Thermal Stability and Dynamic Mechanical Properties of Poly(ε-caprolactone)/Chitosan Composite Membranes
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Preparation of CS–PCL Membranes
2.3. Characteristics of CS–PCL Membranes
3. Results and Discussion
3.1. Mechanical Property
3.2. Porosity and Pore Features
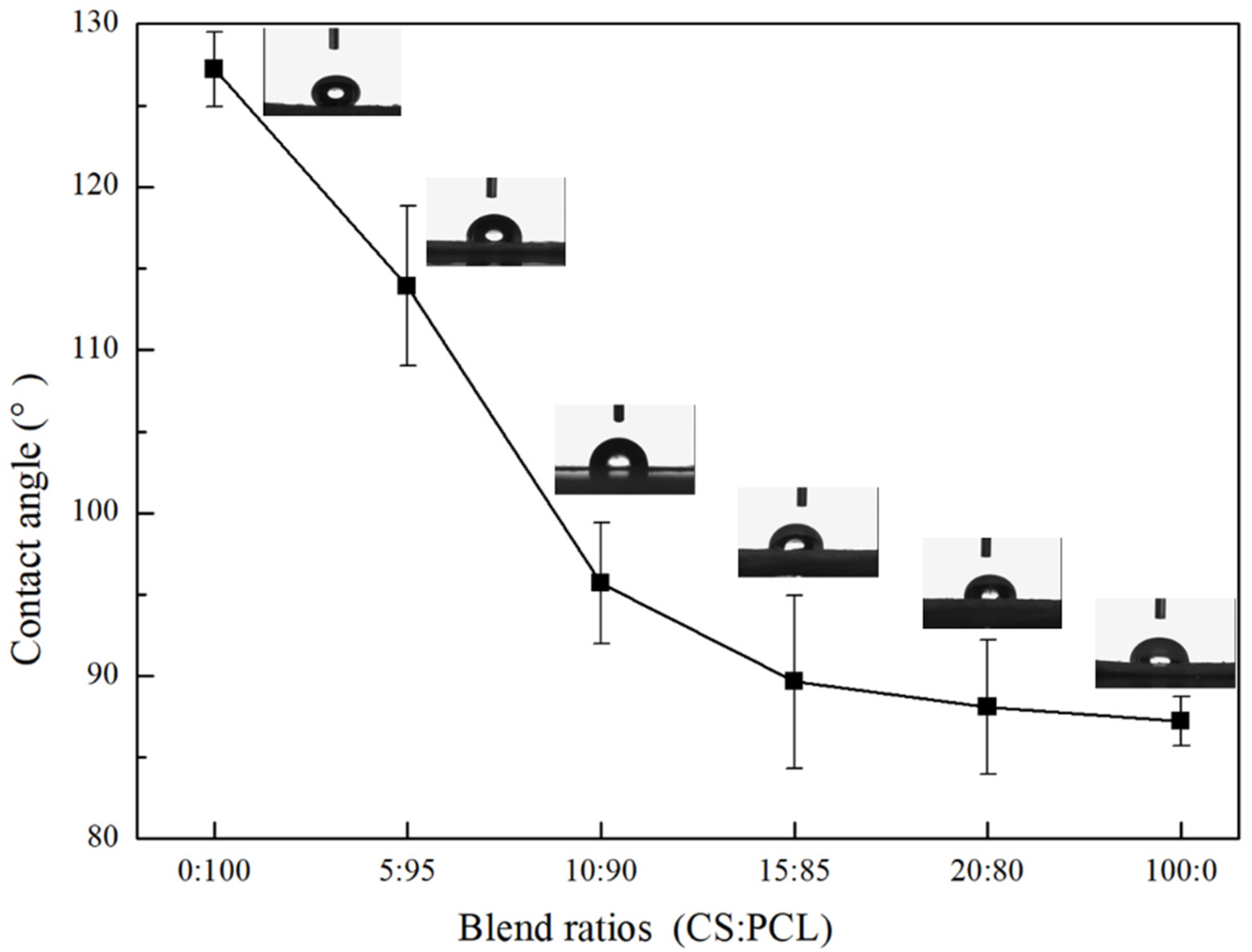
3.3. Contact Angle
3.4. Dynamic Mechanical Analysis
3.5. Thermal Properties and Thermal Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozkan, O.; Sasmazel, H.T. Antibacterial Performance of PCL-Chitosan Core–Shell Scaffolds. J. Nanosci. Nanotechnol. 2018, 18, 2415–2421. [Google Scholar] [CrossRef]
- Xu, N.; Ding, D. Preparation and antibacterial activity of chitosan derivative membrane complexation with iodine. RSC Adv. 2015, 5, 79820–79828. [Google Scholar] [CrossRef]
- Braber, N.L.V.; Vergara, L.I.D.; Vieyra, F.E.M.; Borsarelli, C.D.; Yossen, M.M.; Vega, J.R.; Correa, S.G.; Montenegro, M.A. Physicochemical characterization of water-soluble chitosan derivatives with singlet oxygen quenching and antibacterial capabilities. Int. J. Biol. Macromol. 2017, 102, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.; He, J.; Yan, T.; Liu, C.; Wei, X.; Li, J.; Huang, Y. Antibacterial and hemostatic composite gauze of N,O-carboxymethyl chitosan/oxidized regenerated cellulose. RSC Adv. 2016, 6, 94429–94436. [Google Scholar] [CrossRef]
- Wu, C.; Su, H.; Karydis, A.; Anderson, K.M.; Ghadri, N.; Tang, S.; Wang, Y.-F.; Bumgardner, J.D. Mechanically stable surface-hydrophobilized chitosan nanofibrous barrier membranes for guided bone regeneration. Biomed. Mater. 2017, 13, 015004. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Shukla, R.; Gupta, J.; Shukla, P.; Dwivedi, P.; Tripathi, P.; Bhattacharya, S.M.; Mishra, P.R. Chitosan coated alginate micro particles for the oral delivery of antifilarial drugs and combinations for intervention in Brugia malayi induced lymphatic filariasis. RSC Adv. 2015, 5, 69047–69056. [Google Scholar] [CrossRef]
- Ghorbani, F.M.; Kaffashi, B.; Shokrollahi, P.; Seyedjafari, E.; Ardeshirylajimi, A. PCL/chitosan/Zn-doped nHA electrospun nanocomposite scaffold promotes adipose derived stem cells adhesion and proliferation. Carbohydr. Polym. 2015, 118, 133–142. [Google Scholar] [CrossRef]
- Moghadas, B.; Dashtimoghadam, E.; Mirzadeh, H.; Seidi, F.; Hasani-Sadrabadi, M.M. Novel chitosan-based nanobiohybrid membranes for wound dressing applications. RSC Adv. 2016, 6, 7701–7711. [Google Scholar] [CrossRef]
- Cooper, A.; Bhattarai, N.; Zhang, M. Fabrication and cellular compatibility of aligned chitosan–PCL fibers for nerve tissue regeneration. Carbohydr. Polym. 2011, 85, 149–156. [Google Scholar] [CrossRef]
- Huang, G.-S.; Dai, L.-G.; Yen, B.L.; Hsu, S.-H. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945. [Google Scholar] [CrossRef] [PubMed]
- Periayah, M.H.; Halim, A.S.; Saad, A.Z.M. Chitosan: A Promising Marine Polysaccharide for Biomedical Research. Pharmacogn. Rev. 2016, 10, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sukriti; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Romano, I.; Mele, E.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Hajiali, H.; Goldoni, L.; Marini, L.; Athanassiou, A. Photo-polymerisable electrospun fibres of N-methacrylate glycol chitosan for biomedical applications. RSC Adv. 2015, 5, 24723–24728. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zheng, S.; Dan, W.; Dan, N. Ultrasound-mediated preparation and evaluation of a collagen/PVP-PCL micro- and nanofiber scaffold electrospun from chloroform/ethanol mixture. Fibers Polym. 2016, 17, 1186–1197. [Google Scholar] [CrossRef]
- Badran, M.M.; Alomrani, A.H.; Harisa, G.I.; Ashour, A.; Kumar, A.; Yassin, A.E. Novel docetaxel chitosan-coated PLGA/PCL nanoparticles with magnified cytotoxicity and bioavailability. Biomed. Pharmacother. 2018, 106, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Suwantog, O. Biomedical applications of electrospun polycaprolactone fiber mats. Polym. Adv. Technol. 2016, 27, 1264–1273. [Google Scholar] [CrossRef]
- Daelemans, L.; Steyaert, I.; Schoollaert, E.; Goudenhooft, C.; Rahier, H.; De Clerck, K. Nanostructured Hydrogels by Blend Electrospinning of Polycaprolactone/Gelatin Nanofibers. Nanomaterials 2018, 8, 551. [Google Scholar] [CrossRef] [Green Version]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan–polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef]
- Rijal, N.P.; Adhikari, U.; Khanal, S.; Pai, D.; Sankar, J.; Bhattarai, N. Magnesium oxide-poly(ε-caprolactone)-chitosan-based composite nanofiber for tissue engineering applications. Mater. Sci. Eng. B 2018, 228, 18–27. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Chen, F. Preparation and characterization of photocured poly (ε-caprolactone) diacrylate/poly (ethylene glycol) diacrylate/chitosan for photopolymerization-type 3D printing tissue engineering scaffold application. Mater. Sci. Eng. C 2017, 81, 66–73. [Google Scholar] [CrossRef]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef]
- Malheiro, V.N.; Caridade, S.; Alves, N.M.; Mano, J.F. New poly(ε-caprolactone)/chitosan blend fibers for tissue engineering applications. Acta Biomater. 2010, 6, 418–428. [Google Scholar] [CrossRef]
- Zelenková, T.; Onnainty, R.; Granero, G.E.; Barresi, A.A.; Fissore, D. Use of microreactors and freeze-drying in the manufacturing process of chitosan coated PCL nanoparticles. Eur. J. Pharm. Sci. 2018, 119, 135–146. [Google Scholar] [CrossRef]
- Hild, M.; Al Rez, M.F.; Aibibu, D.; Toskas, G.; Cheng, T.; Laourine, E.; Cherif, C. Pcl/chitosan blended nanofibrous tubes made by dual syringe electrospinning. Autex. Res. 2015, 15, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Kiadeh, S.Z.H.; Ghaee, A.; Mashak, A.; Mohammadnejad, J. Preparation of chitosan-silica/PCL composite membrane as wound dressing with enhanced cell attachment. Polym. Adv. Technol. 2017, 28, 1396–1408. [Google Scholar] [CrossRef]
- Prado, L.B.; Huber, S.C.; Barnabé, A.; Bassora, F.D.S.; Paixão, D.S.; Durán, N.; Annichino-Bizzacchi, J.M. Characterization of PCL and Chitosan Nanoparticles as Carriers of Enoxaparin and Its Antithrombotic Effect in Animal Models of Venous Thrombosis. J. Nanotechnol. 2017, 2017, 4925495. [Google Scholar] [CrossRef] [Green Version]
- Abasalta, M.; Asefnejad, A.; Khorasani, M.T.; Saadatabadi, A.R. Fabrication of carboxymethyl chitosan/poly(ε-caprolactone)/doxorubicin/nickel ferrite core-shell fibers for controlled release of doxorubicin against breast cancer. Carbohydr. Polym. 2021, 257, 117631. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ling, Y.; Li, X.; Yuan, B.; Yu, F.; Xie, W.; Chen, X. Combining amphiphilic chitosan and bioglass for mediating cellular osteogenic growth peptide gene. RSC Adv. 2015, 5, 79239–79248. [Google Scholar] [CrossRef]
- Ju, H.Y.; Dan, W.H.; Dan, N.H.; Liu, X.Y.; Liu, Y.Q. Micromorphology and structural characteristics of chitosan/polycaprolactone composite membrane. Acta Bioch. Bioph. Sin. 2016, 33, 2038–2044. [Google Scholar]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Janarthanan, G.; Mamatha, M.P.; Sivanandam, S.; Singaram, G.; Beliyur, K.D.R.; Kulasekaran, S.S.; Rajendran, S.; Amitava, B. Carbon nanofiber amalgamated 3D poly-ε-caprolactone scaffold functionalized porous-nanoarchitectures for human meniscal tissue engineering: In vitro and in vivo biocompatibility studies. Nanomed. Nanotechnol. 2018, 7, 2247–2258. [Google Scholar]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-Y.; Hung, L.-H.; Chu, I.-M.; Ko, C.-S.; Lee, Y.-D. The application of type II collagen and chondroitin sulfate grafted PCL porous scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2009, 92A, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Gaona, L.A.; Ribelles, J.G.; Perilla, J.E.; Lebourg, M. Hydrolytic degradation of PLLA/PCL microporous membranes prepared by freeze extraction. Polym. Degrad. Stab. 2012, 97, 1621–1632. [Google Scholar] [CrossRef]
- Surucu, S.; Sasmazel, H.T. Development of core-shell coaxially electrospun composite PCL/chitosan scaffolds. Int. J. Biol. Macromol. 2016, 92, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Dell’Erba, R.; Groeninckx, G.; Maglio, G.; Malinconico, M.; Migliozzi, A. Immiscible polymer blends of semicrystalline biocompatible components: Thermal properties and phase morphology analysis of PLLA/PCL blends. Polymer 2001, 42, 7831–7840. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Huang, J.; Zhang, J.; Yin, D.; Zheng, Z.; Liao, C.; Sun, S. Investigation of mechanical properties and degradability of multi-channel chitosan–polycaprolactone/collagen conduits. Polym. Degrad. Stab. 2012, 98, 122–132. [Google Scholar] [CrossRef]
- Urbanek, O.; Sajkiewicz, P.; Pierini, F. The effect of polarity in the electrospinning process on PCL/chitosan nanofibres’ structure, properties and efficiency of surface modification. Polymer 2017, 124, 168–175. [Google Scholar] [CrossRef]
- Naguib, H.F.; Aziz, M.S.A.; Sherif, S.M.; Saad, G.R. Synthesis and thermal characterization of poly(ester-ether urethane)s based on PHB and PCL-PEG-PCL blocks. J. Polym. Res. 2010, 18, 1217–1227. [Google Scholar] [CrossRef]
- Peponi, L.; Sessini, V.; Arrieta, M.P.; Navarro-Baena, I.; Sonseca, A.; Dominici, F.; Gimenez, E.; Torre, L.; Tercjak, A.; López, D.; et al. Thermally-activated shape memory effect on biodegradable nanocomposites based on PLA/PCL blend reinforced with hydroxyapatite. Polym. Degrad. Stab. 2018, 151, 36–51. [Google Scholar] [CrossRef]
- Seggiani, M.; Altieri, R.; Puccini, M.; Stefanelli, E.; Esposito, A.; Castellani, F.; Stanzione, V.; Vitolo, S. Polycaprolactone-collagen hydrolysate thermoplastic blends: Processability and biodegradability/compostability. Polym. Degrad. Stab. 2018, 150, 13–24. [Google Scholar] [CrossRef]



| CS:PCL | Tensile Strength (MPa) | Maximum Elongation (%) | Elongation at Break (%) |
|---|---|---|---|
| 0:100 | 20.981 ± 0.447 | 210.74 ± 0.96 | 210.08 ± 0.53 |
| 5:95 | 20.289 ± 0.198 | 201.26 ± 0.78 | 205.82 ± 0.28 |
| 10:90 | 20.036 ± 0.283 | 196.27 ± 0.49 | 198.72 ± 0.62 |
| 15:85 | 17.764 ± 0.475 | 153.88 ± 0.74 | 162.88 ± 1.03 |
| 20:80 | 16.691 ± 0.231 | 96.61 ± 0.56 | 101.41 ± 0.77 |
| 100:0 | 12.363 ± 0.536 | 13.60 ± 0.73 | 19.29 ± 0.46 |
| CS:PCL | Mean Porosity (%) | Specific Surface Area | Mean Pore Volume | Mean Pore Size | |||
|---|---|---|---|---|---|---|---|
| Cumulative Adsorption (m2/g) | Cumulative Desorption (m2/g) | Cumulative Adsorption (cm3/g) | Cumulative Desorption (cm3/g) | Adsorption (μm) | Desorption (μm) | ||
| 0:100 | 96.74 | 8.6624 | 10.6078 | 0.59773 | 0.68114 | 0.0964 | 0.1093 |
| 5:95 | 89.18 | 4.6281 | 4.8766 | 0.19456 | 0.21097 | 0.0217 | 0.0278 |
| 10:90 | 85.61 | 4.2417 | 4.5961 | 0.18399 | 0.19015 | 0.0199 | 0.0259 |
| 15:85 | 80.96 | 3.9348 | 3.9914 | 0.16932 | 0.18273 | 0.0186 | 0.0194 |
| 20:80 | 67.55 | 2.1565 | 1.9287 | 0.07765 | 0.09563 | 0.0079 | 0.0096 |
| 100:0 | 60.29 | 1.0467 | 1. 3726 | 0.01536 | 0.01756 | 0.0035 | 0.0047 |
| CS:PCL | Tm (°C) | ΔHm (J/g) |
|---|---|---|
| 0:100 | 64.8 | 56.74 |
| 5:95 | 69.8 | 61.93 |
| 10:90 | 77.6 | 79.65 |
| 15:85 | 77.9 | 80.13 |
| 20:80 | 78.1 | 81.97 |
| 100:0 | 86.3 | 97.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wu, Y.; Yang, M.; Zhang, G.; Ju, H. Thermal Stability and Dynamic Mechanical Properties of Poly(ε-caprolactone)/Chitosan Composite Membranes. Materials 2021, 14, 5538. https://doi.org/10.3390/ma14195538
Zhang Y, Wu Y, Yang M, Zhang G, Ju H. Thermal Stability and Dynamic Mechanical Properties of Poly(ε-caprolactone)/Chitosan Composite Membranes. Materials. 2021; 14(19):5538. https://doi.org/10.3390/ma14195538
Chicago/Turabian StyleZhang, Yanbo, Yaqi Wu, Ming Yang, Gang Zhang, and Haiyan Ju. 2021. "Thermal Stability and Dynamic Mechanical Properties of Poly(ε-caprolactone)/Chitosan Composite Membranes" Materials 14, no. 19: 5538. https://doi.org/10.3390/ma14195538
APA StyleZhang, Y., Wu, Y., Yang, M., Zhang, G., & Ju, H. (2021). Thermal Stability and Dynamic Mechanical Properties of Poly(ε-caprolactone)/Chitosan Composite Membranes. Materials, 14(19), 5538. https://doi.org/10.3390/ma14195538






