | - | |
| Blend PVK:PBD b | 310 sh, 344 sh | - | 391, 481 | 2.1, 2.4 | - | |
| Blend PVK:PBD c | 310 sh, 344 sh | - | 373, 492 | 2.0, 1.8 | - | |
| AzNI-4 | CHCl3 | 339 sh, 389 sh | 328 sh, 342, 409, 426 sh | 520 | 1.0 | 14.6 |
| CH2Cl2 | 330 (3.25), 330 sh | 327 sh, 340, 407, 424 sh | 512 | - | - | |
| CO(CH3)2 | 338 (2.76), 377 sh | 342, 411 sh, 427 | 506 | - | - | |
| CH3OH | 326 (3.02), 376 sh, 430 (0.11) | 342, 406 sh, 427 | 525 | - | - | |
| CH3CN | 327 (2.80), 378 sh | 324 sh, 339, 410, 427 sh | 416, 492 | - | - | |
| Film | 338 sh, 376 sh, 397 sh | - | - | - | - | |
| Blend PVK:PBD b | 310 sh, 344 sh | - | 392, 465 sh | 1.3 | - | |
| Blend PVK:PBD c | 310 sh, 344 sh | - | 376, 493 | 0.8, 1.1 | - | |
| AzNI-5 | CHCl3 | 341 (3.11), 375 sh | 328 sh, 340, 409, 425 sh | 524 | 1.0 | 10.4 |
| CH2Cl2 | 337 (3.11), 377 sh | 326 sh, 341, 409, 424 sh | 511 | - | - | |
| CO(CH3)2 | 339 (3.00), 375 sh | 341, 410 sh, 425 | 509 | - | - | |
| CH3OH | 335 (3.05), 380 sh | 342, 409 sh, 428 | 548 | - | - | |
| CH3CN | 336 (3.20), 375 sh | 326 sh, 340, 409 sh, 425 | 507 | - | - | |
| Film | 354, 395 sh, 423 sh | - | - | - | - | |
| Blend PVK:PBD b | 310 sh, 344 sh | - | 380, 469 | 1.2, 0.9 | - | |
| Blend PVK:PBD c | 310 sh, 344 sh | - | 377, 469 | 1.4, 1,1 | - | |
| AzNI-6 | CHCl3 | 331 (3.30), 381 sh | 342, 408, 424 sh | 430, 514 | 3.2 | 11.5 |
| CH2Cl2 | 332 (3.38), 383 sh | 326 sh, 340, 406, 424 sh | 432, 504 | - | - | |
| CO(CH3)2 | 337 (3.15), 382 sh | 340, 410 sh, 425 | 426, 500 | - | - | |
| CH3OH | 335 sh, 330 sh, 450 sh | 342, 410 sh, 425 | 434, 536 | - | - | |
| CH3CN | 334 sh, 379 sh | 324 sh, 340, 412, 424 | 428, 502 | - | - | |
| Film | 328 | - | 504 | 1.2 | - | |
| Blend PVK:PBD b | 310 sh, 344 sh | - | 395, 468 | 3.6, 2.8 | - | |
| Blend PVK:PBD c | 310 sh, 344 sh | - | 380, 492 | 3.3, 4.2 | - |
| The Active Layer Construction | ||||
|---|---|---|---|---|
| Molecules | Parameters | /AzNI/ | /PVK:PBD:AzNI 2 wt.%/ | /PVK:PBD:AzNI 15 wt.%/ |
| AzNI-1 | λEL (nm) | - | 497 | 565 |
| UELMax (V) | - | 14 | 26 | |
| EL intensity (counts) | - | 7332 | 2425 | |
| AzNI-2 | λ EL (nm) | 636 | 507 | 525 |
| UELMax (V) | 16 | 27 | 20 | |
| EL intensity (counts) | 6390 | 17410 | 19,602 | |
| AzNI-3 | λ EL (nm) | - | 504 | 507 |
| UELMax (V) | - | 25 | 17 | |
| EL intensity (counts) | - | 62,103 | 25,123 | |
| AzNI-4 | λ EL (nm) | - | 495 | 525 |
| UELMax (V) | - | 17 | 25 | |
| EL intensity (counts) | - | 44,236 | 3058 | |
| AzNI-5 | λ EL (nm) | - | 503 | - |
| UELMax (V) | - | 26 | - | |
| EL intensity (counts) | - | 8956 | - | |
| AzNI-6 | λ EL (nm) | - | 500 | 536 |
| UELMax (V) | - | 25 | 24 | |
| EL intensity (counts) | - | 39205 | 1280 | |
| Molecules | Activity [µM] | |||
|---|---|---|---|---|
| MCF-7 | HCT 116 | U-87 | NHDF | |
| AzNI-1 | >25 | >25 | >25 | >25 |
| AzNI-2 | >25 | >25 | >25 | >25 |
| AzNI-3 | >25 | >25 | >25 | >25 |
| AzNI-4 | >25 | >25 | >25 | >25 |
| AzNI-5 | >25 | >25 | >25 | >25 |
| AzNI-6 | >25 | >25 | >25 | >25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotowicz, S.; Korzec, M.; Malarz, K.; Krystkowska, A.; Mrozek-Wilczkiewicz, A.; Golba, S.; Siwy, M.; Maćkowski, S.; Schab-Balcerzak, E. Luminescence and Electrochemical Activity of New Unsymmetrical 3-Imino-1,8-naphthalimide Derivatives. Materials 2021, 14, 5504. https://doi.org/10.3390/ma14195504
Kotowicz S, Korzec M, Malarz K, Krystkowska A, Mrozek-Wilczkiewicz A, Golba S, Siwy M, Maćkowski S, Schab-Balcerzak E. Luminescence and Electrochemical Activity of New Unsymmetrical 3-Imino-1,8-naphthalimide Derivatives. Materials. 2021; 14(19):5504. https://doi.org/10.3390/ma14195504
Chicago/Turabian StyleKotowicz, Sonia, Mateusz Korzec, Katarzyna Malarz, Aleksandra Krystkowska, Anna Mrozek-Wilczkiewicz, Sylwia Golba, Mariola Siwy, Sebastian Maćkowski, and Ewa Schab-Balcerzak. 2021. "Luminescence and Electrochemical Activity of New Unsymmetrical 3-Imino-1,8-naphthalimide Derivatives" Materials 14, no. 19: 5504. https://doi.org/10.3390/ma14195504
APA StyleKotowicz, S., Korzec, M., Malarz, K., Krystkowska, A., Mrozek-Wilczkiewicz, A., Golba, S., Siwy, M., Maćkowski, S., & Schab-Balcerzak, E. (2021). Luminescence and Electrochemical Activity of New Unsymmetrical 3-Imino-1,8-naphthalimide Derivatives. Materials, 14(19), 5504. https://doi.org/10.3390/ma14195504