Cr-Based Sputtered Decorative Coatings for Automotive Industry
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
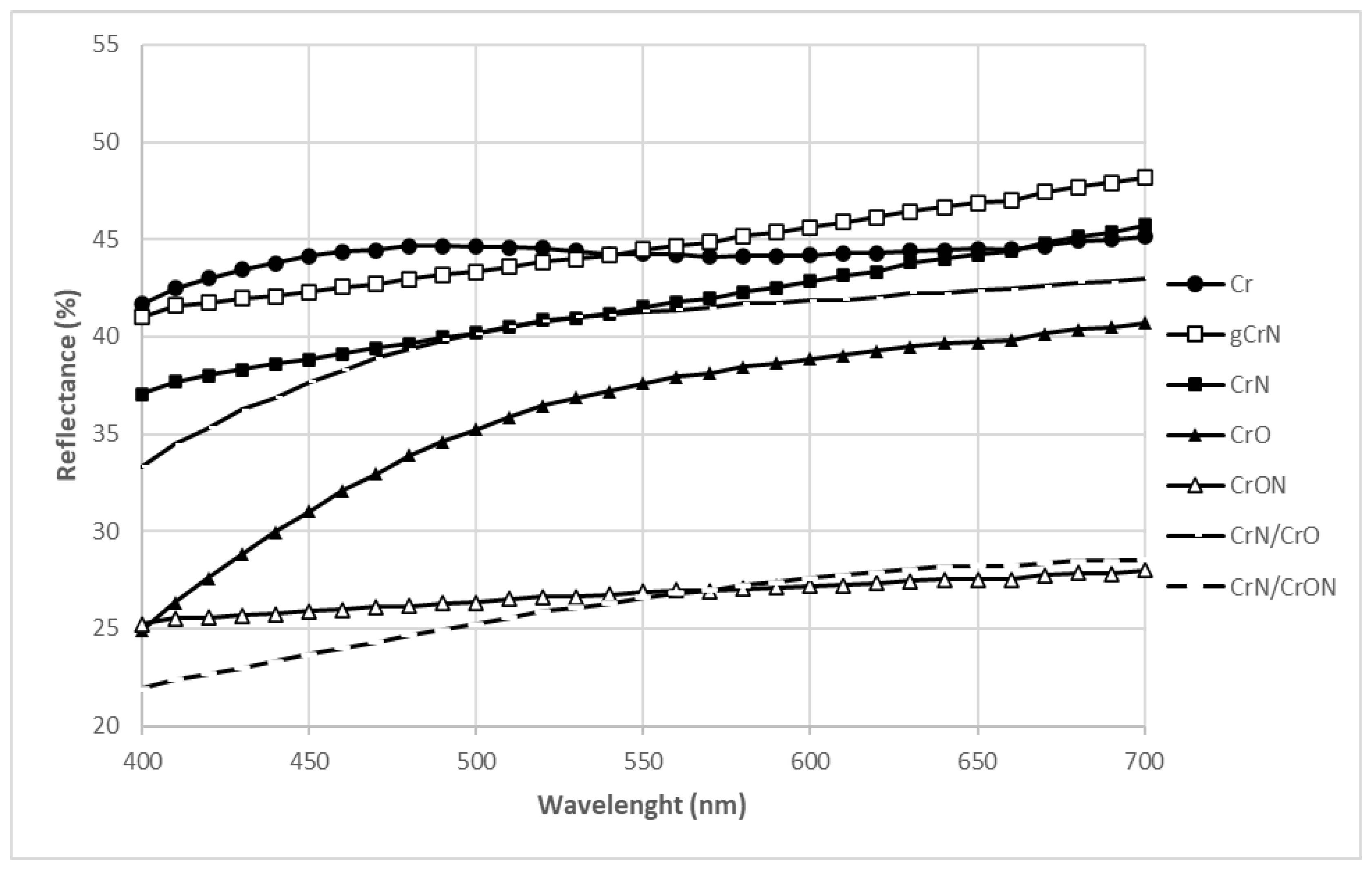
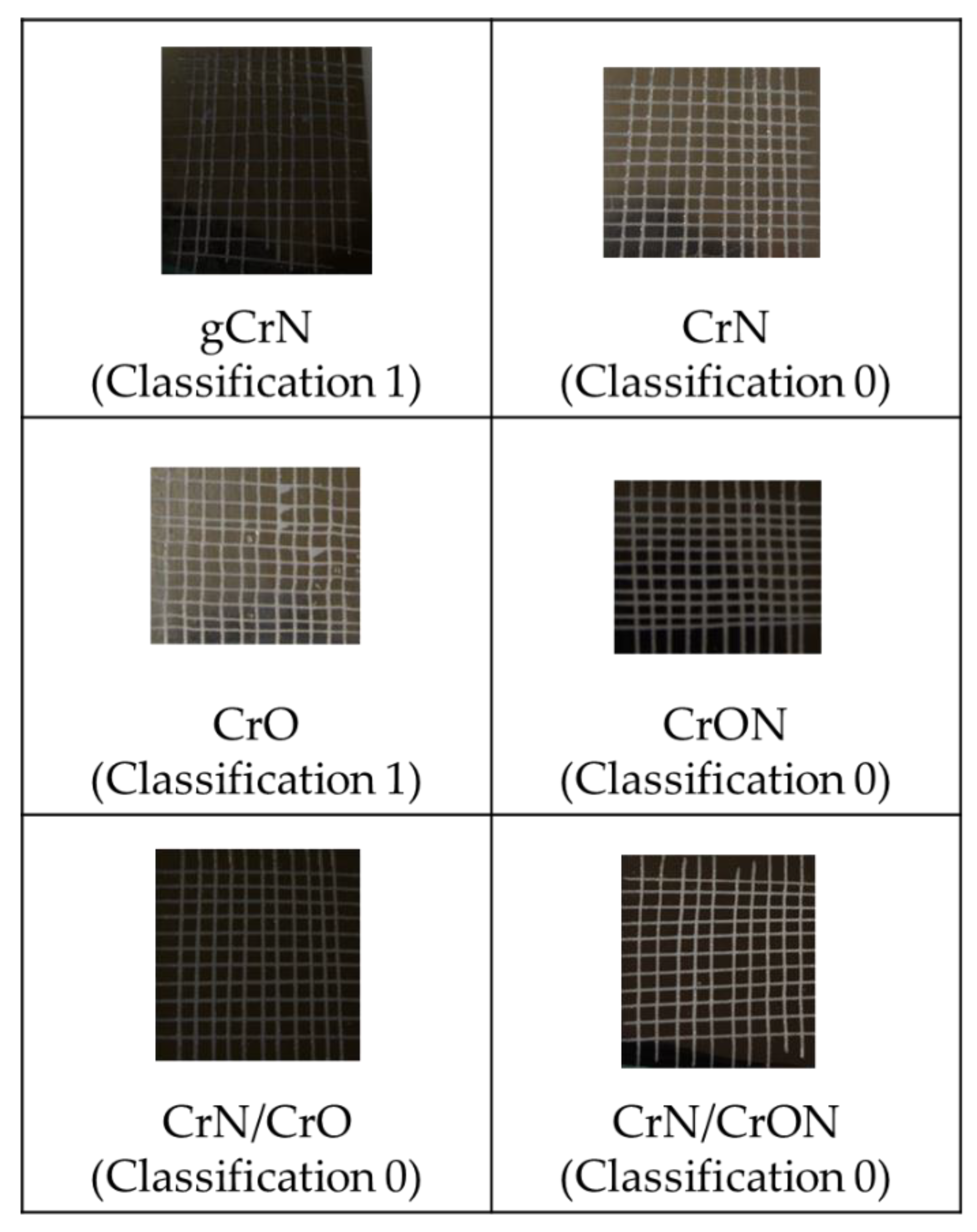
3.1. Functional Characterization: Colour and Adhesion
3.2. Deposition Conditions and Basic Characterization
3.2.1. Chemical Composition
- (1)
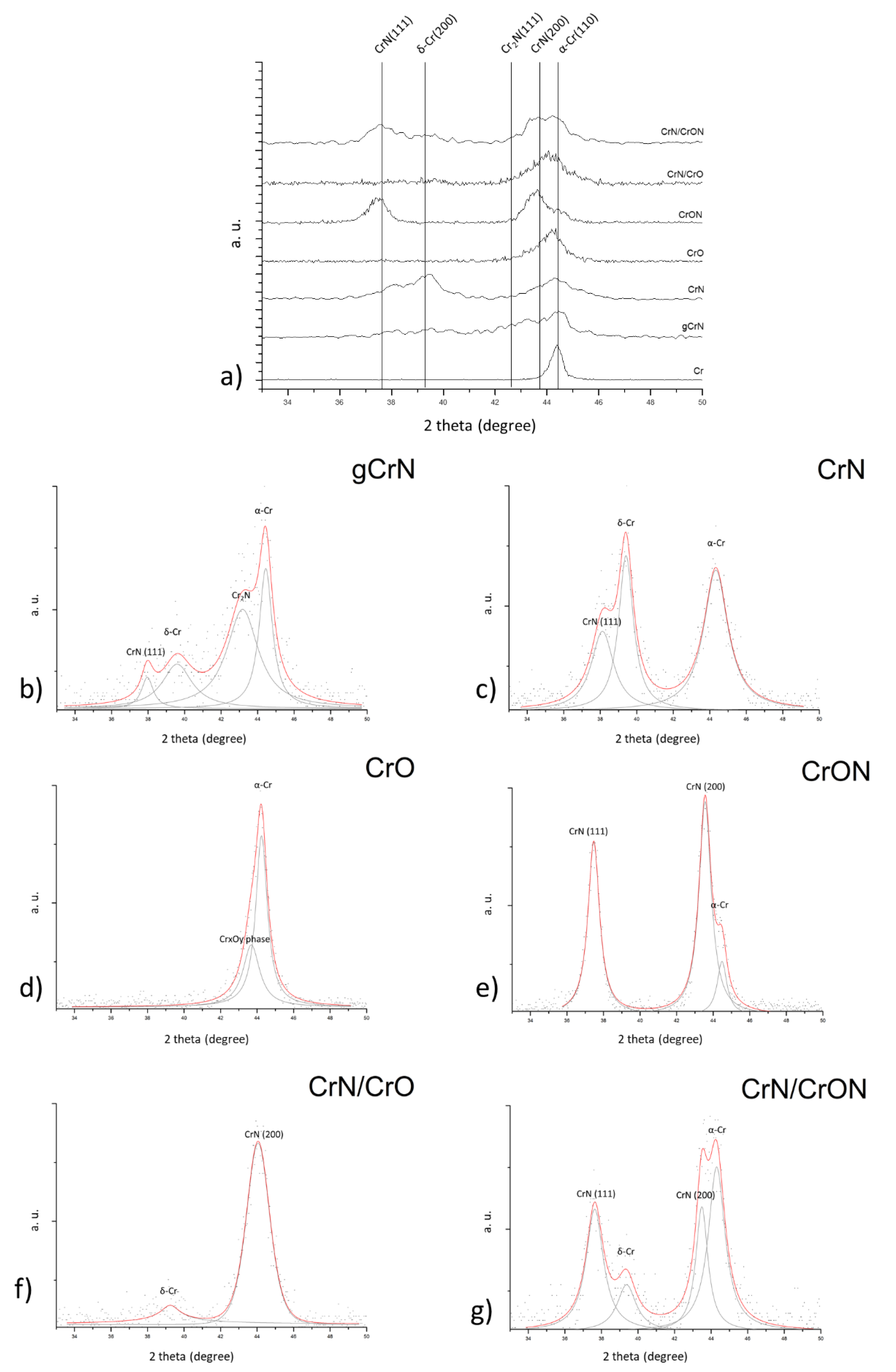
- gCrN and CrN coatings: For graded CrN coatings, gCrN, deposited with increasing nitrogen flux presented lower N content in comparison with the CrN coating. The 33 at.% N introduced in the CrN coating on a N2/Ar flow-rate ratio of 0.35 was in line with previous work by Mayhofer et al. [25];
- (2)
- CrO coating: By adding O2 as the reactive gas inside the deposition chamber, it is possible to incorporate a significate amount of oxygen (30 at.% of O), even with a much lower gas flow than for N2-presence coatings, which is explained by the higher reactivity of oxygen to Cr [26];
- (3)
- CrON coating: When a gas mixture of 85% N2 + 15% O2 was added to the atmosphere for the CrON coating, the number of reactive species inside the deposition chamber became higher and this led to the highest non-metallic elements amount being added to the coatings in the present study (~21 at.% of O and ~33 at.% of N). Note that, proportionally, the amount of O in the coating was higher than the amount of O2 in the gas mixture (85% N2 and 15% O2) which can be explained by the higher affinity of O to Cr than N to Cr, as indicated by the standard molar enthalpy of formation of Cr2O3 and CrN: ΔfH° (Cr2O3) = −1139.7 kJ/mol and ΔfH° (CrN) = −117.2 kJ/mol [26], also observed in previous studies from our research team on oxynitrides coatings [27];
- (4)
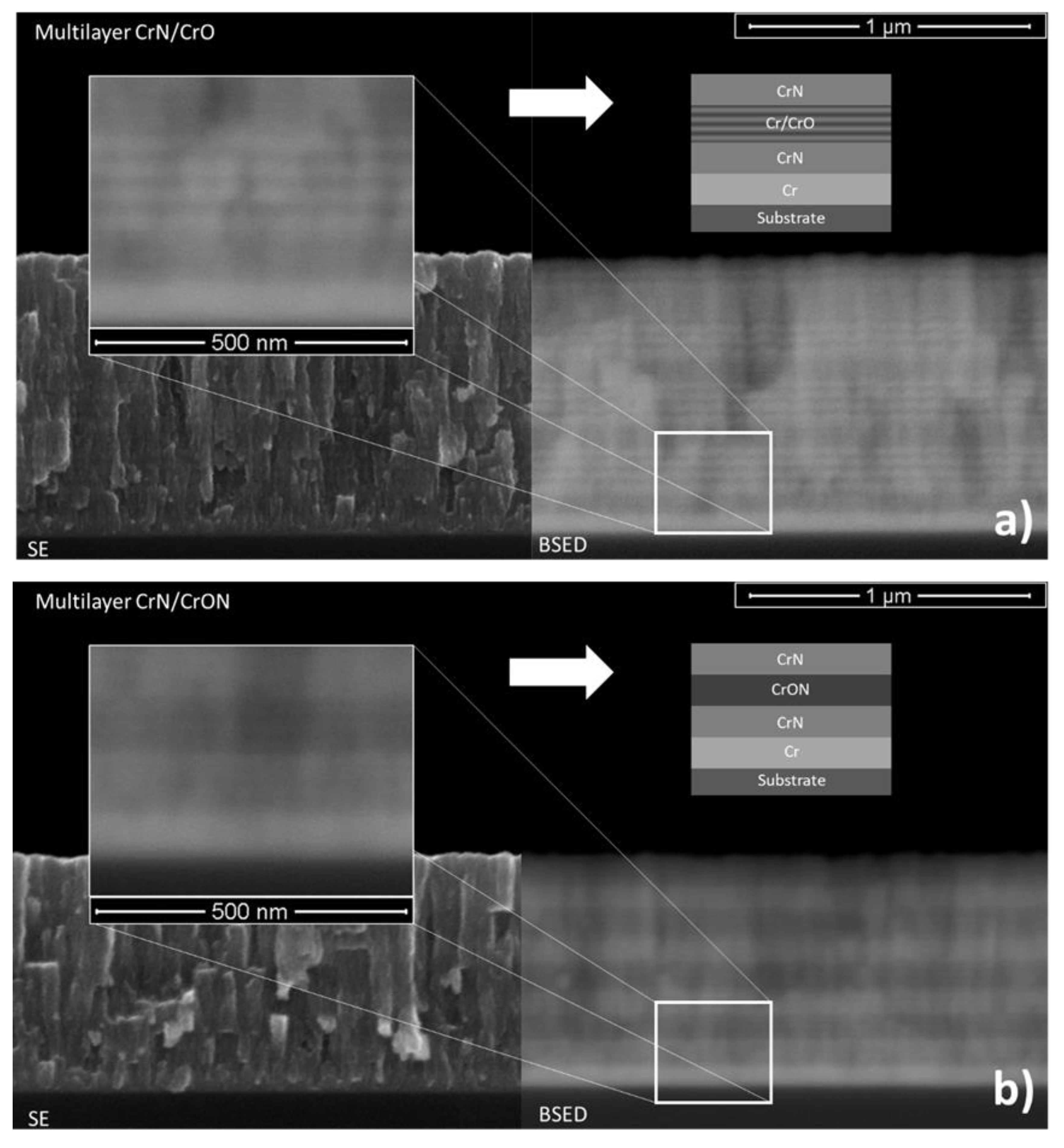
- CrN/CrO and CrN/CrON coatings: In the case of multilayered coatings, CrN/CrO and CrN/CrON, the chemical composition measured using this method showed a value similar to the average of the chemical composition of individual layers (CrN and CrO or CrON), which indicated that the analytical method had been influenced by the several layers of depth.
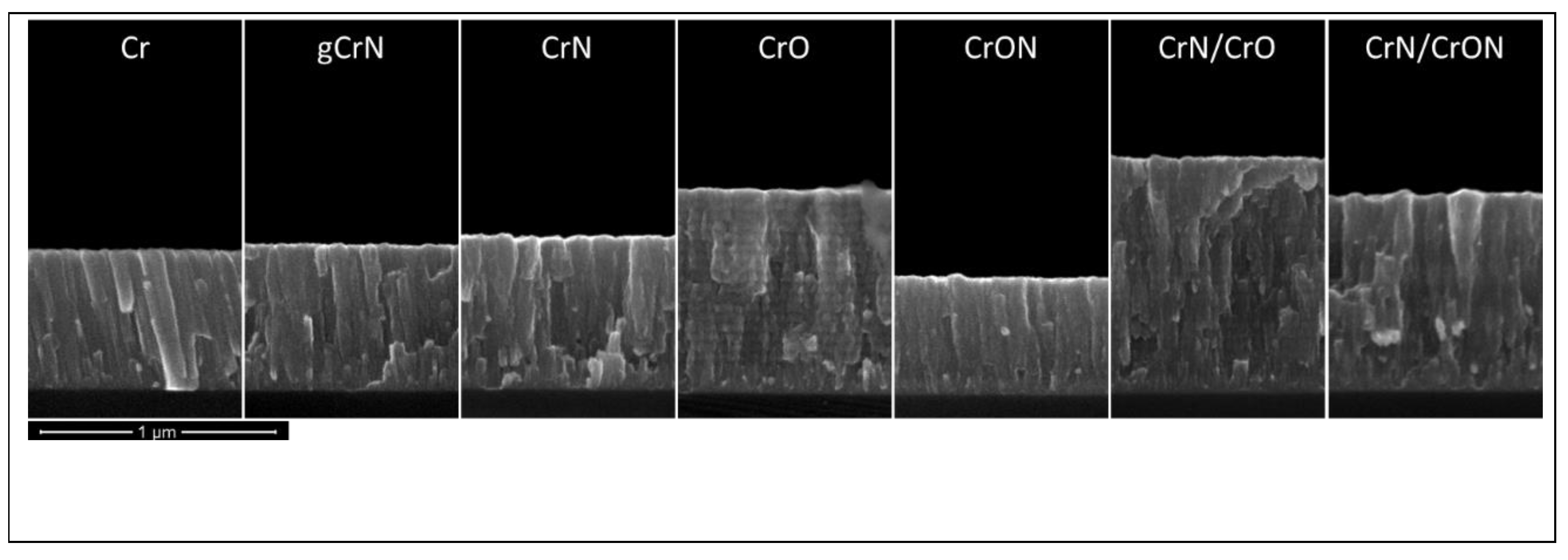
3.2.2. Morphology
3.2.3. Structural Characterization
3.3. Mechanical and Tribological Characterization
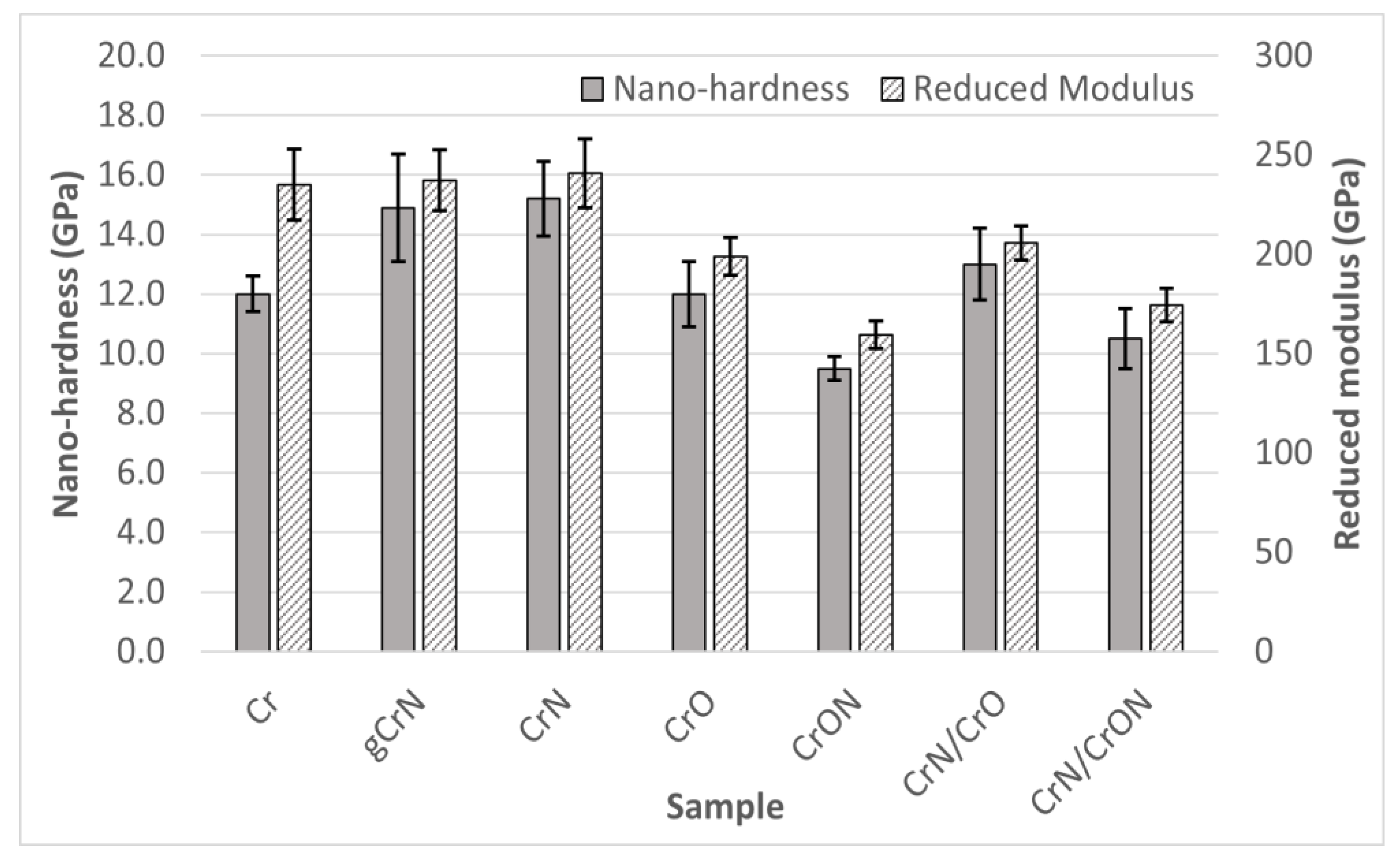
3.3.1. Mechanical Characterization
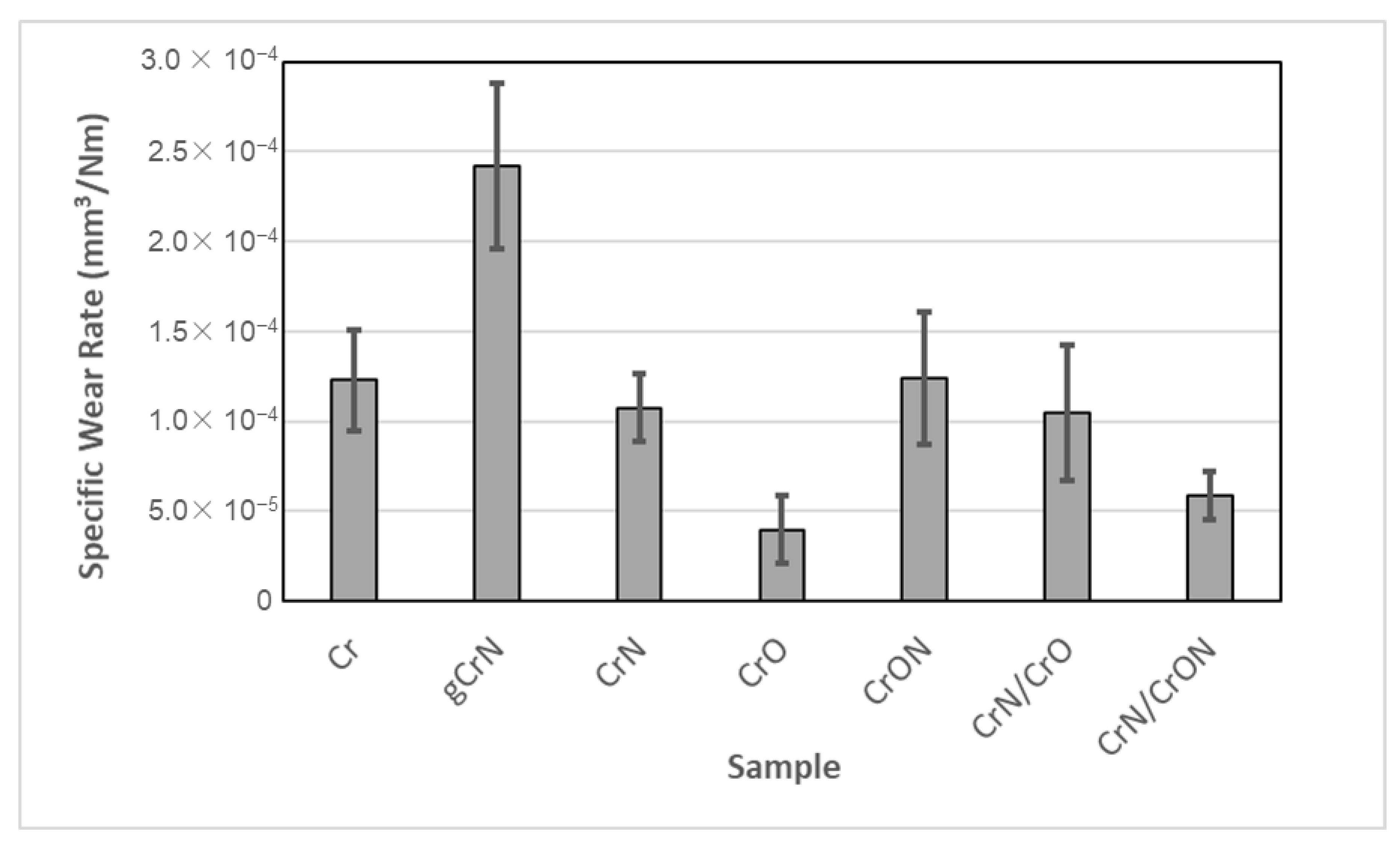
3.3.2. Tribological Characterization by Dry Sliding Reciprocating Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reheem, A.A.; Maksoud, M.I.A.A.; Ashour, A. Surface modification and metallization of polycarbonate using low energy ion beam. Radiat. Phys. Chem. 2016, 125, 171–175. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Gopalakrishna, K.; Vivek, C.S. Plating on acrylonitrile–butadiene–styrene (ABS) plastic: A review. J. Mater. Sci. 2016, 51, 3657–3674. [Google Scholar] [CrossRef]
- Charbonnier, M.; Romand, M.; Harry, E.; Alami, M. Surface plasma functionalization of polycarbonate: Application to electroless nickel and copper plating. J. Appl. Electrochem. 2001, 31, 57–63. [Google Scholar] [CrossRef]
- White, J.; Tenore, C.; Pavich, A.; Scherzer, R.; Stagon, S. Environmentally benign metallization of material extrusion technology 3D printed acrylonitrile butadiene styrene parts using physical vapor deposition. Addit. Manuf. 2018, 22, 279–285. [Google Scholar] [CrossRef]
- Tang, X.; Wang, J.; Wang, C.; Shen, B. A novel surface activation method for Ni/Au electroless plating of acrylonitrile–butadiene–styrene. Surf. Coat. Technol. 2011, 206, 1382–1388. [Google Scholar] [CrossRef]
- Garcia, A.; Berthelot, T.; Viel, P.; Mesnage, A.; Jégou, P.; Nekelson, F.; Roussel, S.; Palacin, S. ABS Polymer Electroless Plating through a One-Step Poly(acrylic acid) Covalent Grafting. ACS Appl. Mater. Interfaces 2010, 2, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Directive 2005/90/EC of the European Parliament and of the Council; European Parliament: Brussels, Belgium, 2006. [Google Scholar]
- Vernhes, L.; Azzi, M.; Klemberg-Sapieha, J. Alternatives for hard chromium plating: Nanostructured coatings for severe-service valves. Mater. Chem. Phys. 2013, 140, 522–528. [Google Scholar] [CrossRef]
- Sahraoui, T.; Fenineche, N.-E.; Montavon, G.; Coddet, C. Alternative to chromium: Characteristics and wear behavior of HVOF coatings for gas turbine shafts repair (heavy-duty). J. Mater. Process. Technol. 2004, 152, 43–55. [Google Scholar] [CrossRef]
- Forsich, C.; Dipolt, C.; Heim, D.; Mueller, T.; Gebeshuber, A.; Holecek, R.; Lugmair, C. Potential of thick a-C:H:Si films as substitute for chromium plating. Surf. Coat. Technol. 2014, 241, 86–92. [Google Scholar] [CrossRef]
- Yasbandha, H. Surface Engineering of Coinage Dies. Ph.D. Thesis, Faculty of Engineering, University of Wollongong, Wollongong, Australia, 2001. [Google Scholar]
- Bewilogua, K.; Bräuer, G.; Dietz, A.; Gäbler, J.; Goch, G.; Karpuschewski, B.; Szyszka, B. Surface technology for automotive engineering. CIRP Ann. Manuf. Technol. 2009, 58, 608–627. [Google Scholar] [CrossRef]
- Antonakou, E.V.; Achilias, D.S. Recent Advances in Polycarbonate Recycling: A Review of Degradation Methods and Their Mechanisms. Waste Biomass Valoriz. 2013, 4, 9–21. [Google Scholar] [CrossRef]
- Grimberg, I.; Bouaifi, B.; Draugelates, U.; Soifer, K.; Weiss, B. Microstructure and adhesion mechanisms of TiN coatings on metallized acrylonitrile-butadiene-styrene. Surf. Coat. Technol. 1994, 68–69, 166–175. [Google Scholar] [CrossRef]
- Sukwisute, P.; Sakdanuphab, R.; Sakulkalavek, A. Hardness and wear resistance improvement of ABS surface by CrN thin film. Mater. Today Proc. 2017, 4, 6553–6561. [Google Scholar] [CrossRef]
- Pedrosa, P.; Rodrigues, M.S.; Neto, M.A.; Oliveira, F.J.; Silva, R.; Borges, J.; Amaral, M.; Ferreira, A.; Godinho, L.H.; Carvalho, S.; et al. Properties of CrN thin films deposited in plasma-activated ABS by reactive magnetron sputtering. Surf. Coat. Technol. 2018, 349, 858–866. [Google Scholar] [CrossRef]
- Carneiro, E.; Castro, J.D.; Marques, S.; Cavaleiro, A.; Carvalho, S. REACH regulation challenge: Development of alternative coatings to hexavalent chromium for minting applications. Surf. Coat. Technol. 2021, 418, 127271. [Google Scholar] [CrossRef]
- Subramanian, B.; Jayachandran, M. Preparation of chromium oxynitride and chromium nitride films by DC reactive magnetron sputtering and their material properties. Corros. Eng. Sci. Technol. 2011, 46, 554–561. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, B.; Sun, J.; Jonnard, P.; Le Guen, K.; Tu, Y.; Yan, C.; Lan, R. Structure and optical properties of CrOxNy films with composition modulation. Surf. Eng. 2020, 36, 411–417. [Google Scholar] [CrossRef]
- Mientus, R.; Grötschel, R.; Ellmer, K. Optical and electronic properties of CrOxNy films, deposited by reactive DC magnetron sputtering in Ar/N2/O2(N2O) atmospheres. Surf. Coat. Technol. 2005, 200, 341–345. [Google Scholar] [CrossRef]
- Kato, K. Friction and wear of passive metals and coatings. In Tribocorrosion of Passive Metals and Coatings; Woodhead Publishing: Sawston, UK, 2011; pp. 65–99. [Google Scholar] [CrossRef]
- Colombo, D.A.; Echeverría, M.D.; Laino, S.; Dommarco, R.C.; Massone, J.M. Sliding Wear Behavior of PVD CrN and TiN Coated Austempered Ductile Iron. ISIJ Int. 2014, 54, 2860–2867. [Google Scholar] [CrossRef] [Green Version]
- Beck, U.; Reiners, G.; Kopacz, U.; Jehn, H. Decorative hard coatings: Interdependence of optical, stoichiometric and structural properties. Surf. Coat. Technol. 1993, 60, 389–395. [Google Scholar] [CrossRef]
- Budke, E.; Krempel-Hesse, J.; Maidhof, H.; Schüssler, H. Decorative hard coatings with improved corrosion resistance. Surf. Coat. Technol. 1999, 112, 108–113. [Google Scholar] [CrossRef]
- Mayrhofer, P.; Tischler, G.; Mitterer, C. Microstructure and mechanical/thermal properties of Cr–N coatings deposited by reactive unbalanced magnetron sputtering. Surf. Coat. Technol. 2001, 142–144, 78–84. [Google Scholar] [CrossRef]
- Handbook of chemistry and physics. J. Frankl. Inst. 1930, 209, 847. [CrossRef]
- Parreira, N.M.G.; Polcar, T.; Martin, N.; Banakh, O.; Cavaleiro, A. Optical and Electrical Properties of W-O-N Coatings Deposited by DC Reactive Sputtering. Plasma Process. Polym. 2007, 4 (Suppl. 1), S69–S75. [Google Scholar] [CrossRef]
- Xu, J.; Umehara, H.; Kojima, I. Effect of deposition parameters on composition, structures, density and topography of CrN films deposited by r.f. magnetron sputtering. Appl. Surf. Sci. 2002, 201, 208–218. [Google Scholar] [CrossRef]
- Rothhaar, U.; Oechsner, H. R.f. magnetron sputter deposition of Cr2O3 layers on ceramic Al2O3 substrates. Surf. Coat. Technol. 1993, 59, 183–186. [Google Scholar] [CrossRef]
- Kharrazi, M.; Azens, A.; Kullman, L.; Granqvist, C. High-rate dual-target d.c. magnetron sputter deposition of electrochromic MoO3 films. Thin Solid Film. 1997, 295, 117–121. [Google Scholar] [CrossRef]
- Parreira, N.; Carvalho, N.; Cavaleiro, A. Synthesis, structural and mechanical characterization of sputtered tungsten oxide coatings. Thin Solid Film. 2006, 510, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Daniel, R.; Martinschitz, K.; Keckes, J.; Mitterer, C. The origin of stresses in magnetron-sputtered thin films with zone T structures. Acta Mater. 2010, 58, 2621–2633. [Google Scholar] [CrossRef]
- Arias, D.; Gómez, A.G.; Souza, R.; Velez, J.M. Residual stress gradient of Cr and CrN thin films. Mater. Chem. Phys. 2018, 204, 269–276. [Google Scholar] [CrossRef]
- Alves, C.A.; Marques, L.; Calderon V., S.; Ferreira, P.; Schneider, D.; Cavaleiro, A.; Carvalho, S. An experimental and theoretical study on the crystal structure and elastic properties of Ta1−xOx coatings. Surf. Coat. Technol. 2019, 364, 289–297. [Google Scholar] [CrossRef]
- Kowoll, T.; Müller, E.; Fritsch-Decker, S.; Hettler, S.; Störmer, H.; Weiss, C.; Gerthsen, D. Contrast of Backscattered Electron SEM Images of Nanoparticles on Substrates with Complex Structure. Scanning 2017, 2017, 4907457. [Google Scholar] [CrossRef]
- Kimoto, K.; Nishida, I. An Electron Diffraction Study on the Crystal Structure of a New Modification of Chromium. J. Phys. Soc. Jpn. 1967, 22, 744–756. [Google Scholar] [CrossRef]
- Peralta, J.; Esteve, J.; Lousa, A. δ-A15 and bcc phases coexist in sputtered chromium coatings with moderate oxygen contents. Thin Solid Films 2020, 693, 137676. [Google Scholar] [CrossRef]
- Parreira, N.M.; Carvalho, N.J.; Cavaleiro, A. On the Structural Evaluation of Unbiased W-O-N Sputtered Coatings. Mater. Sci. Forum 2006, 514–516, 825–832. [Google Scholar] [CrossRef]
- Parreira, N.; Carvalho, N.; Vaz, F.; Cavaleiro, A. Mechanical evaluation of unbiased W–O–N coatings deposited by d.c. reactive magnetron sputtering. Surf. Coat. Technol. 2006, 200, 6511–6516. [Google Scholar] [CrossRef]
- Song, H.J.; Yan, Q. The Characteristics of CrNX Coatings with Different Interlayer. Adv. Mater. Res. 2011, 204–210, 938–941. [Google Scholar] [CrossRef]
- Marulanda, D.; Lousa, A.; Martinez-De-Olcoz, L.; Olaya, J. Microstructure characterization of nano-structured Cr/Cr2N multilayer films produced through radio frequency magnetron sputtering. Thin Solid Films 2014, 550, 272–277. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G. X-ray Diffraction A Practical Approach; Plenum Press: New York, NY, USA, 1998. [Google Scholar]
- Collard, S.; Küpfer, H.; Hecht, G.; Hoyer, W.; Moussaoui, H. The reactive magnetron deposition of CrNxOy films: First results of property investigations. Surf. Coat. Technol. 1999, 112, 181–184. [Google Scholar] [CrossRef]
- Hones, P.; Diserens, M.; Lévy, F. Characterization of sputter-deposited chromium oxide thin films. Surf. Coat. Technol. 1999, 120–121, 277–283. [Google Scholar] [CrossRef]
- Forniés, E.; Galindo, R.E.; Sánchez, O.; Albella, J. Growth of CrNx films by DC reactive magnetron sputtering at constant N2/Ar gas flow. Surf. Coat. Technol. 2006, 200, 6047–6053. [Google Scholar] [CrossRef]
- Hones, P.; Sanjines, R.; Levy, F. Characterization of sputter-deposited chromium nitride thin films for hard coatings. Surf. Coat. Technol. 1997, 94–95, 398–402. [Google Scholar] [CrossRef]
- Elangovan, T.; Kuppusami, P.; Thirumurugesan, R.; Ganesan, V.; Mohandas, E.; Mangalaraj, D. Nanostructured CrN thin films prepared by reactive pulsed DC magnetron sputtering. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 167, 17–25. [Google Scholar] [CrossRef]
- Pang, X.; Gao, K.; Volinsky, A. Microstructure and mechanical properties of chromium oxide coatings. J. Mater. Res. 2007, 22, 3531–3537. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, F.; Yaqub, T.; Cavaleiro, A. Influence of Ag additions on the structure, mechanical properties and oxidation behaviour of Cr-O coatings deposited by HiPIMS. Surf. Coat. Technol. 2018, 339, 167–180. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Rajam, K. Growth and characterization of chromium oxide coatings prepared by pulsed-direct current reactive unbalanced magnetron sputtering. Appl. Surf. Sci. 2008, 255, 2925–2931. [Google Scholar] [CrossRef]
- Castaldi, L.; Kurapov, D.; Reiter, A.; Shklover, V.; Schwaller, P.; Patscheider, J. Effect of the oxygen content on the structure, morphology and oxidation resistance of Cr–O–N coatings. Surf. Coat. Technol. 2008, 203, 545–549. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Soc. Sect. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Lehoczky, S.L. Retardation of Dislocation Generation and Motion in Thin-Layered Metal Laminates. Phys. Rev. Lett. 1978, 41, 1814–1818. [Google Scholar] [CrossRef]
- Marulanda, D.; Olaya, J.; Piratoba, U.; Mariño, A.; Camps, E. The effect of bilayer period and degree of unbalancing on magnetron sputtered Cr/CrN nano-multilayer wear and corrosion. Thin Solid Film. 2011, 519, 1886–1893. [Google Scholar] [CrossRef]
- Kot, M.; Rakowski, W.; Major, Ł.; Major, R.; Morgiel, J. Effect of bilayer period on properties of Cr/CrN multilayer coatings produced by laser ablation. Surf. Coat. Technol. 2008, 202, 3501–3506. [Google Scholar] [CrossRef]
- Arias, D.; Gómez, A.; Velez, J.M.; Souza, R.; Olaya, J. A mechanical and tribological study of Cr/CrN multilayer coatings. Mater. Chem. Phys. 2015, 160, 131–140. [Google Scholar] [CrossRef]
- Parreira, N.; Polcar, T.; Cavaleiro, A. Characterization of W–O coatings deposited by magnetron sputtering with reactive gas pulsing. Surf. Coat. Technol. 2007, 201, 5481–5486. [Google Scholar] [CrossRef]
- He, X.-M.; Baker, N.; Kehler, B.A.; Walter, K.C.; Nastasi, M.; Nakamura, Y. Structure, hardness, and tribological properties of reactive magnetron sputtered chromium nitride films. J. Vac. Sci. Technol. A 2000, 18, 30–36. [Google Scholar] [CrossRef]
- Urgen, M.; Ezirmik, V.; Senel, E.; Kahraman, Z.; Kazmanli, K. The effect of oxygen content on the temperature dependent tribological behavior of Cr–O–N coatings. Surf. Coat. Technol. 2009, 203, 2272–2277. [Google Scholar] [CrossRef]


| Coating | Gas Flow (sccm) | Deposition Time (s) | Thickness | Deposition Rate | Chemical Composition (at.%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar | N2 | O2 | N2 + O2 | Interlayer | Coating | (nm) | (nm/s) | Cr | O | N | |
| Cr | 100 | 360 | 603 | 1.7 | 92 | 8 | - | ||||
| gCrN | 70 | 5→25 | 360 | 629 | 1.8 | 66 | 6 | 28 | |||
| CrN | 70 | 25 | 50 | 310 | 638 | 1.8 | 60 | 8 | 32 | ||
| CrO | 100 | 15 | 50 | 310 | 862 | 2.4 | 69 | 31 | - | ||
| CrON | 70 | 40 | 50 | 310 | 497 | 1.4 | 46 | 21 | 33 | ||
| CrN/CrO | 70 | 25 | 15 | 50 | 480 | 1020 | 1.9 | 63 | 25 | 12 | |
| CrN/CrON | 70 | 25 | 40 | 50 | 480 | 858 | 1.6 | 49 | 18 | 33 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, E.; Parreira, N.M.G.; Vuchkov, T.; Cavaleiro, A.; Ferreira, J.; Andritschky, M.; Carvalho, S. Cr-Based Sputtered Decorative Coatings for Automotive Industry. Materials 2021, 14, 5527. https://doi.org/10.3390/ma14195527
Carneiro E, Parreira NMG, Vuchkov T, Cavaleiro A, Ferreira J, Andritschky M, Carvalho S. Cr-Based Sputtered Decorative Coatings for Automotive Industry. Materials. 2021; 14(19):5527. https://doi.org/10.3390/ma14195527
Chicago/Turabian StyleCarneiro, Edgar, Nuno M. G. Parreira, Todor Vuchkov, Albano Cavaleiro, Jorge Ferreira, Martin Andritschky, and Sandra Carvalho. 2021. "Cr-Based Sputtered Decorative Coatings for Automotive Industry" Materials 14, no. 19: 5527. https://doi.org/10.3390/ma14195527







