Annealing-Induced Off-Stoichiometric and Structural Alterations in Ca2+- and Y3+-Stabilized Zirconia Ceramics
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. X-ray Diffraction
3.2. Raman Spectroscopy
3.3. XPS Spectroscopy
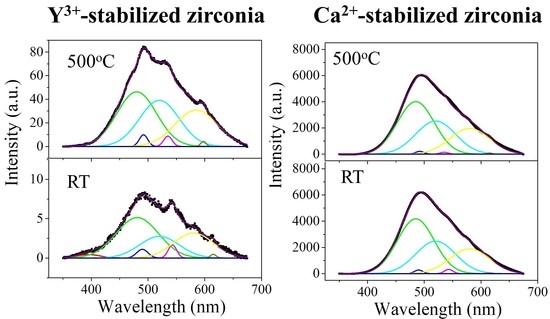
3.4. CL Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kim, D.-Y.; Kwon, S.; Yoon, B.-H.; Lee, J.-H. High-temperature corrosion characteristics of yttria-stabilized zirconia material in molten salts of LiCl-Li2O and LiCl-Li2O-Li. J. Nucl. Sci. Technol. 2017, 55, 97–103. [Google Scholar] [CrossRef]
- Panadero, R.A.; Roman-Rodriguez, J.L.; Ferreiroa, A.; Sola-Ruiz, M.F.; Fons-Font, A. Zirconia in fixed prosthesis: A literature review. J. Clin. Exp. Dent. 2014, 6, 66–73. [Google Scholar] [CrossRef]
- Butz, B. Yttria-Doped Zirconia as Solid Electrolyte for Fuel-Cell Applications: Fundamental Aspects; Südwestdt Verlag für Hochschulschr: Verlag, Germany, 2011. [Google Scholar]
- Hao, S.-J.; Wang, C.; Liu, T.-L.; Mao, Z.-M.; Mao, Z.-Q.; Wang, J.-L. Fabrication of nanoscale yttria stabilized zirconia for solid oxide fuel cell. Int. J. Hydrogen Energy 2017, 42, 29949–29959. [Google Scholar] [CrossRef]
- Yeh, W.; Patuwathavithane, C.; Zee, R.H. Electrical properties of vapor-deposited yttria-stabilized zirconia thin films. J. Appl. Phys. 1996, 79, 7809. [Google Scholar] [CrossRef]
- Dey, S.; Mardinly, J.; Wang, Y.; Valdez, J.A.; Holesinger, T.G.; Uberuaga, B.P.; Ditto, J.J.; Drazin, J.W.; Castro, R.H.R. Irradiation-induced grain growth and defect evolution in nanocrystalline zirconia with doped grain boundaries. Phys. Chem. Chem. Phys. 2016, 18, 16921–16929. [Google Scholar] [CrossRef] [PubMed]
- Tateiwa, T.; Marin, E.; Rondinella, A.; Ciniglio, M.; Zhu, W.; Affatato, S.; Pezzotti, G.; Bock, R.M.; McEntire, B.J.; Bal, B.S.; et al. Burst strength of BIOLOX®delta femoral heads and its dependence on low-temperature environmental degradation. Materials 2020, 13, 350. [Google Scholar] [CrossRef] [Green Version]
- Butz, B.; Schneider, R.; Gerthsen, D.; Schowalter, M.; Rosenauer, A. Decomposition of 8.5 mol.% Y2O3-doped zirconia and its contribution to the degradation of ionic conductivity. Acta Mater. 2009, 57, 5480–5490. [Google Scholar] [CrossRef]
- Pezzotti, G. Advanced Materials for Joint Implants; Pan Stanford: Singapore, 2013. [Google Scholar]
- Savin, A.; Craus, M.-L.; Turchenko, V.; Bruma, A.; Dubos, P.-A.; Malo, S.; Konstantinova, T.E.; Burkhovetsky, V.V. Monitoring Techniques of Cerium Stabilized Zirconia for Medical Prosthesis. Appl. Sci. 2015, 5, 1665–1682. [Google Scholar] [CrossRef]
- Butz, B.; Kruse, P.; Störmer, H.; Gerthsen, D.; Müller, A.; Weber, A.; Ivers-Tiffée, E. Correlation between microstructure and degradation in conductivity for cubic Y2O3-doped ZrO2. Solid State Ion. 2006, 177, 3275–3284. [Google Scholar] [CrossRef]
- Zhu, W.; Fujiwara, A.; Nishiike, N.; Marin, E.; Sugano, N.; Pezzotti, G. Transition metals increase hydrothermal stability of yttria-tetragonal zirconia polycrystals (3Y-TZP). J. Eur. Ceram. Soc. 2018, 38, 3573–3577. [Google Scholar] [CrossRef]
- Chen, W.; Hu, D.; Gu, H.; Qian, P.; Vleugels, J.; Jiang, Y.; Xing, J. Bi-modal distribution of stabilizers to regulate the dual-phase microstructure and transformability in Nd2O3/Y2O3-doped zirconia ceramics. J. Eur. Ceram. Soc. 2019, 39, 43330–44337. [Google Scholar] [CrossRef]
- Borik, M.A.; Chislov, A.S.; Kulebyakin, A.V.; Lomonova, E.E.; Milovich, F.O.; Myzina, V.A.; Ryabochkina, P.A.; Sidorova, N.V.; Tabachkova, N.Y. Effect of heat treatment on the structure and mechanical properties of partially gadolinia-stabilized zirconia crystals. J. Asian Ceram. Soc. 2021, 9, 559–569. [Google Scholar] [CrossRef]
- Schulz, U. Phase Transformation in EB-PVD Yttria Partially Stabilized Zirconia Thermal Barrier Coatings during Annealing. J. Am. Ceram. Soc. 2004, 83, 904–910. [Google Scholar] [CrossRef]
- Zhu, W.; Fujiwara, A.; Nishiike, N.; Nakashima, S.; Gu, H.; Marin, E.; Sugano, N.; Pezzotti, G. Mechanisms induced by transition metal contaminants and their effect on the hydrothermal stability of zirconia-containing bioceramics: An XPS study. Phys. Chem. Chem. Phys. 2018, 20, 28929–28940. [Google Scholar] [CrossRef]
- Zhu, W.; Nakashima, S.; Marin, E.; Gu, H.; Pezzotti, G. Microscopic mapping of dopant content and its link to the structural and thermal stability of yttria-stabilized zirconia polycrystals. J. Mater. Sci. 2020, 55, 524–534. [Google Scholar] [CrossRef]
- Garvie, R.C.; Nicholson, P.S. Phase Analysis in Zirconia Systems. J. Am. Ceram. Soc. 1972, 55, 303. [Google Scholar] [CrossRef]
- Verma, S.; Rani, S.; Kumar, S. Tetragonal zirconia quantum dots in silica matrix prepared by a modified sol–gel protocol. Appl. Phys. A 2018, 124, 387. [Google Scholar] [CrossRef]
- Middleburgh, S.C.; Ipatova, I.; Evitts, L.J.; Rushton, M.J.D.; Assinder, B.; Grimes, R.W.; Lee, W.E. Evidence of excess oxygen accommodation in yttria partially-stabilized zirconia. Scr. Mater. 2020, 175, 7–10. [Google Scholar] [CrossRef]
- Chevalier, J.; Deville, S.; Münch, E.; Jullian, R.; Lair, F. Critical effect of cubic phase on aging in 3 mol% yttria-stabilized zirconia ceramics for hip replacement prosthesis. Biomaterials 2004, 25, 5539–5545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smits, K.; Grigorjeva, L.; Millers, D.; Sarakovskis, A.; Grabis, J.; Lojkowski, W. Intrinsic defect related luminescence in ZrO2. J. Lumin. 2011, 131, 2058–2062. [Google Scholar] [CrossRef]
- Králik, B.; Chang, E.K.; Louie, S.G. Structural properties and quasiparticle band structure of zirconia. Phys. Rev. B 1998, 57, 7027. [Google Scholar] [CrossRef]
- Petrik, N.G.; Taylor, D.P.; Orlando, T.M. Laser-stimulated luminescence of yttria-stabilized cubic zirconia crystals. J. Appl. Phys. 1999, 85, 6770–6776. [Google Scholar] [CrossRef]
- Boffelli, M.; Zhu, W.; Back, M.; Sponchia, G.; Francese, T.; Riello, P.; Benedetti, A.; Pezzotti, G. Oxygen Hole States in Zirconia Lattices: Quantitative Aspects of Their Cathodoluminescence Emission. J. Phys. Chem. A 2014, 118, 9828–9836. [Google Scholar] [CrossRef]
- Leto, A.; Porporati, A.A.; Zhu, W.; Green, M.; Pezzotti, G. High-resolution stress assessments of interconnect/dielectric electronic patterns using optically active point defects of silica glass as a stress sensor. J. Appl. Phys. 2007, 101, 093514. [Google Scholar] [CrossRef]
- Munisso, M.C.; Zhu, W.; Leto, A.; Pezzotti, G. Stress Dependence of Sapphire Cathodoluminescence from Optically Active Oxygen Defects as a Function of Crystallographic Orientation. J. Phys. Chem. A 2007, 111, 3526–3533. [Google Scholar] [CrossRef]
- Zhu, W.; Nakashima, S.; Matsuura, M.; Gu, H.; Marin, E.; Pezzotti, G. Raman and X-ray photoelectron spectroscopic characterization of thermal stability of 3 mol% yttria stabilized zirconia ceramics. J. Eur. Ceram. Soc. 2019, 39, 4928–4935. [Google Scholar] [CrossRef]
- Fabris, S.; Paxton, A.T.; Finnis, M.W. A stabilization mechanism of zirconia based on oxygen vacancies only. Acta Mater. 2002, 50, 5171–5178. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Liang, K.; Gu, S.; Zheng, Y.; Fang, H. Effect of oxygen vacancies on transformation of zirconia at low temperatures. J. Mater. Sci. 1997, 32, 6653–6656. [Google Scholar] [CrossRef]
- Weckman, T.; Laasonen, K. First principles study of the atomic layer deposition of alumina by TMA–H2O-process. Phys. Chem. Chem. Phys. 2015, 17, 17322–17334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagida, H.; Koumoto, K.; Miyayama, M. The Chemistry of Ceramics; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Ahamer, C.; Opitz, A.K.; Rupp, G.M.; Fleig, J. Revisiting the Temperature Dependent Ionic Conductivity of Yttria Stabilized Zirconia (YSZ). J. Electrochem. Soc. 2017, 164, F790–F803. [Google Scholar] [CrossRef]
- Merle-Mejean, T.; Barberis, P.; Othmane, S.B.; Nardou, F.; Quintard, P.E. Chemical forms of hydroxyls on/in zirconia: An FT-IR study. J. Eur. Ceram. Soc. 1998, 18, 1579–1586. [Google Scholar] [CrossRef]
- Balint, I.; Springuel-Huet, M.-A.; Aika, K.-i.; Fraissard, J. Evidence for oxygen vacancy formation in HZSM-5 at high temperature. Phys. Chem. Chem. Phys. 1999, 1, 3845–3851. [Google Scholar] [CrossRef]
- Lackner, P.; Zou, Z.; Mayr, S.; Choi, J.-I.J.; Diebold, U.; Schmid, M. Surface structures of ZrO2 films on Rh(111): From two layers to bulk termination. Surf. Sci. 2019, 679, 180–187. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Nakashima, S.; Marin, E.; Gu, H.; Pezzotti, G. Annealing-Induced Off-Stoichiometric and Structural Alterations in Ca2+- and Y3+-Stabilized Zirconia Ceramics. Materials 2021, 14, 5555. https://doi.org/10.3390/ma14195555
Zhu W, Nakashima S, Marin E, Gu H, Pezzotti G. Annealing-Induced Off-Stoichiometric and Structural Alterations in Ca2+- and Y3+-Stabilized Zirconia Ceramics. Materials. 2021; 14(19):5555. https://doi.org/10.3390/ma14195555
Chicago/Turabian StyleZhu, Wenliang, Shizuka Nakashima, Elia Marin, Hui Gu, and Giuseppe Pezzotti. 2021. "Annealing-Induced Off-Stoichiometric and Structural Alterations in Ca2+- and Y3+-Stabilized Zirconia Ceramics" Materials 14, no. 19: 5555. https://doi.org/10.3390/ma14195555
APA StyleZhu, W., Nakashima, S., Marin, E., Gu, H., & Pezzotti, G. (2021). Annealing-Induced Off-Stoichiometric and Structural Alterations in Ca2+- and Y3+-Stabilized Zirconia Ceramics. Materials, 14(19), 5555. https://doi.org/10.3390/ma14195555