Preparation of Crystalline LaFeO3 Nanoparticles at Low Calcination Temperature: Precursor and Synthesis Parameter Effects
Abstract
:1. Introduction
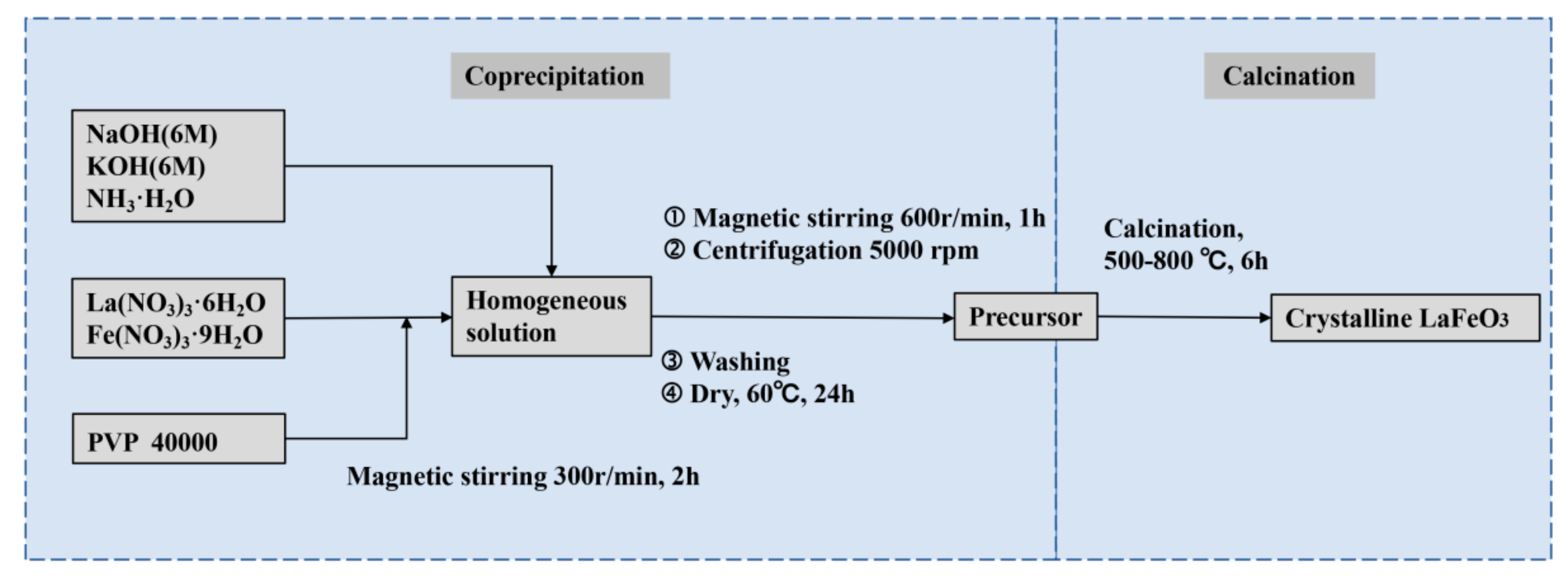
2. Experimental Section
3. Results
3.1. Preparation of Crystalline LFO Nanoparticles
3.2. Influences of La/Fe Stoichiometric Ratios
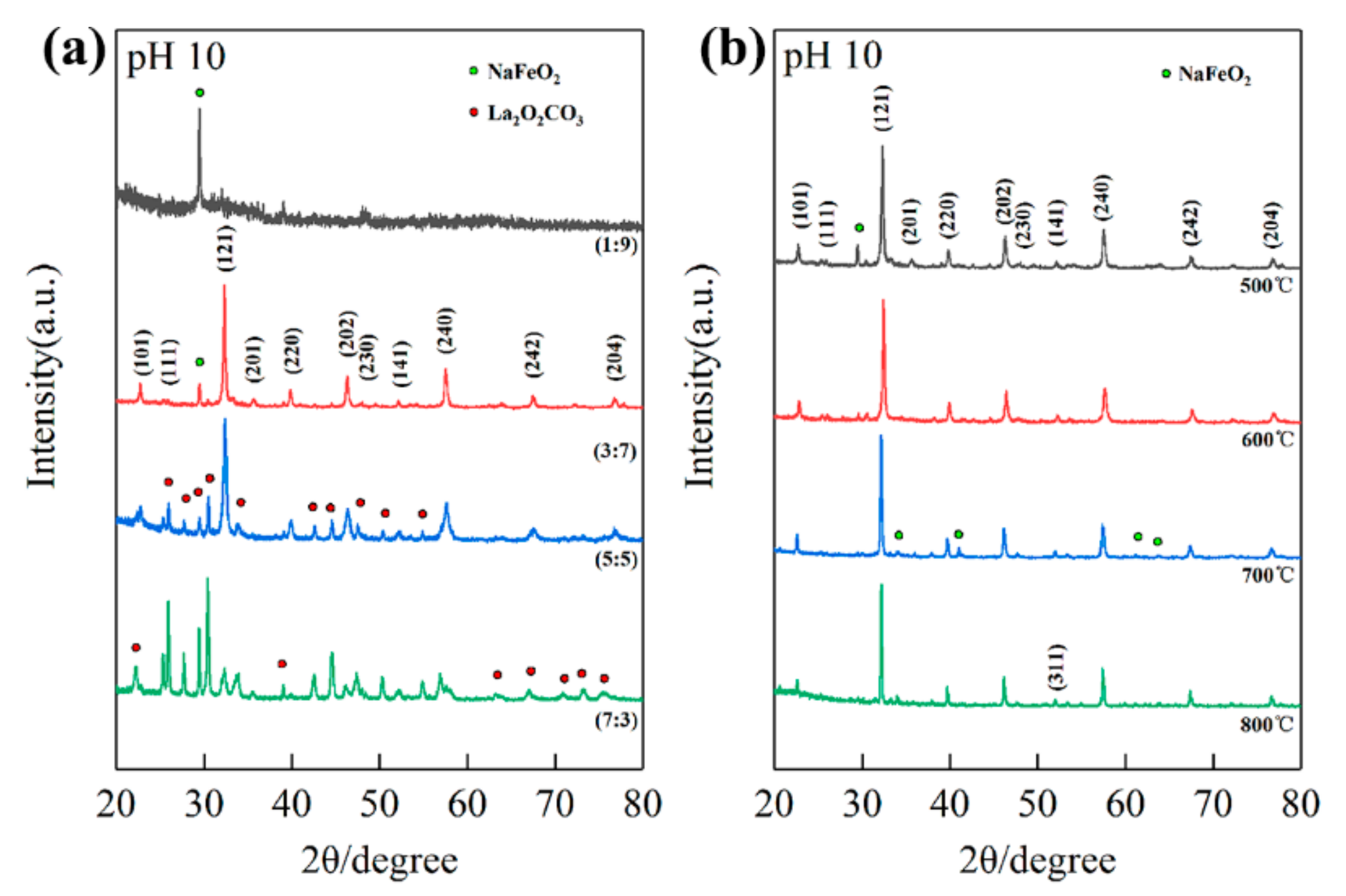
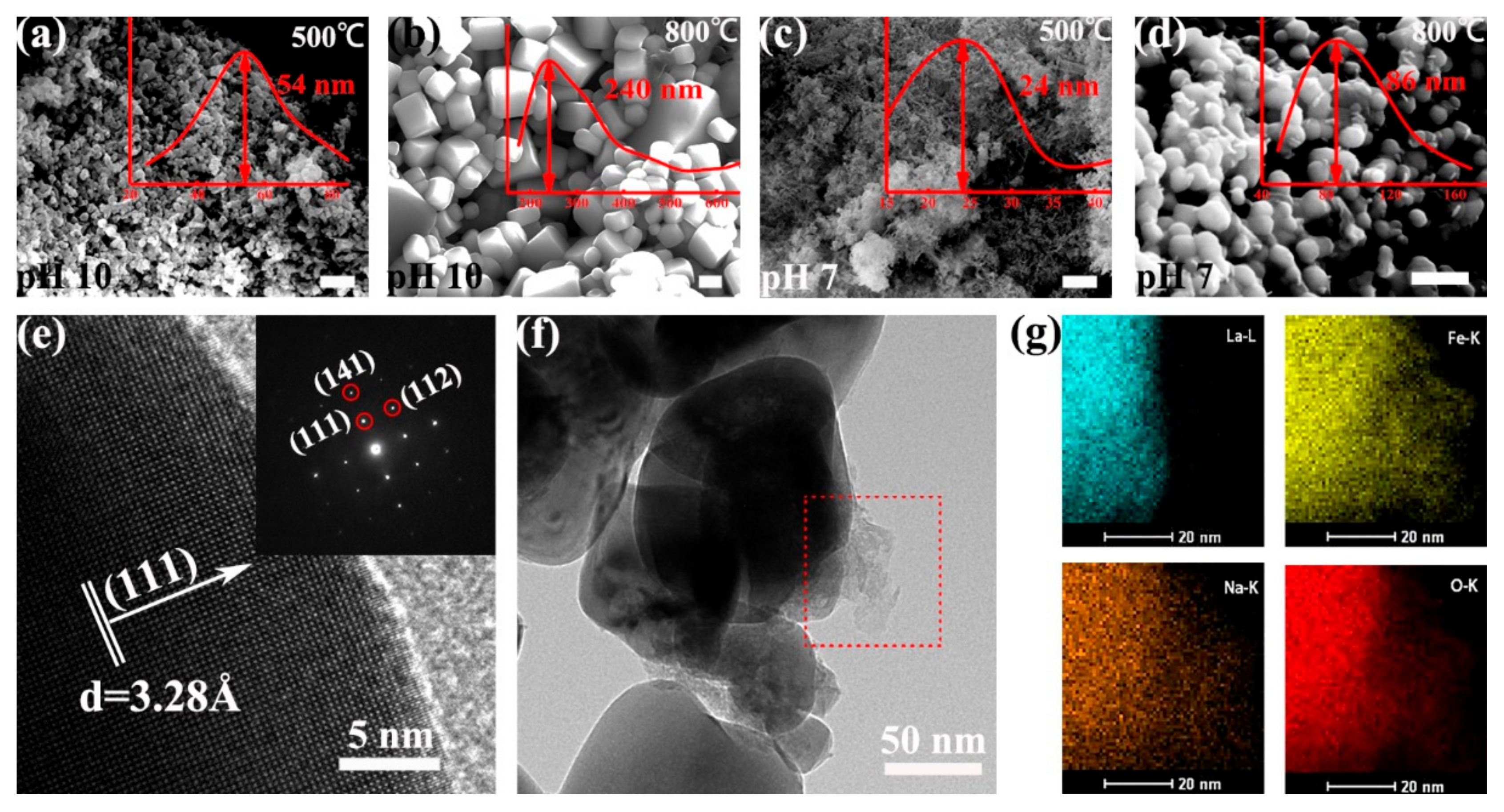
3.3. Influence of pH Values and Calcination Temperatures
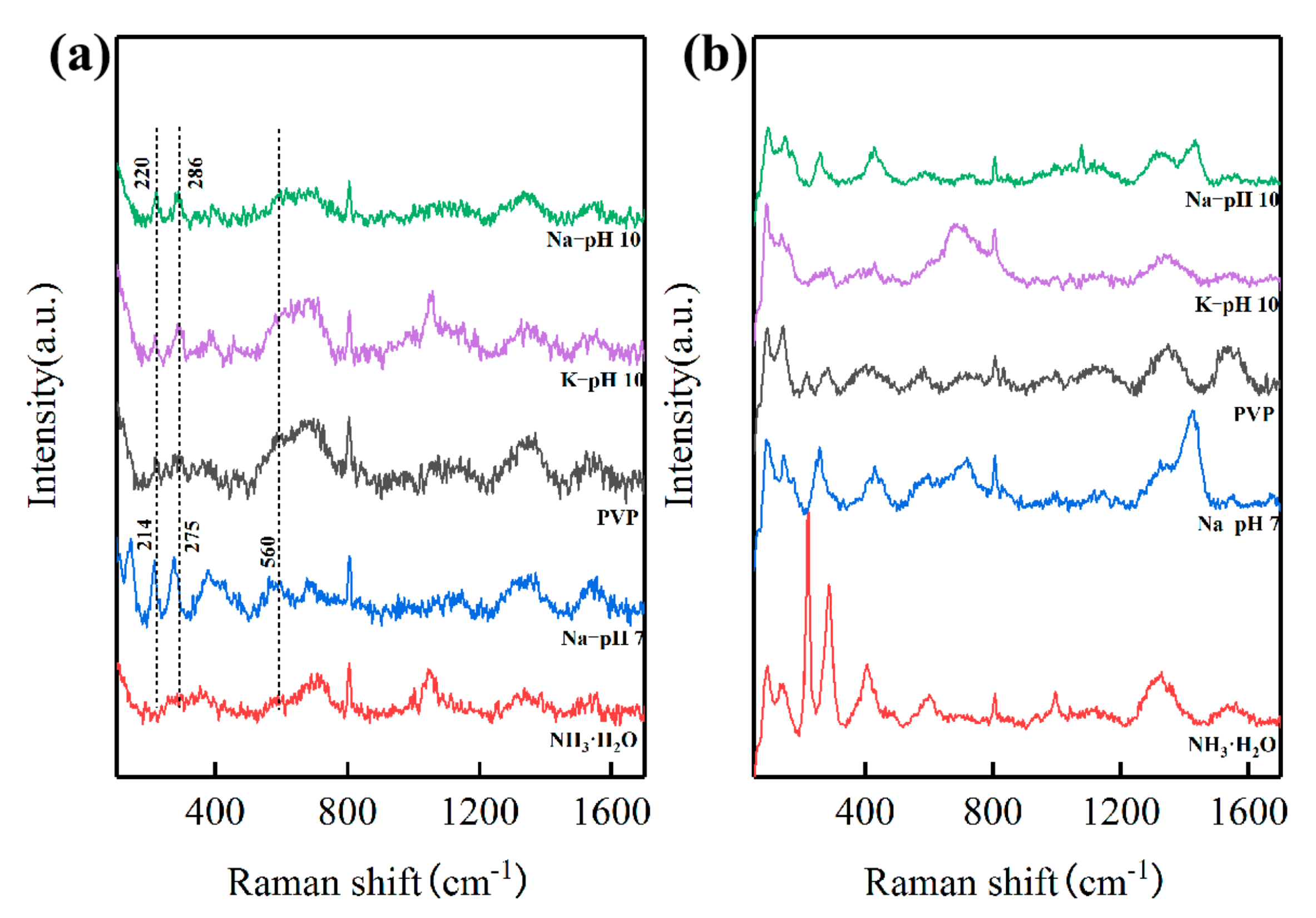
3.4. Influence of Precipitants
3.5. Influence of Polyvinylpyrrolidone
3.6. Mechanism Investigation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, J.; Norby, T. Cation self-diffusion in LaFeO3 measured by the solid state reaction method. Solid State Ionics 2006, 177, 639–646. [Google Scholar] [CrossRef]
- Yan, B.; Feng, Q.; Liu, J.; Chou, K.C. A Kinetic Study on LaFeO3-δ Preparation with Solid-state Reaction Technique. High Temp. Mater. Process. 2009, 28, 101–108. [Google Scholar] [CrossRef]
- Chen, J.; Yu, R.; Li, L.; Sun, C.; Zhang, T.; Chen, H.; Xing, X. Structure and Shape Evolution of Bi1-xLaxFeO3Perovskite Microcrystals by Molten Salt Synthesis. Eur. J. Inorg. Chem. 2008, 2008, 3655–3660. [Google Scholar] [CrossRef]
- Wang, D.; Chu, X.; Gong, M. Single-crystalline LaFeO3 nanotubes with rough tube walls: Synthesis and gas-sensing properties. Nanotechnology 2006, 17, 5501–5505. [Google Scholar] [CrossRef]
- Romero, M.; Gómez, R.; Marquina, V.; Pérez-Mazariego, J.L.; Escamilla, R. Synthesis by molten salt method of the AFeO3 system (A = La, Gd) and its structural, vibrational and internal hyperfine magnetic field characterization. Phys. B Condens. Matter 2014, 443, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, G.; Baltazar, V.U.; Smart, T.J.; Radmilovic, A.; Ping, Y.; Choi, K.-S. Combined Theoretical and Experimental Investigations of Atomic Doping to Enhance Photon Absorption and Carrier Transport of LaFeO3 Photocathodes. Chem. Mater. 2019, 31, 5890–5899. [Google Scholar] [CrossRef]
- Wheeler, G.; Choi, K.-S. Photoelectrochemical Properties and Stability of Nanoporous p-Type LaFeO3 Photoelectrodes Prepared by Electrodeposition. ACS Energy Lett. 2017, 2, 2378–2382. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Q.; Deng, X.; Jiao, H.; Wang, P.; Gengzang, D. Synthesis and characterization of In-doped LaFeO3 hollow nanofibers with enhanced formaldehyde sensing properties. Mater. Lett. 2019, 236, 229–232. [Google Scholar] [CrossRef]
- Wiranwetchayan, O.; Promnopas, S.; Phadungdhitidhada, S.; Phuruangrat, A.; Thongtem, T.; Singjai, P.; Thongtem, S. Characterization of perovskite LaFeO3 synthesized by microwave plasma method for photocatalytic applications. Ceram. Int. 2019, 45, 4802–4809. [Google Scholar] [CrossRef]
- Cao, E.; Wu, A.; Wang, H.-H.; Zhang, Y.; Hao, W.; Sun, L. Enhanced Ethanol Sensing Performance of Au and Cl Comodified LaFeO3 Nanoparticles. ACS Appl. Nano Mater. 2019, 2, 1541–1551. [Google Scholar] [CrossRef]
- Cui, X.; Yi, D.; Li, N.; Zhang, L.; Zhang, X.; Yang, D. Novel LaFeO3 Coating Modification for a LiFePO4 Cathode. Energy Fuels 2020, 34, 7600–7606. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Lefevre, C.; Robert, D.; Keller, N. Ti-substituted LaFeO3 perovskite as photoassisted CWPO catalyst for water treatment. Appl. Catal. B Environ. 2019, 248, 120–128. [Google Scholar] [CrossRef]
- Li, W.; Yang, F.; Xiong, P.; Jia, Y.; Liu, J.; Yan, X.; Chen, X. Effect of Bi-doping on the electrocatalytic properties of LaFeO3 powders prepared by sol–gel method. J. Mater. Sci. 2019, 54, 7460–7468. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, K.; Xiao, C.; Jia, L. C-doped LaFeO3 Porous Nanostructures for Highly Selective Detection of Formaldehyde. Sens. Actuators B Chem. 2021, 347, 130550. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Chen, X.; Chen, H.; Wu, Y.; Wang, Y.; Tang, L.; Cui, G.; Chen, D. Catalytic behavior of LaFeO3 perovskite oxide during low-pressure gas nitriding. Appl. Surf. Sci. 2020, 506, 145045. [Google Scholar] [CrossRef]
- Parida, K.; Reddy, K.; Martha, S.; Das, D.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sol–gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrog. Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Zhang, L.; Yang, X.; Lu, L.; Wang, X. Preparation and characterization of perovskite LaFeO3 nanocrystals. Mater. Lett. 2006, 60, 1767–1770. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Wu, J.; Yang, J.; Chen, Y.; Liu, G.; Niu, T. Hydrothermal synthesis of graphene-LaFeO3 composite supported with Cu-Co nanocatalyst for higher alcohol synthesis from syngas. Appl. Surf. Sci. 2016, 364, 388–399. [Google Scholar]
- Kostyukhin, E.; Kustov, A.L.; Kustov, L. One-step hydrothermal microwave-assisted synthesis of LaFeO3 nanoparticles. Ceram. Int. 2019, 45, 14384–14388. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, S.; Sachdev, K. Effect of precursors on the morphology and surface area of LaFeO3. Ceram. Int. 2019, 45, 7217–7225. [Google Scholar] [CrossRef]
- Nakayama, S. LaFeO3 perovskite-type oxide prepared by oxide-mixing, co-precipitation and complex synthesis methods. J. Mater. Sci. 2001, 36, 5643–5648. [Google Scholar] [CrossRef]
- Kucharczyk, B.; Winiarski, J.; Szczygiel, I.; Adamska, K. The physicochemical properties of LaFeO3 perovskite prepared by various methods and its activity in the oxidation of hydrocarbons. Ind. Eng. Chem. Res. 2020, 59, 16603–16613. [Google Scholar] [CrossRef]
- Nishikawa, H.; Itani, F.; Kawaguchi, T.; Tanaka, H.; Iwata, N. Metallic conductivity of the heterointerface between LaFeO3 and SrTiO3. Solid State Commun. 2021, 323, 114105. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, X.; Yuan, L.; Wang, M.; Han, M.; Luo, L.; Zheng, B.; Huang, K.; Feng, S. Insight into the enhanced photoelectrocatalytic activity in reduced LaFeO3 films. Chem. Commun. 2017, 53, 2499–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukee, A.; Alharbi, A.; Staerz, A.; Wisitsoraat, A.; Liewhiran, C.; Weimar, U.; Barsan, N. Effect of AgO loading on flame-made LaFeO3 p-type semiconductor nanoparticles to acetylene sensing. Sens. Actuators B Chem. 2020, 312, 127990. [Google Scholar] [CrossRef]
- Azouzi, W.; Sigle, W.; Labrim, H.; Benaissa, M. Sol-gel synthesis of nanoporous LaFeO3 powders for solar applications. Mater. Sci. Semicond. Process. 2019, 104, 104682. [Google Scholar] [CrossRef]
- Dumitru, R.; Negrea, S.; Ianculescu, A.; Păcurariu, C.; Vasile, B.; Surdu, A.; Manea, F. Lanthanum Ferrite Ceramic Powders: Synthesis, Characterization and Electrochemical Detection Application. Materials 2020, 13, 2061. [Google Scholar] [CrossRef]
- Bielecki, J.; Svedlindh, P.; Tibebu, D.; Eriksson, T.; Cai, S. Structural and magnetic properties of isovalently substituted multiferroic BiFe03: Insights from Raman spectroscopy. Phys. Rev. B. 2012, 86, 184422. [Google Scholar] [CrossRef] [Green Version]
- Thirumalairajan, S.; Girija, K.; Ganesh, V.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Novel Synthesis of LaFeO3 Nanostructure Dendrites: A Systematic Investigation of Growth Mechanism, Properties, and Biosensing for Highly Selective Determination of Neurotransmitter Compounds. Cryst. Growth Des. 2012, 13, 291–302. [Google Scholar] [CrossRef]
- Triyono, D.; Purnamasari, I.; Rafsanjani, R.A. Effect of the Zr-Substitution on the Structural and Electrical Properties of LaFeO3: XRD, Raman Scattering, SEM, and Impedance Spectroscopy Study. Crystals 2020, 10, 399. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Liu, X.; Li, S.; Zhao, M. XPS study on O(1s) and Fe(2p) for nanocrystalline composite oxide LaFeO3 with the perovskite structure. Mater. Chem. Phys. 1994, 38, 355–362. [Google Scholar] [CrossRef]
- Özkan, D.C.; Türk, A.; Çelik, E. Synthesis and characterizations of sol–gel derived LaFeO3 perovskite powders. J. Mater. Sci. Mater. Electron. 2020, 31, 22789–22809. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, J.; Feng, Z.; Du, C.; Wen, Y.; Shan, B.; Chen, R. Enhanced Photoelectrochemical Water Oxidation by Fabrication of p-LaFeO3/n-Fe2O3 Heterojunction on Hematite Nanorods. J. Phys. Chem. C 2017, 121, 12991–12998. [Google Scholar] [CrossRef]

| La:Fe | pH | 500 °C | 600 °C | 700 °C | 800 °C |
|---|---|---|---|---|---|
| 1:9 | 7 | LFO/Fe2O3 | LFO/Fe2O3 | ||
| 10 | NFO | LFO/NFO | LFO/NFO | LFO/NFO | |
| 3:7 | 7 | LFO | LFO/NFO | LFO/NFO | |
| 10 | LFO/NFO | LFO/NFO | LFO/NFO | LFO/NFO | |
| 5:5 | 7 | LCO | LFO/LCO | LFO/La2O3 | LFO/La2O3 |
| 10 | LFO/LCO | LFO/LCO | LFO/La2O3 | LFO/La2O3 | |
| 7:3 | 7 | LFO/LCO | LFO/LCO | LFO/La2O3 | LFO/La2O3 |
| 10 | LFO/LCO | LFO/LCO | LFO/La2O3 | LFO/La2O3 | |
| 3:7/PVP | 10 | LFO/Fe2O3/LCO | LFO/Fe2O3/LCO | LFO/Fe2O3/LCO | LFO/Fe2O3/La2O3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Cheng, L.; Gao, J.; Zhang, S.; Wang, H.; Jin, Z.; Tang, Z.; Peng, C. Preparation of Crystalline LaFeO3 Nanoparticles at Low Calcination Temperature: Precursor and Synthesis Parameter Effects. Materials 2021, 14, 5534. https://doi.org/10.3390/ma14195534
Jiang W, Cheng L, Gao J, Zhang S, Wang H, Jin Z, Tang Z, Peng C. Preparation of Crystalline LaFeO3 Nanoparticles at Low Calcination Temperature: Precursor and Synthesis Parameter Effects. Materials. 2021; 14(19):5534. https://doi.org/10.3390/ma14195534
Chicago/Turabian StyleJiang, Wen, Liwei Cheng, Jianghui Gao, Shiyu Zhang, Hao Wang, Zhihao Jin, Zhongfeng Tang, and Cheng Peng. 2021. "Preparation of Crystalline LaFeO3 Nanoparticles at Low Calcination Temperature: Precursor and Synthesis Parameter Effects" Materials 14, no. 19: 5534. https://doi.org/10.3390/ma14195534






