Assessment of the Functional Properties of 316L Steel Alloy Subjected to Ion Implantation Used in Biotribological Systems
Abstract
:1. Introduction
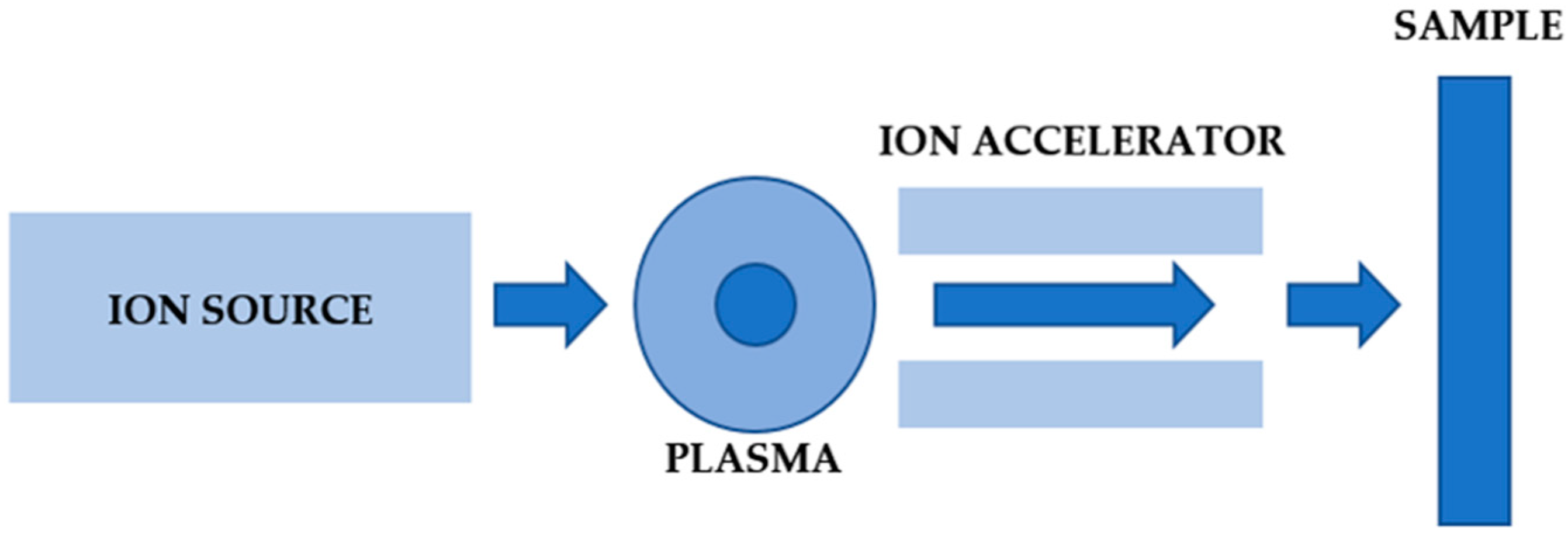
2. Materials and Methods
- where:
- E, v—Young’s modulus and Poisson’s ratio of the tested material;
- Ei, vi—Young’s modulus and Poisson’s ratio of the indenter’s material;
- S—contact stiffness (tangent of the inclination angle of the unloading curve);
- A—contact area calculated from the contact depth and indenter’s geometry calibration.
3. Results
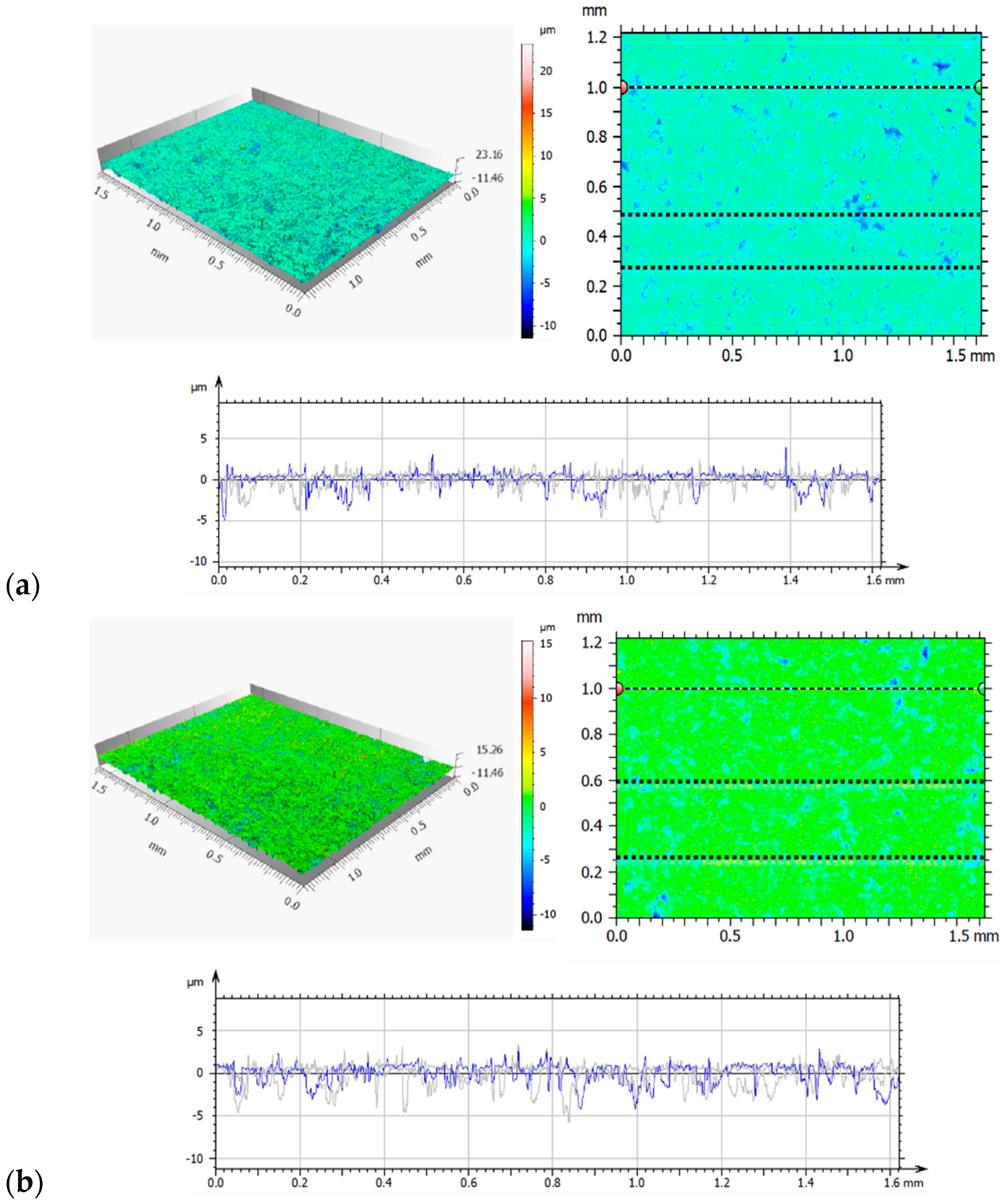
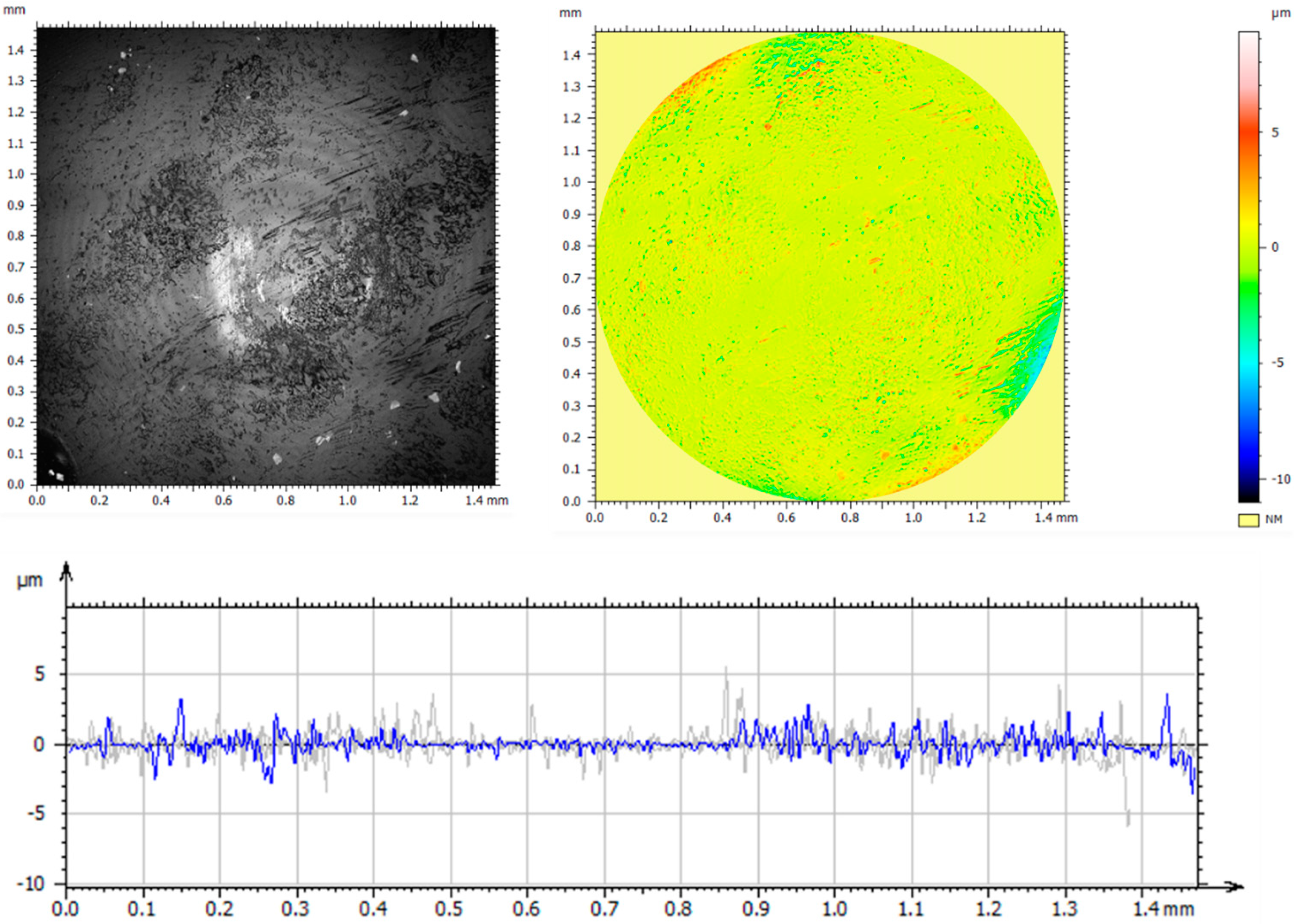
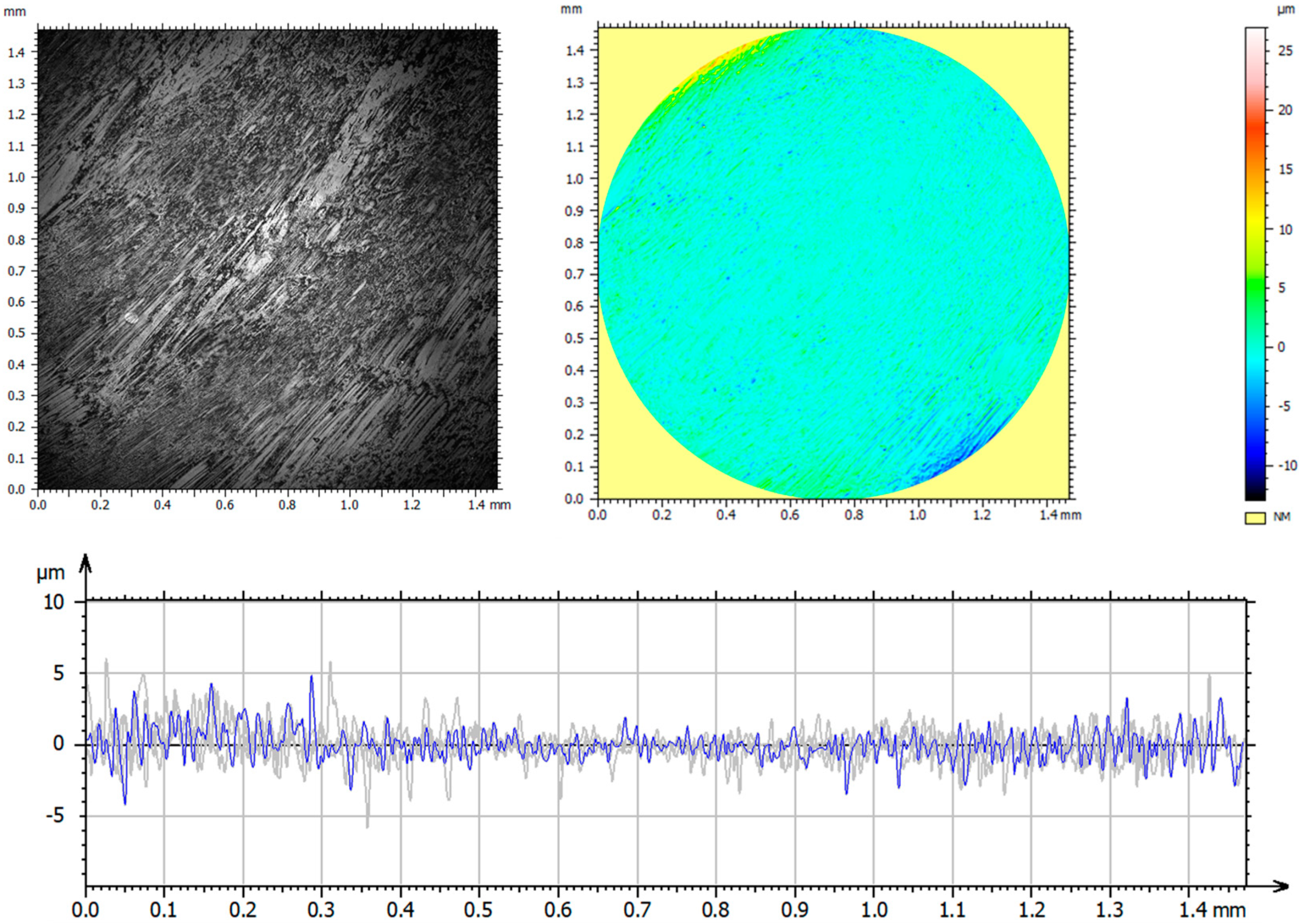
3.1. Confocal Microscopy Results
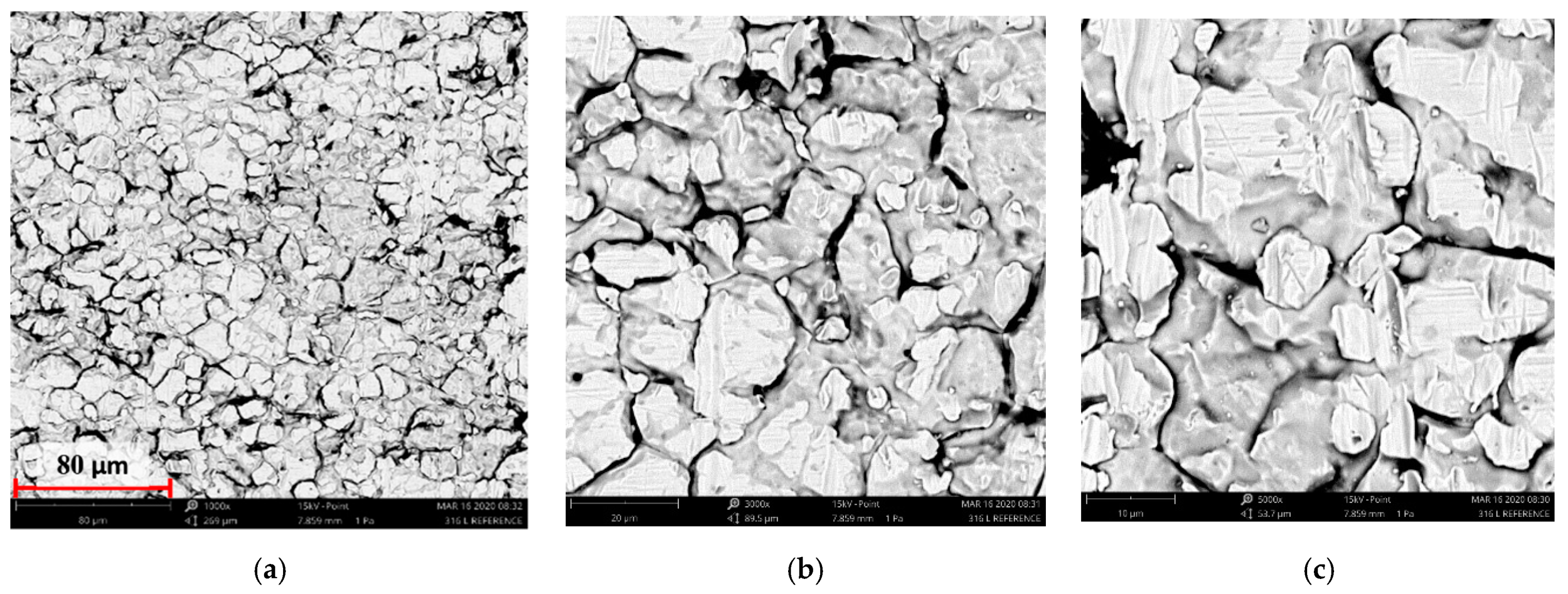
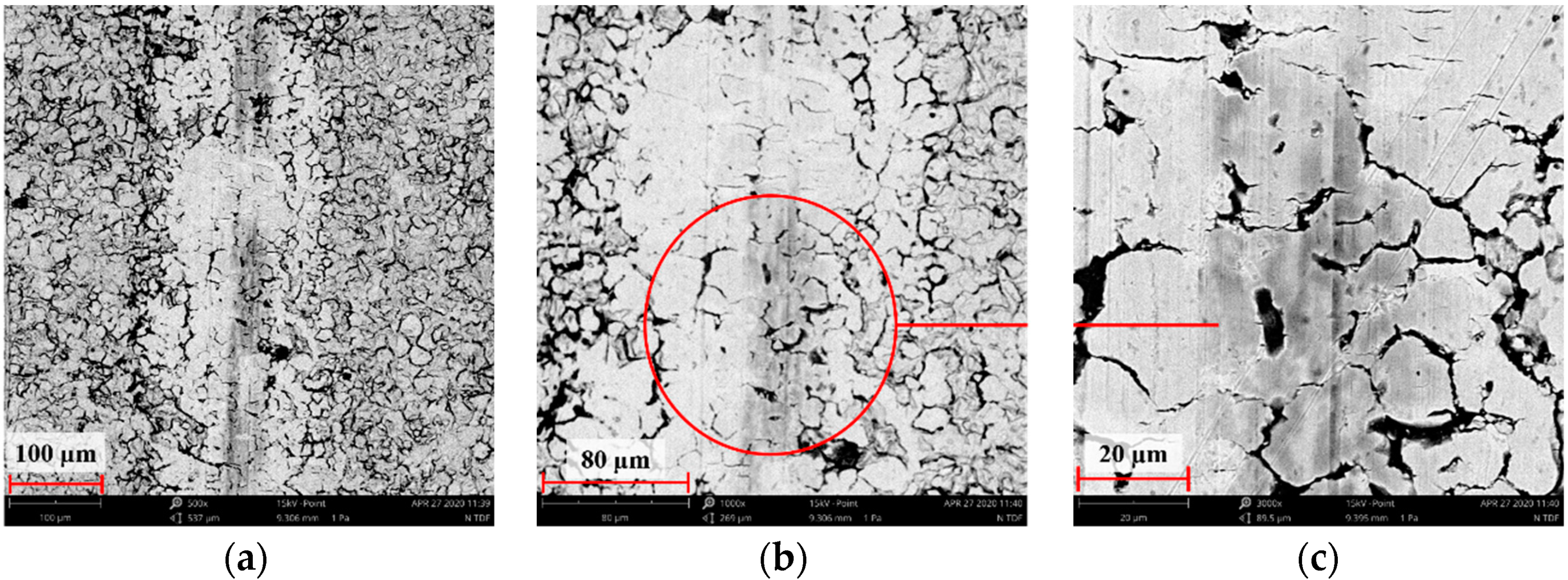
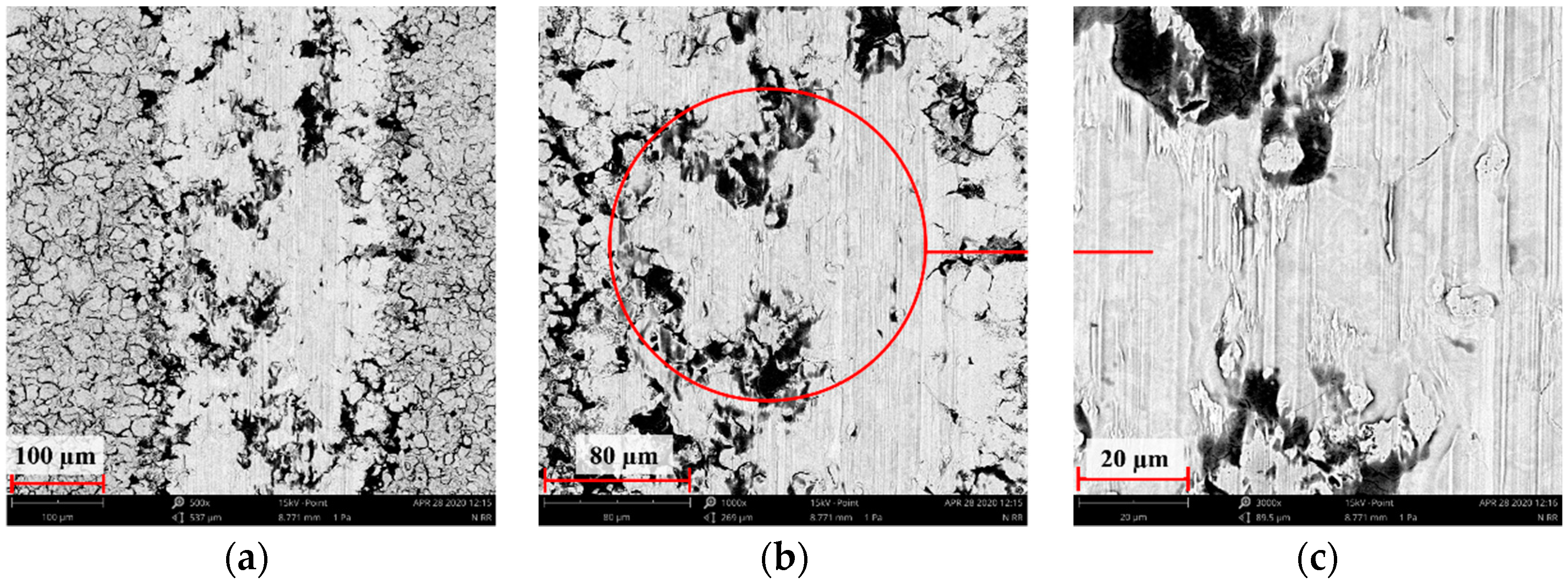
3.2. Scanning Electron Microscopy Results from Surface Morphology Observations
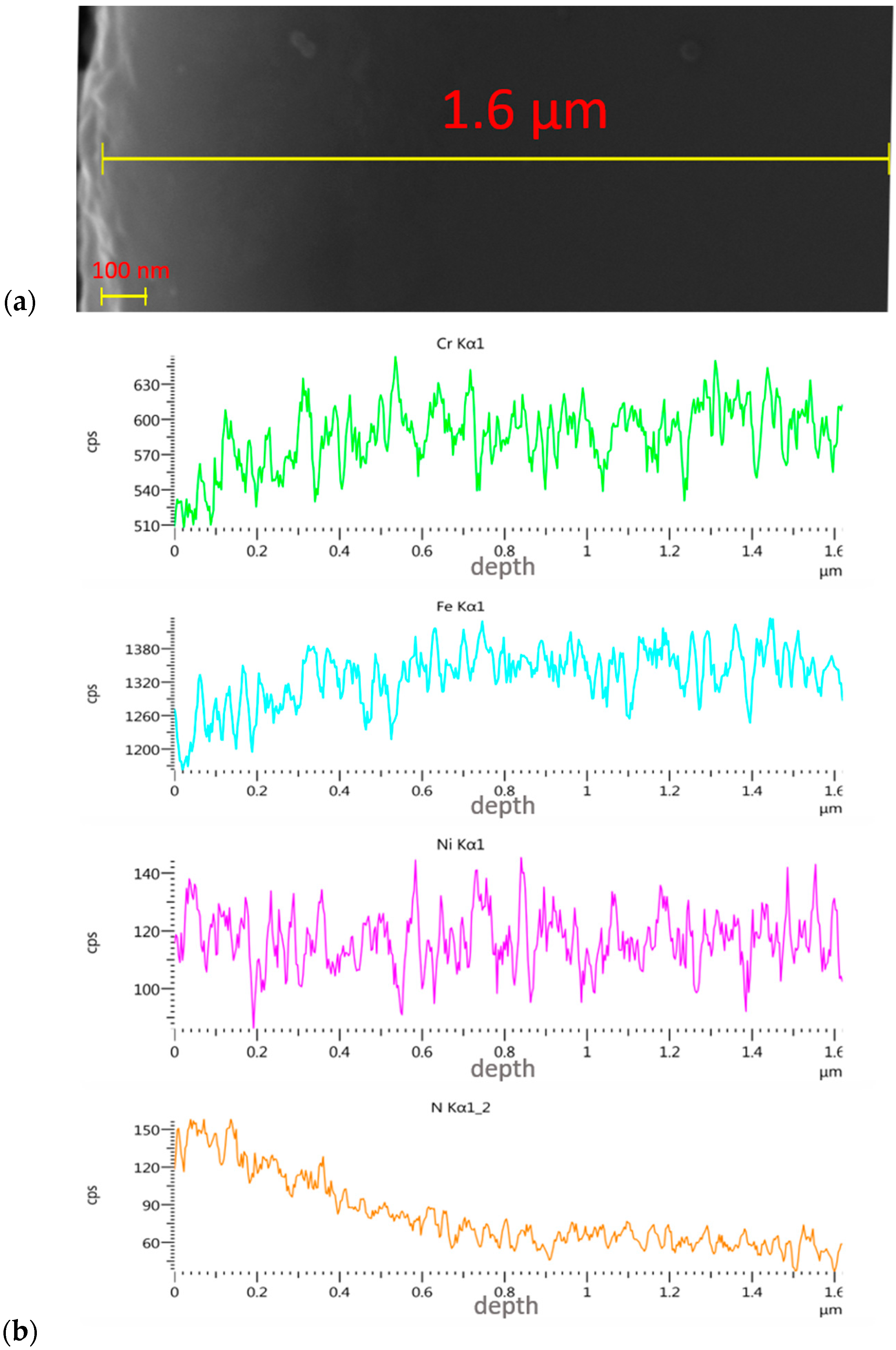
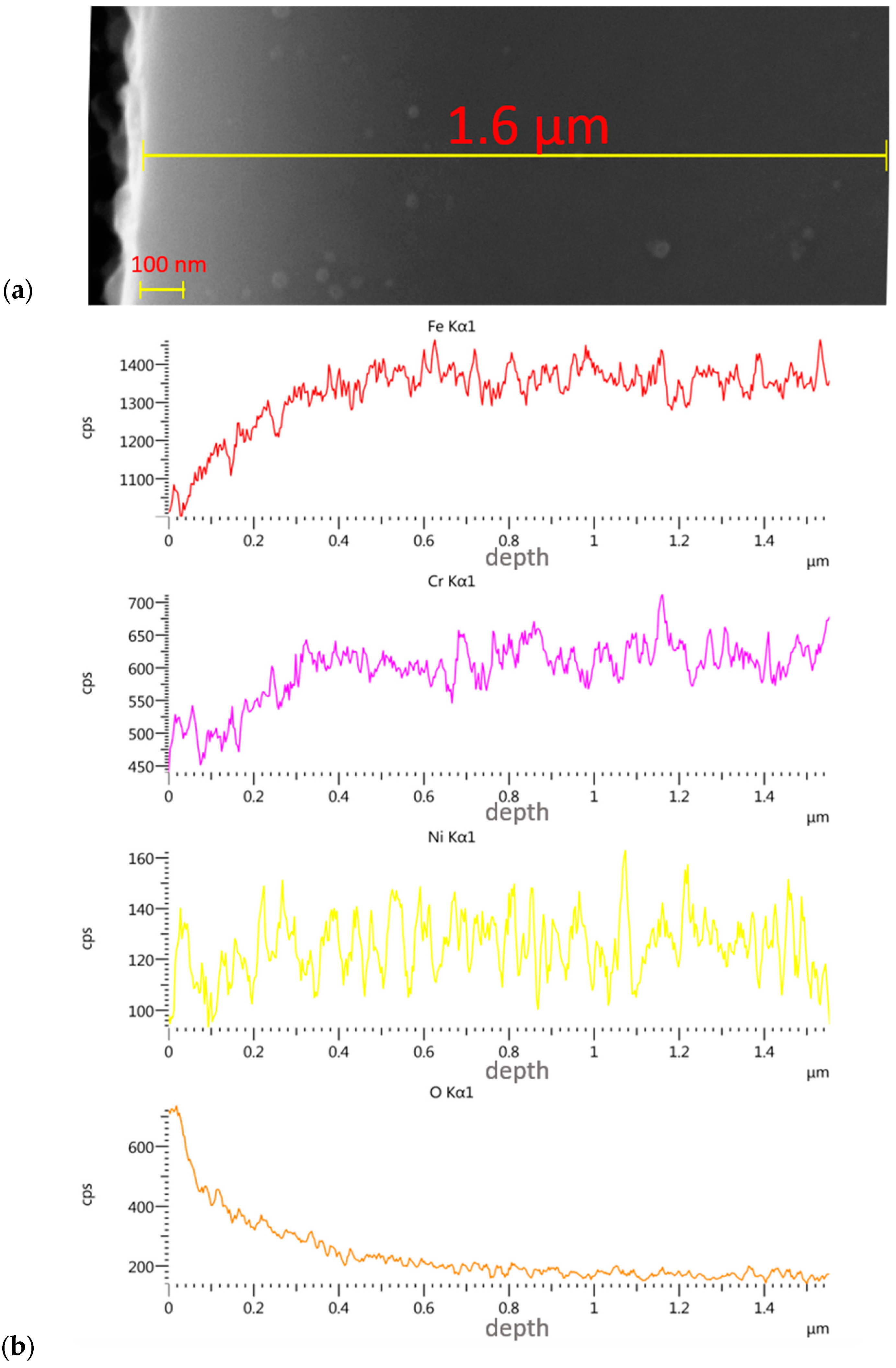
3.3. Assessment of Implanted Layers
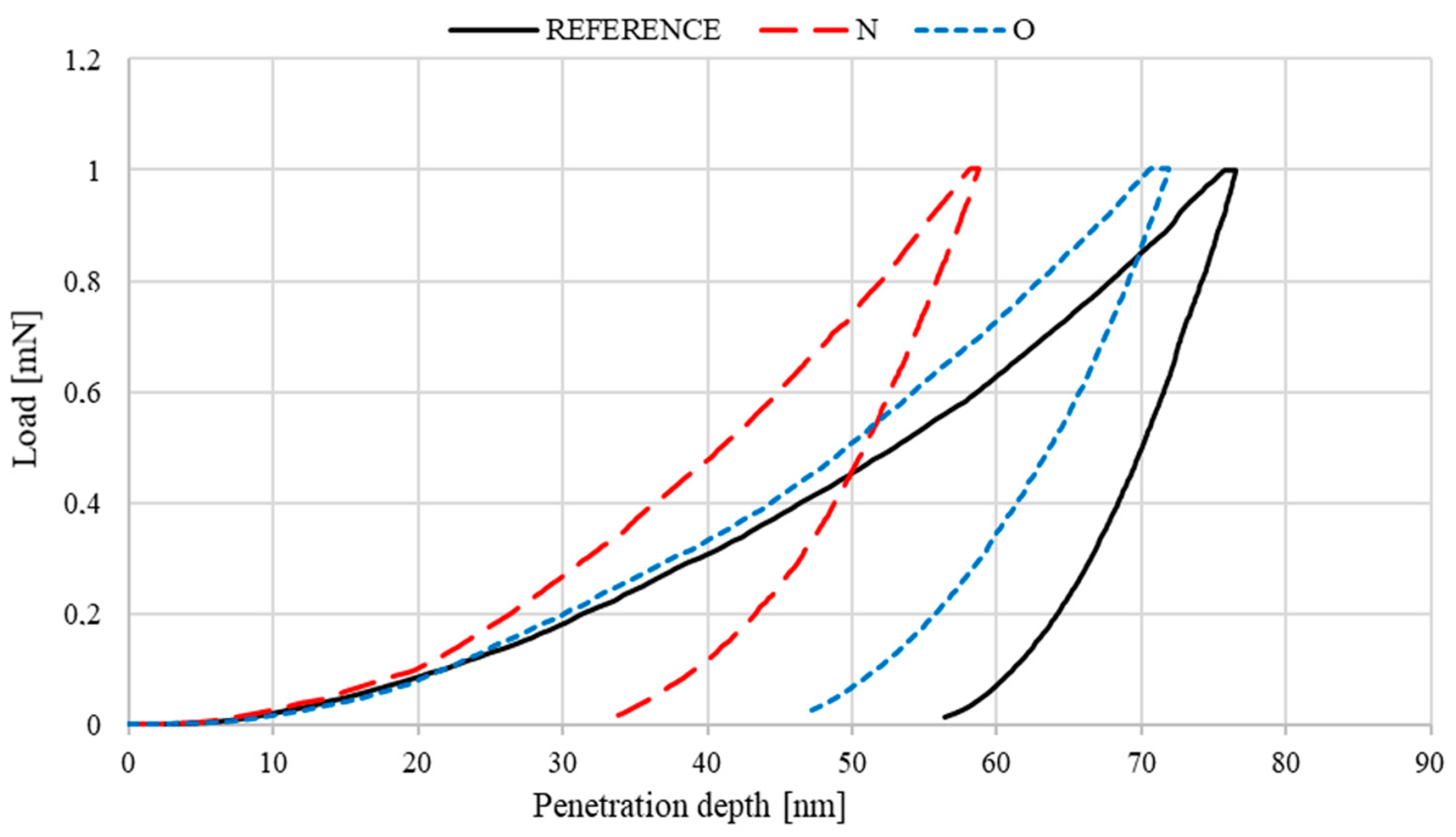
3.4. Nanohardness of Deposited Layers
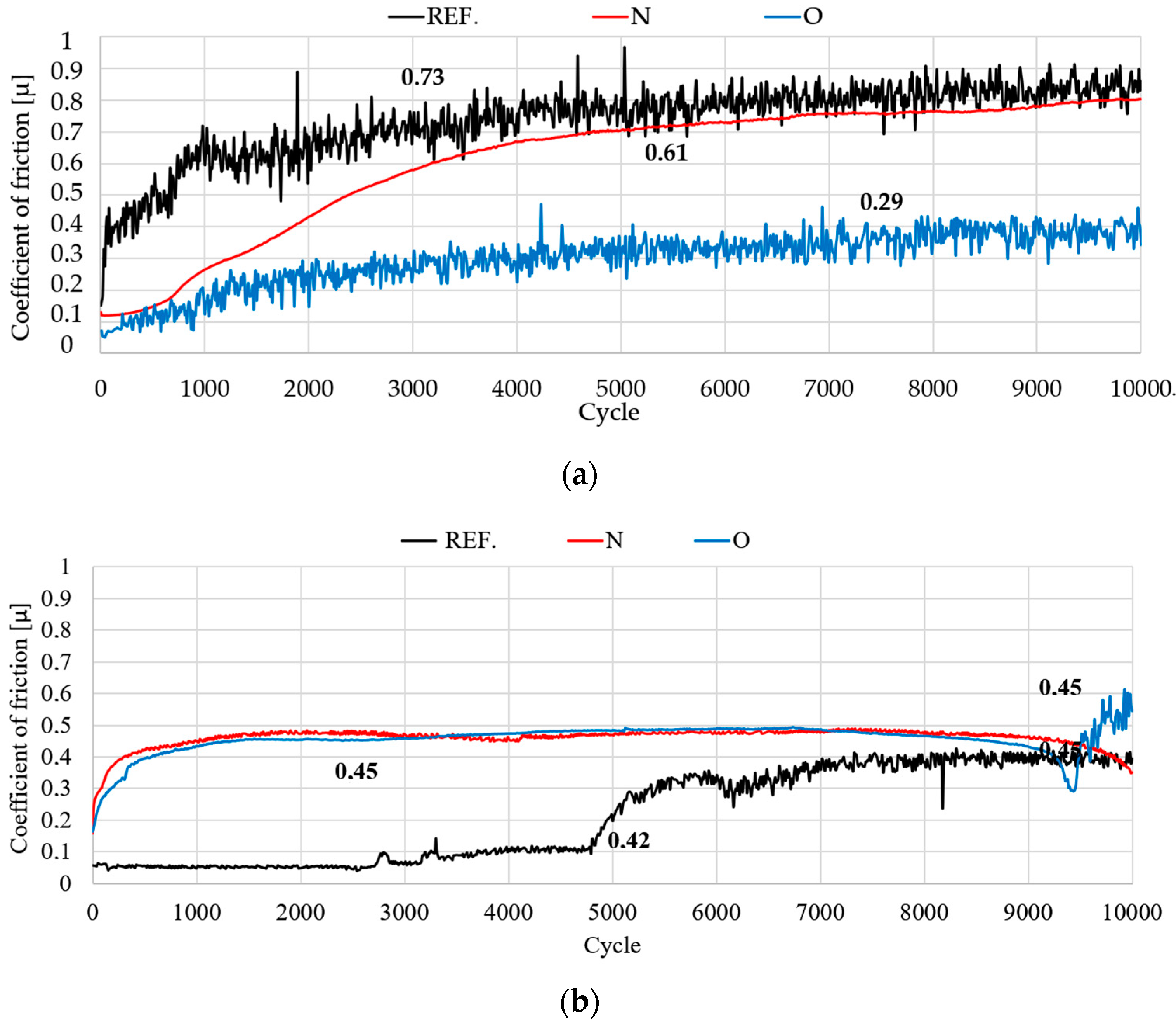
3.5. Tribological Test
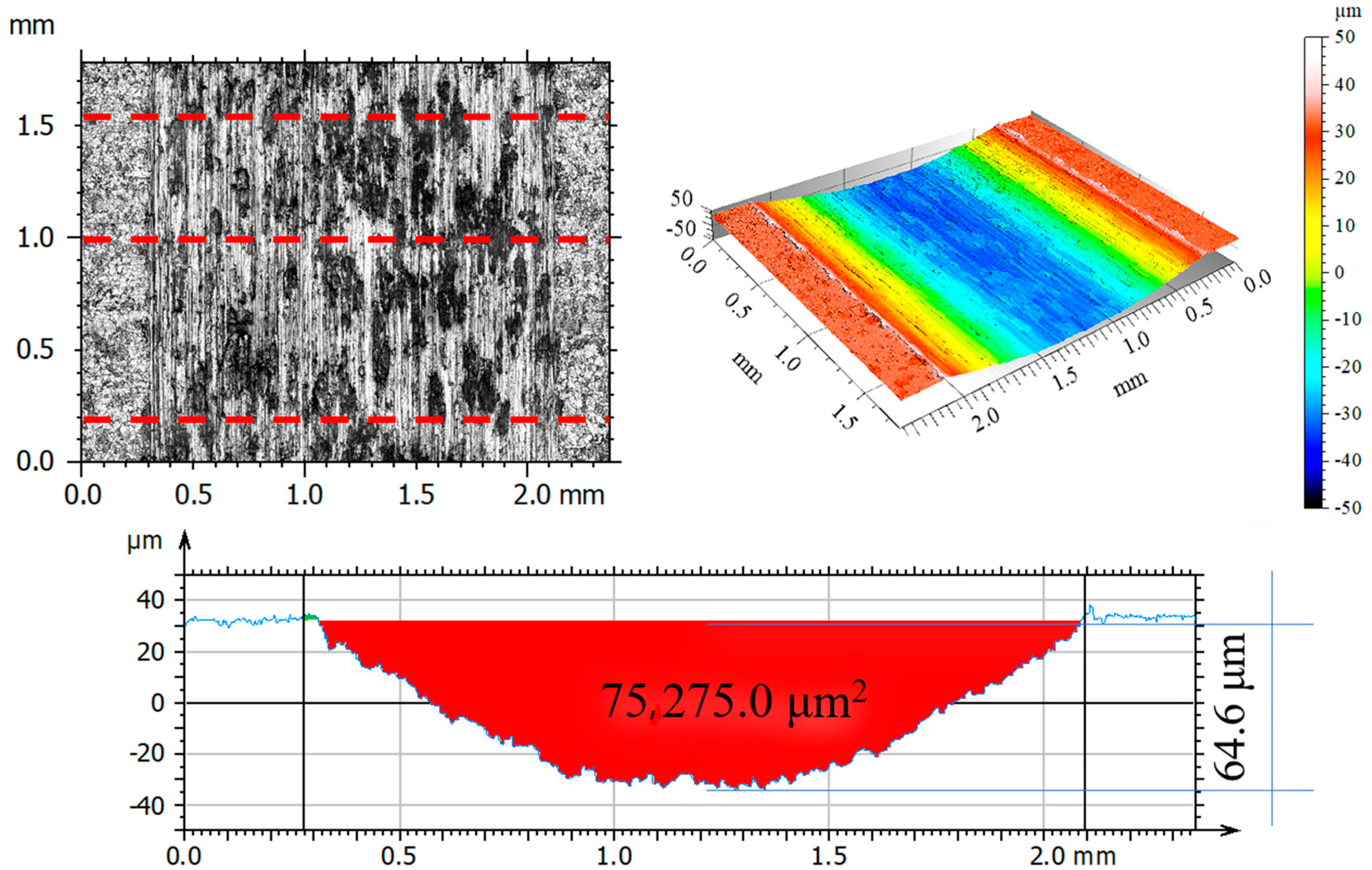
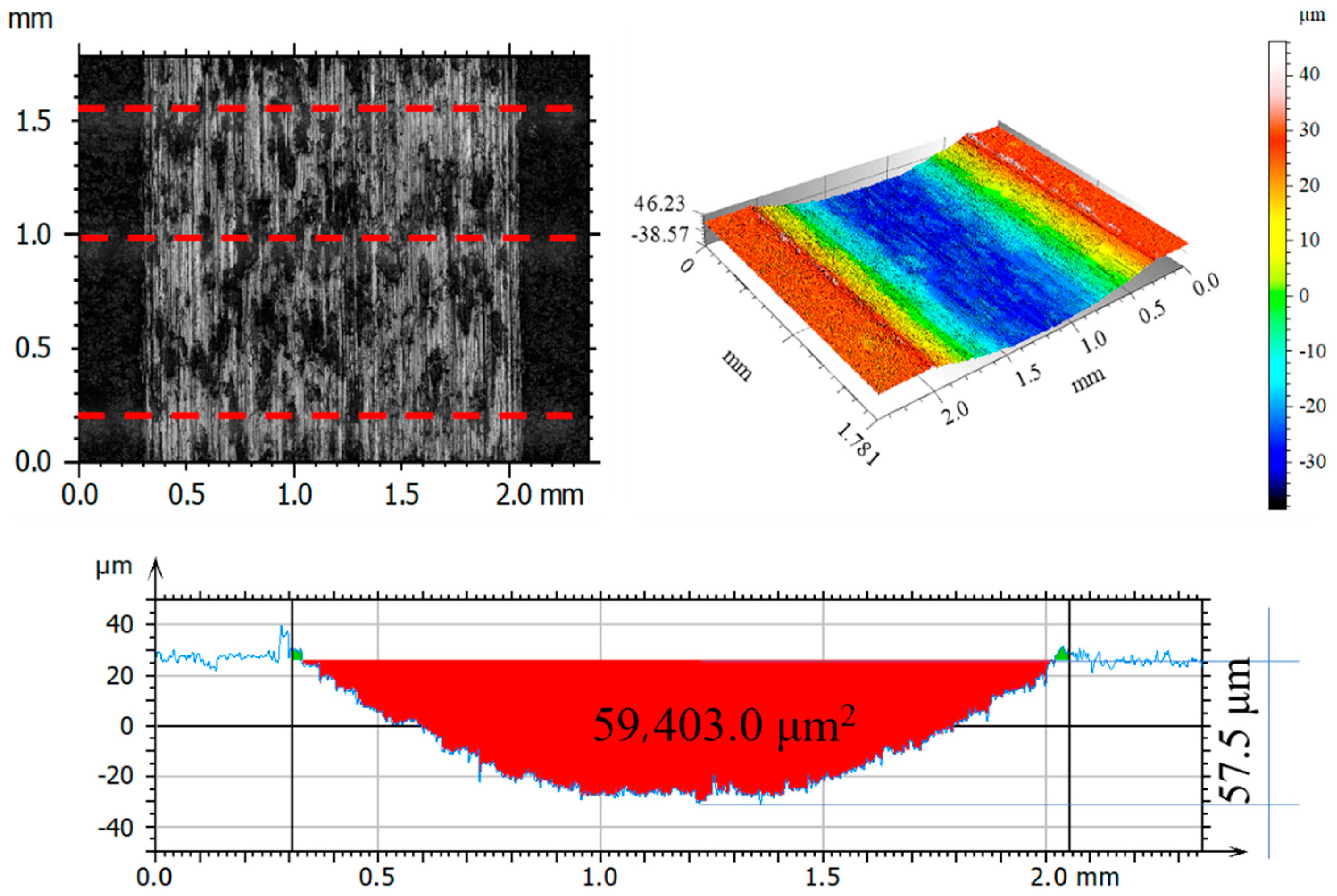
3.6. Assessment of Surface Geometric Structure of Samples and Counter-Samples
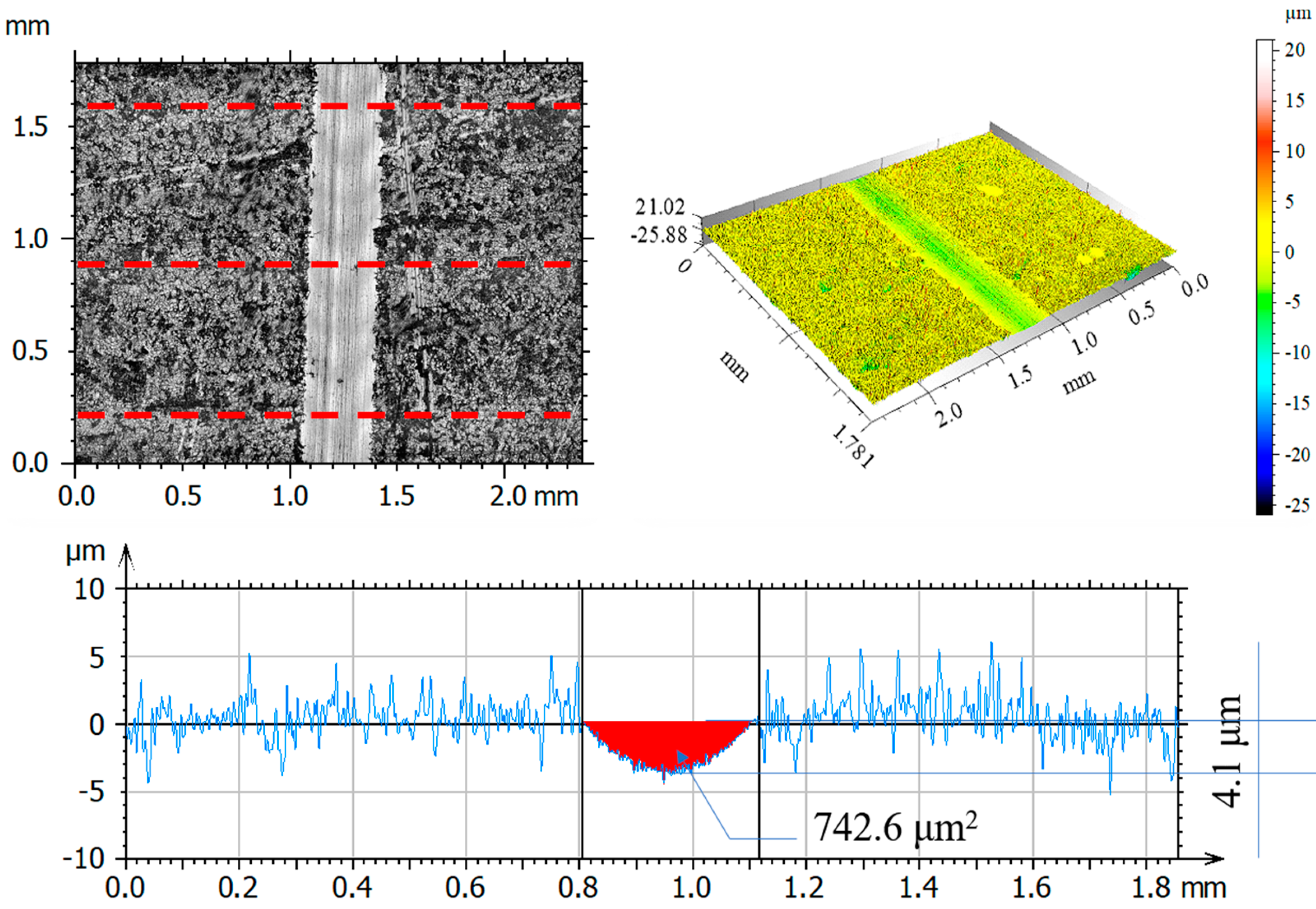
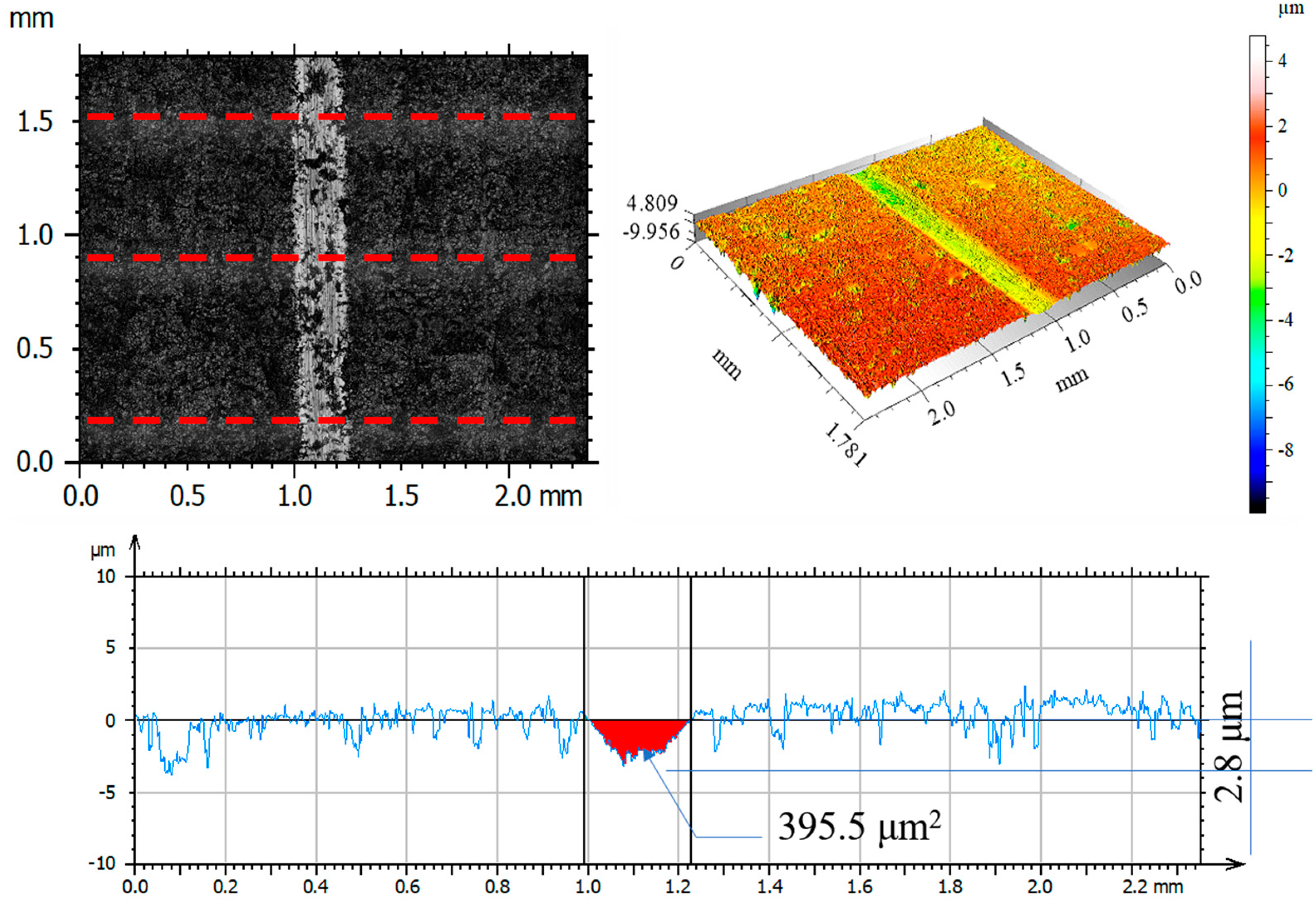
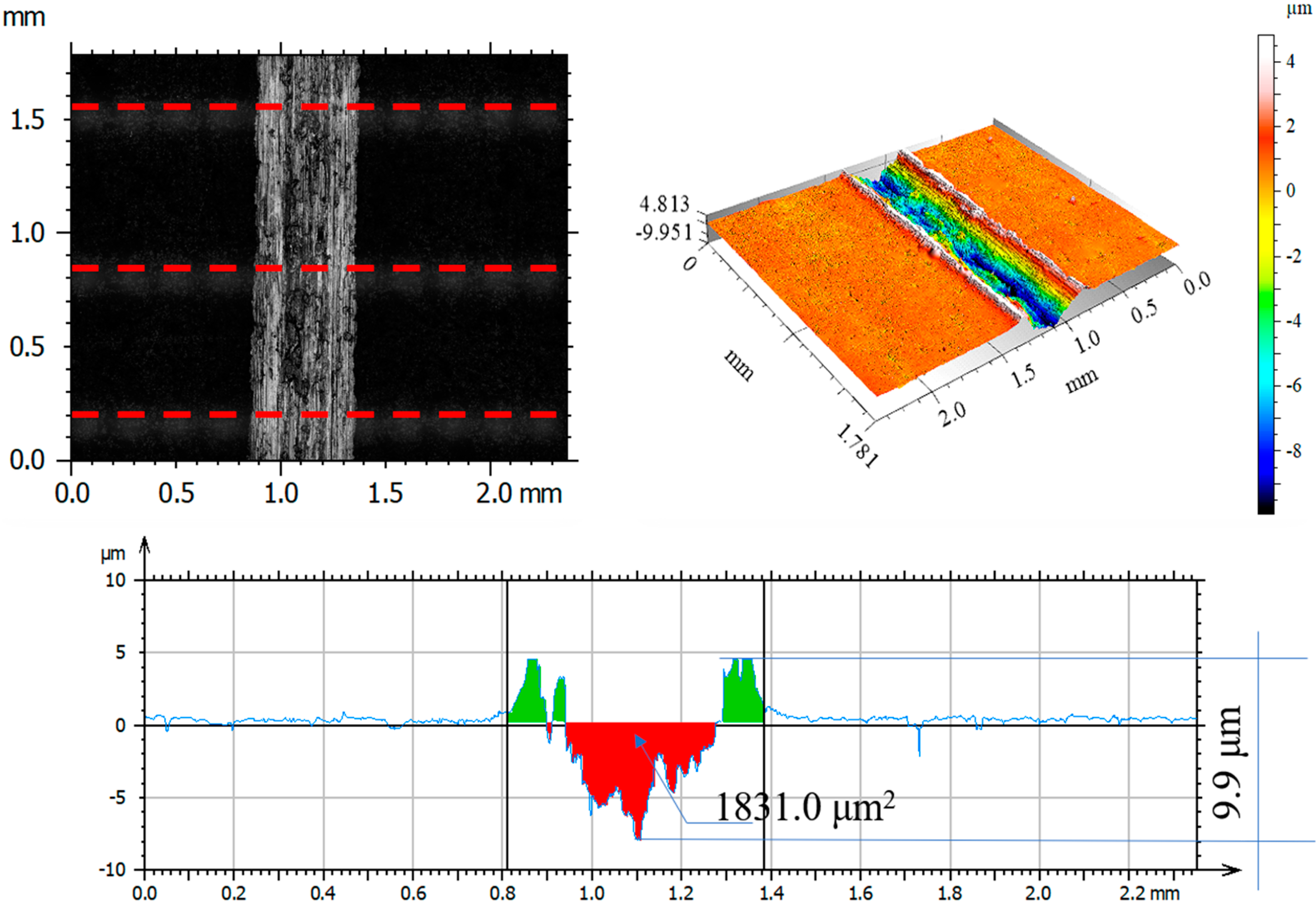
3.7. Assessment of Wear Mechanism
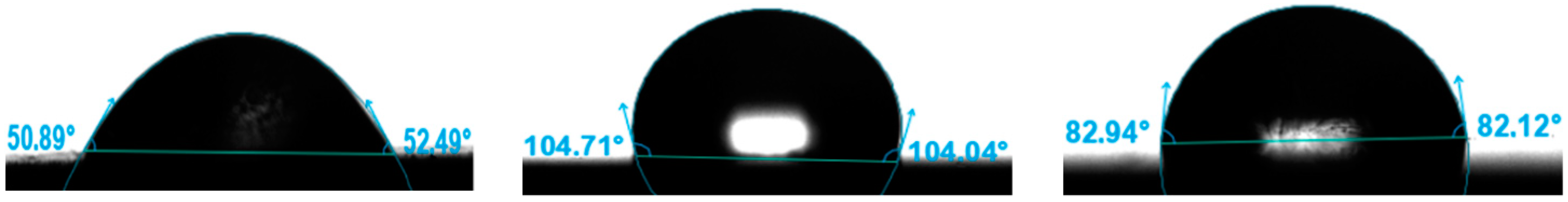
3.8. Contact Angle
4. Conclusions
- The use of ion implantation had a positive effect on the geometric structure of the implanted surface, which became smooth as a result of the process. The smoothing was demonstrated both by the values of all amplitude parameters and the results of the microstructure tests.
- The linear EDS analysis of the elemental distribution demonstrated that, with the same implantation parameters (5 × 1017 N+/cm2 and 5 × 1017 O+/cm2 at an energy of 35 kV), the nitrogen ions were implanted to a depth of about 500 nm and the oxygen ions to a depth of about 350 nm.
- Mechanical tests showed a beneficial effect of the proposed process on both hardness and Young’s modulus. Implantation with nitrogen ions provided the highest effectiveness—an approximately 45% increase in hardness and 20% modulus reduction. In addition, the load–penetration depth curves indicated the greater elasticity of the implanted layers compared to those of the reference sample.
- Under dry friction conditions, implanted samples displayed the most beneficial tribological characteristics. The friction coefficient mean values were about 17% lower when nitrogen ions were used and about 60% lower for oxygen ions. The coefficients of friction were comparable in the case of RS lubricated friction.
- The analysis of the surface geometric structure after tribological tests showed that the reference sample was the most worn material in the friction pair with Al2O3 (III). Moreover, the wear of the sample implanted with oxygen ions was also high despite displaying the lowest resistance to motion.
- The microstructure analysis of wear tracks identified abrasive wear as the dominant wear mechanism in the case of the reference sample and that implanted with oxygen ions. The wear tracks were much wider and deeper compared to the 316L N+ sample. The dislodging of loose wear products between friction surfaces resulted in cracks and grooves.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Marciniak, J. Biomateriały w Chirurgii Kostnej, Wyd; Politechniki Śląskiej: Gliwice, Polska, 2002. [Google Scholar]
- Muley, S.; Vidvans, A.N.; Chaudhari, G.P.; Udainiya, S. An assessment of ultra fine grained 316L stainless steel for implant applications. Acta Biomater. 2016, 30, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Sieczka, Ł.; Bohatyrewicz, A.; Pituch, S. Protheses of the hip arthroplasty yesterday and today. Forum Reumatol. 2017, 3, 216–221. [Google Scholar]
- Chin, P.Y.Y.; Cheok, Q.; Głowacz, A.; Caesarendra, W. A Review of In-Vivo and In-Vitro Real-Time Corrosion Monitoring Systems of Biodegradable Metal Implants. Appl. Sci. 2020, 10, 3141. [Google Scholar] [CrossRef]
- Żurek-Świeczko, B. Biomateriały, Gdańsk: Wyd; Politechniki Gdańskiej: Gdańsk, Polska, 2009. [Google Scholar]
- Gołaska, M.B.; Kalemba, I.; Radzikowska, J.; Warmuzek, M.; Rajchel, B.; Rakowski, W. Modification of the titanium surface with a diamond-like carbon coating. Inżynieria Biomateriałów 2006, 56–57, 1–11. [Google Scholar]
- Kajzer, W.; Jaworska, J.; Jelonek, K.; Szeewczenko, J.; Kajzer, A.; Nowińska, K.; Hercog, A.; Kaczmarek, M.; Kasperczyk, J. Corrosion resistance of Ti6Al4V alloy coated with caprolactone-based biodegradable polymeric coatings. Maint. Reliab. 2018, 20, 30–38. [Google Scholar] [CrossRef]
- Li, L.H.; Kim, H.W.; Lee, S.H.; Kong, Y.M.; Kim, H.E. Biocompatibility of titanium implants modified by microarc oxidation and hydroxyapatite coating. J. Biomed. Mater. Res. 2005, 75, 48–54. [Google Scholar] [CrossRef]
- Harle, J.; Kim, H.W.; Mordan, N.; Knowles, J.C.; Salih, V. Initial responses of human osteoblasts to sol–gel modified titanium with hydroxyapatite and titania composition. Acta Biomater. 2006, 2, 547–556. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K.; Filippi, M. Biomaterial Studies on AISI 316L Stainless Steel after Magnetoelectropolishing. Materials 2009, 2, 129–145. [Google Scholar] [CrossRef] [Green Version]
- Pokrowiecki, R.; Szaraniec, B.; Chłopek, J.; Zaleska, M. Recent trends in surface modification of the titanium biomaterials used for endoosseus dental implants. Eng. Biomater. 2014, 124, 2–10. [Google Scholar]
- Vaca, L.S.; Quintana, J.P.; Vega, D.; Marquez, A.; Bruhl, S.P. Tribological and corrosion Behavior of Duplex Coated AISI 316L using plasma based ion implantation and deposition. Mater. Today Commun. 2021, 26, 101892. [Google Scholar] [CrossRef]
- Kula, Z.; Semenov, M.; Klimek, L. Carbon Coatings Deposited on Prosthodontic Ni-Cr Alloy. Appl. Sci. 2021, 11, 4551. [Google Scholar] [CrossRef]
- Bianchi, S.; Fantozzi, G.; Bernardi, S.; Antonouli, S.; Continenza, M.A.; Macchiarelli, G. Commercial oral hygiene products and implant collar surfaces: Scanning electron microscopy observations. Can. J. Dent. Hyg. 2020, 54, 26–31. [Google Scholar] [PubMed]
- Lin, Z.; Li, S.J.; Sun, F.; Ba, D.C.; Li, X.C. Surface characteristics of a dental implant modified by low energy oxygen ion implantation. Surf. Coat. Technol. 2019, 365, 622. [Google Scholar] [CrossRef]
- Komorowski, P.; Siatkowska, M.; Kamińska, M.; Jakubowski, W.; Walczyńska, M.; Walkowiak-Przybyło, M.; Szymański, W.; Piersa, K.; Wielowski, P.; Sokołowska, P.; et al. Comprehensive Biological Evaluation of Biomaterials Used in Spinal and Orthopedic Surgery. Materials 2020, 13, 4796. [Google Scholar] [CrossRef] [PubMed]
- Rajchel, B. Bioaktywne Powłoki na Powierzchni Polietylenu Oraz Poliuretanu; IF PAN: Kraków, Polska, 2013. [Google Scholar]
- Łępicka, M.; Ciszewski, A.; Golak, K.; Dahlke-Grądzka, M. A Comparative Study of Friction and Wear Processes of Model Metallic Biomaterials Including Registration of Friction-Induced Temperature Response of a Tribological Pair. Materials 2019, 12, 4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeztu, M.; Alava, J.I.; Gay-Escoda, C. Ion implantation: Surface treatment for improving the bone integration of titanium and Ti6Al4V dental implants. Clin. Oral Implant. Res. 2003, 14, 57–62. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Jang, J.H.; Park, J.W. Surface Engineering of Nanostructured Titanium Implants with Bioactive Ions. J. Dent. Res. 2016, 95, 558–565. [Google Scholar] [CrossRef]
- Wang, W.; Fu, Z.; Zhu, L.; Yue, W.; Kang, J.; She, D.; Ren, X.; Wang, C. Effects of Titanium-Implanted Dose on the Tribological Properties of 316L Stainless Steel. Materials 2021, 14, 1482. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface modification of biomaterial titanium micromorphology, microstructure evolution biomedical applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, Z. Morfologia Powierzchni Rozpylanej Jonami-Implikacje Technologiczne i Biomedyczne; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Polska, 1989. [Google Scholar]
- Levintant, N. Analysis of the mechanical and shape memory behavior of nitrogen ion-implanted NiTi alloy. Vacuum 2007, 81, 1183–1187. [Google Scholar] [CrossRef]
- Pawelec, K.; Baranowicz, P.; Wysokińska-Miszczuk, J.; Madej, M.; Skóra, M. The tribological properties of the Ti6Al4V alloy by nitrogen ions. Tribologia 2019, 1, 37–47. [Google Scholar] [CrossRef]
- Sylwanowicz, W.; Michajlik, A.; Ramotowski, W. Anatomia i Fizjologia Człowieka; Państwowy Zakład Wydawnictw Lekarskich: Warszawa, Polska, 1991. [Google Scholar]
- Kot, M.; Lacki, P. Contact mechanics of coating-substrate systems: I—Methods of analysis and FEM modeling of nanoindentation tests. J. Balk. Tribol. Assoc. 2012, 18, 598–614. [Google Scholar]
- Tarnowski, J.; Gawędzki, W.; Kot, M. Analysis of elasticity modulus and microhardness of engine piston surface layers in the aspect of its wear. Tribologia 2013, 5, 115–125. [Google Scholar]
- Niemczewska-Wójcik, M.; Wójcik, A. The multi-scale analysis of ceramic surface topography created in abrasive machining process. Measurement 2020, 166, 108217. [Google Scholar] [CrossRef]










| Alloy 316L [% Content] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | Cr | Ni | Mo | C | Si | Mn | P | S | N |
| Ba-lance | 16.5–18.5 | 10.0–13.0 | 2.0–2.5 | <0.03 | <1.0 | <2.0 | <0.045 | <0.015 | <0.011 |
| Parameter | Unit | Friction Pair |
|---|---|---|
| Al2O3 Ball—316L (Reference) Al2O3 Ball—316L Implanted with N+ Ions Al2O3 Ball—316L Implanted with O+ Ions | ||
| Load | N | 5 |
| Linear viscosity | m/s | 0.0159 |
| Cycles | - | 10,000 |
| Frequency | Hz | 1 |
| Humidity | % | 50 ± 1 |
| Temperature | °C | 23 ± 1 |
| Lubrication | - | no lubrication (DF) Ringer’s solution (RS) |
| Chemical Composition [g/dm3] | ||
|---|---|---|
| NaCl | KCl | CaCl2 |
| 8.6 | 0.3 | 0.243 |
| Parametr | Unit | Sample | ||
|---|---|---|---|---|
| 316L | 316L N+ | 316L O+ | ||
| Sp | μm | 23.16 | 12.83 | 15.26 |
| Sv | μm | 12.46 | 9.33 | 10.46 |
| Sz | μm | 35.68 | 22.16 | 25.72 |
| Sa | μm | 0.70 | 0.75 | 0.82 |
| Sq | μm | 1.7 | 1.03 | 1.14 |
| Ssk | 1.48 | -0.67 | 0.96 | |
| Sku | 9.20 | 7.67 | 8.06 | |
| Parameter | Unit | Sample | |||||
|---|---|---|---|---|---|---|---|
| 316L | 316L N+ | 316L O+ | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Instrumented hardness [HIT] | GPa | 6.2 | 0.1 | 11.2 | 0.2 | 7.0 | 0.2 |
| Young’s modulus [EIT] | GPa | 195.4 | 21.9 | 231.2 | 39.9 | 200.0 | 9.3 |
| Contact area [Ap] | μm2 | 0.162 | 0.003 | 0.089 | 0.022 | 0.143 | 0.056 |
| Plastic behaviour [Wplast] | pJ | 17.8 | 2.3 | 10.6 | 3.7 | 14.4 | 1.6 |
| Elastic behaviour [Welast] | pJ | 8.1 | 1.0 | 9.2 | 0.7 | 9.0 | 0.6 |
| Total behaviour [Wtot] | pJ | 25.9 | 1.3 | 19.8 | 4.4 | 23.4 | 2.3 |
| Maximum indentation [hm] | nm | 76.6 | 0.1 | 58.8 | 5.9 | 72.6 | 0.1 |
| Depth of indenter-sample contact at Fmax [hc] | nm | 68.4 | 0.8 | 48.6 | 5.5 | 63.6 | 1.5 |
| Parameter | Unit | Sample | |||||
|---|---|---|---|---|---|---|---|
| 316L | 316L N+ | 316L O+ | |||||
| DF | RS | DF | RS | DF | RS | ||
| Sp | μm | 58.04 | 13.24 | 6.97 | 3.66 | 59.0 | 10.55 |
| Sv | μm | 33.64 | 18.57 | 7.45 | 4.51 | 36.21 | 11.16 |
| Sz | μm | 91.69 | 1.81 | 14.43 | 8.17 | 95.21 | 21.71 |
| Sa | μm | 18.58 | 0.99 | 0.49 | 0.72 | 15.90 | 3.57 |
| Sq | μm | 21.34 | 1.23 | 0.74 | 0.85 | 18.23 | 4.22 |
| Ssk | 0.62 | 1.10 | -2.16 | 0.6 | 0.652 | 0.05 | |
| Sku | 2.07 | 4.73 | 16.94 | 2.53 | 2.14 | 2.12 | |
| Parameter | Unit | Friction Pair | |||
|---|---|---|---|---|---|
| Al2O3 before Test | 316L— Al2O3 after Test | 316L N+— Al2O3 after Test | 316L O+— Al2O3 after Test | ||
| Rp | μm | 1.67 | 2.56 | 2.26 | 2.81 |
| Rv | μm | 1.94 | 2.87 | 1.88 | 3.86 |
| Rz | μm | 3.58 | 5.43 | 4.14 | 6.50 |
| Ra | μm | 0.32 | 0.65 | 0.39 | 0.68 |
| Rq | μm | 0.49 | 0.87 | 0.54 | 0.99 |
| Rsk | −0.54 | −0.26 | 0.26 | −0.71 | |
| Rku | 9.78 | 4.42 | 7.48 | 5.85 | |
| Contact Angle [°] | ||
|---|---|---|
| Mean | SD | |
| 316L | 51.69 | 0.58 |
| 316L N+ | 103.73 | 1.25 |
| 316L O+ | 82.53 | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska, K.; Madej, M.; Ozimina, D. Assessment of the Functional Properties of 316L Steel Alloy Subjected to Ion Implantation Used in Biotribological Systems. Materials 2021, 14, 5525. https://doi.org/10.3390/ma14195525
Piotrowska K, Madej M, Ozimina D. Assessment of the Functional Properties of 316L Steel Alloy Subjected to Ion Implantation Used in Biotribological Systems. Materials. 2021; 14(19):5525. https://doi.org/10.3390/ma14195525
Chicago/Turabian StylePiotrowska, Katarzyna, Monika Madej, and Dariusz Ozimina. 2021. "Assessment of the Functional Properties of 316L Steel Alloy Subjected to Ion Implantation Used in Biotribological Systems" Materials 14, no. 19: 5525. https://doi.org/10.3390/ma14195525
APA StylePiotrowska, K., Madej, M., & Ozimina, D. (2021). Assessment of the Functional Properties of 316L Steel Alloy Subjected to Ion Implantation Used in Biotribological Systems. Materials, 14(19), 5525. https://doi.org/10.3390/ma14195525







