Synthesis of Micro-Spikes and Herringbones Structures by Femtosecond Laser Pulses on a Titanium Plate—A New Material for Water Organic Pollutants Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
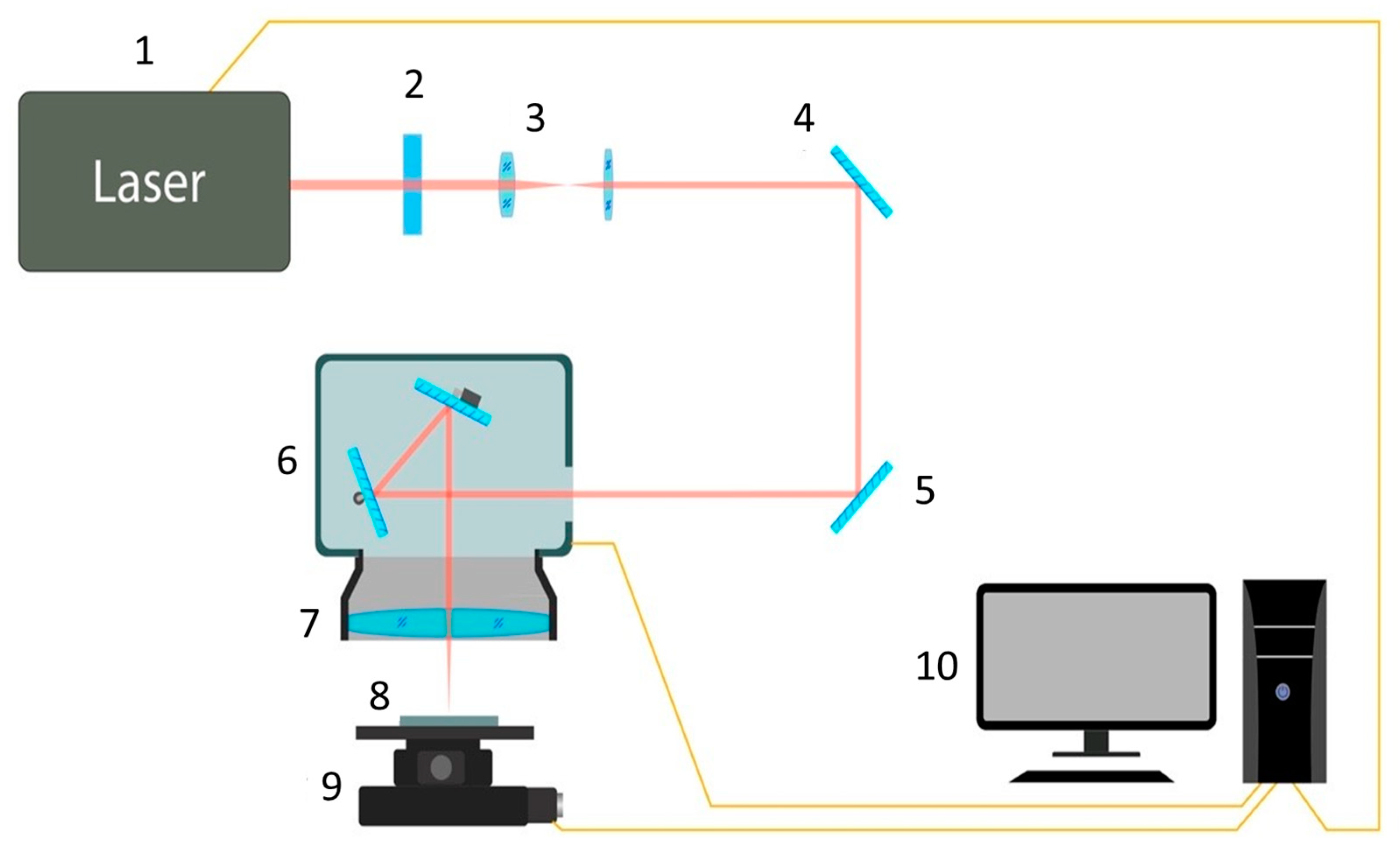
2.2. Laser Surface Treatment
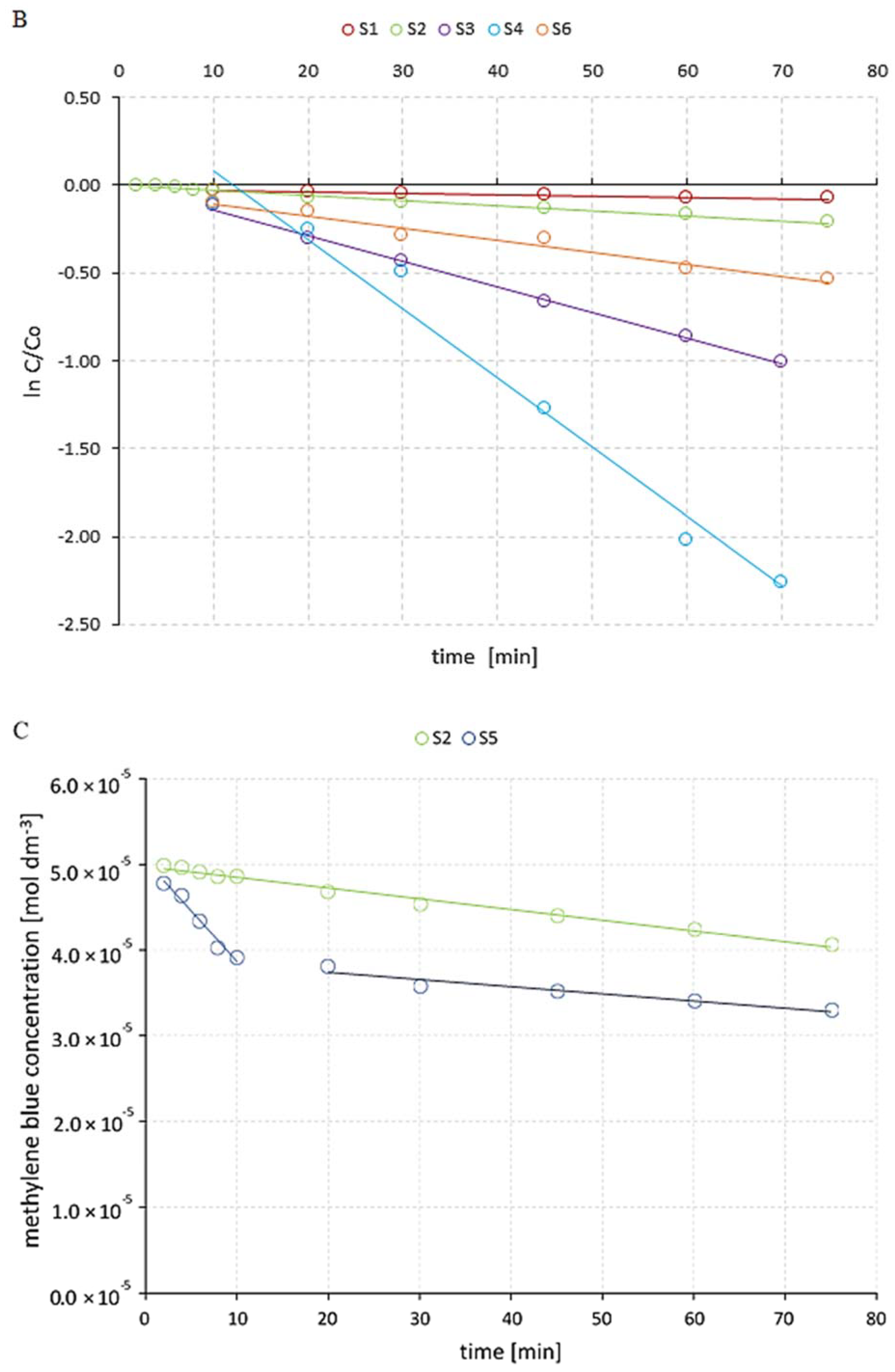
2.3. Photocatalytic Experiments
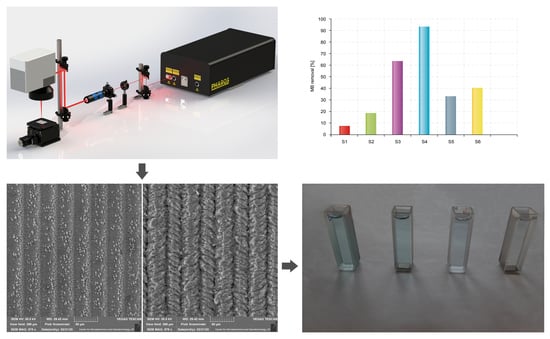
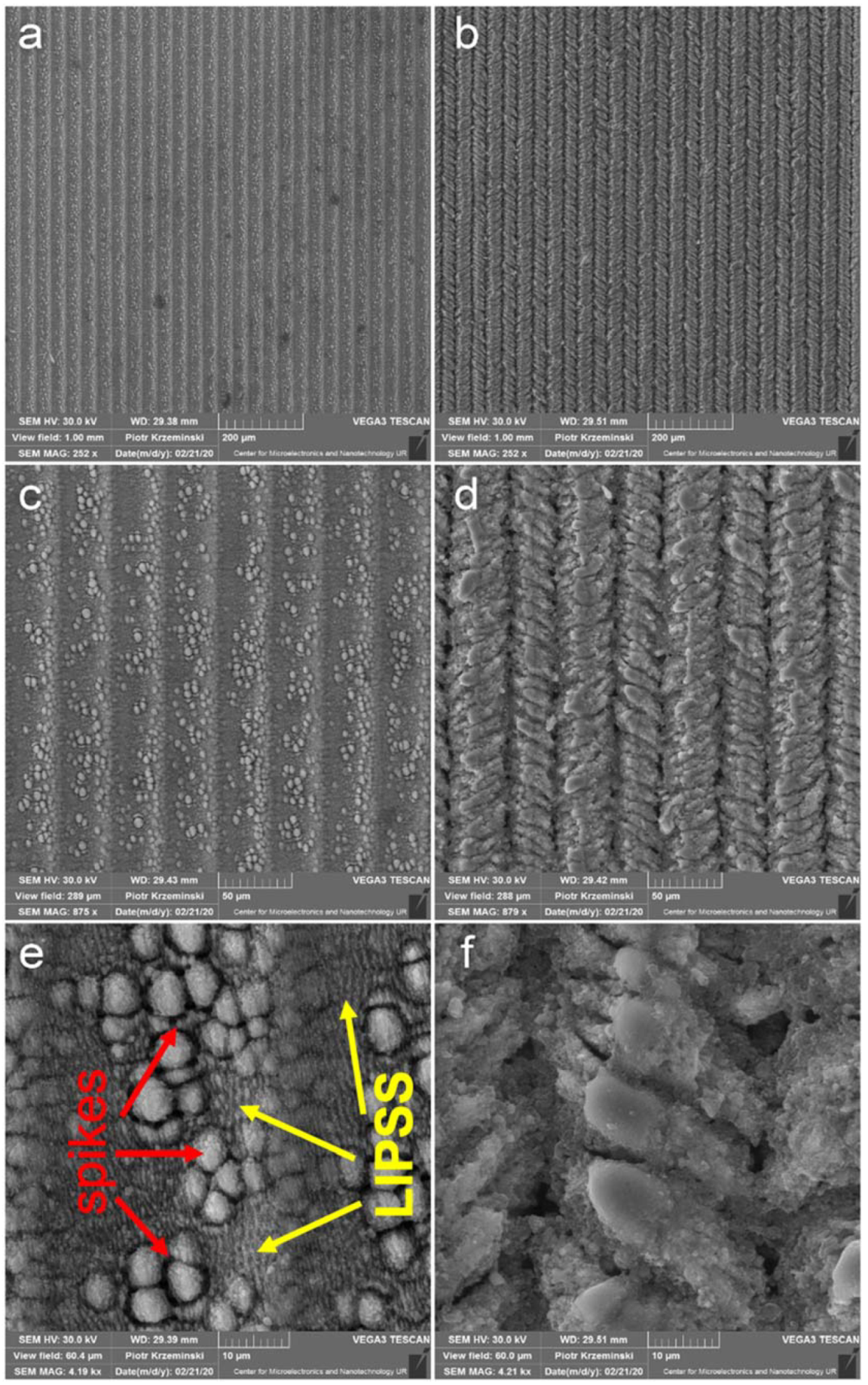
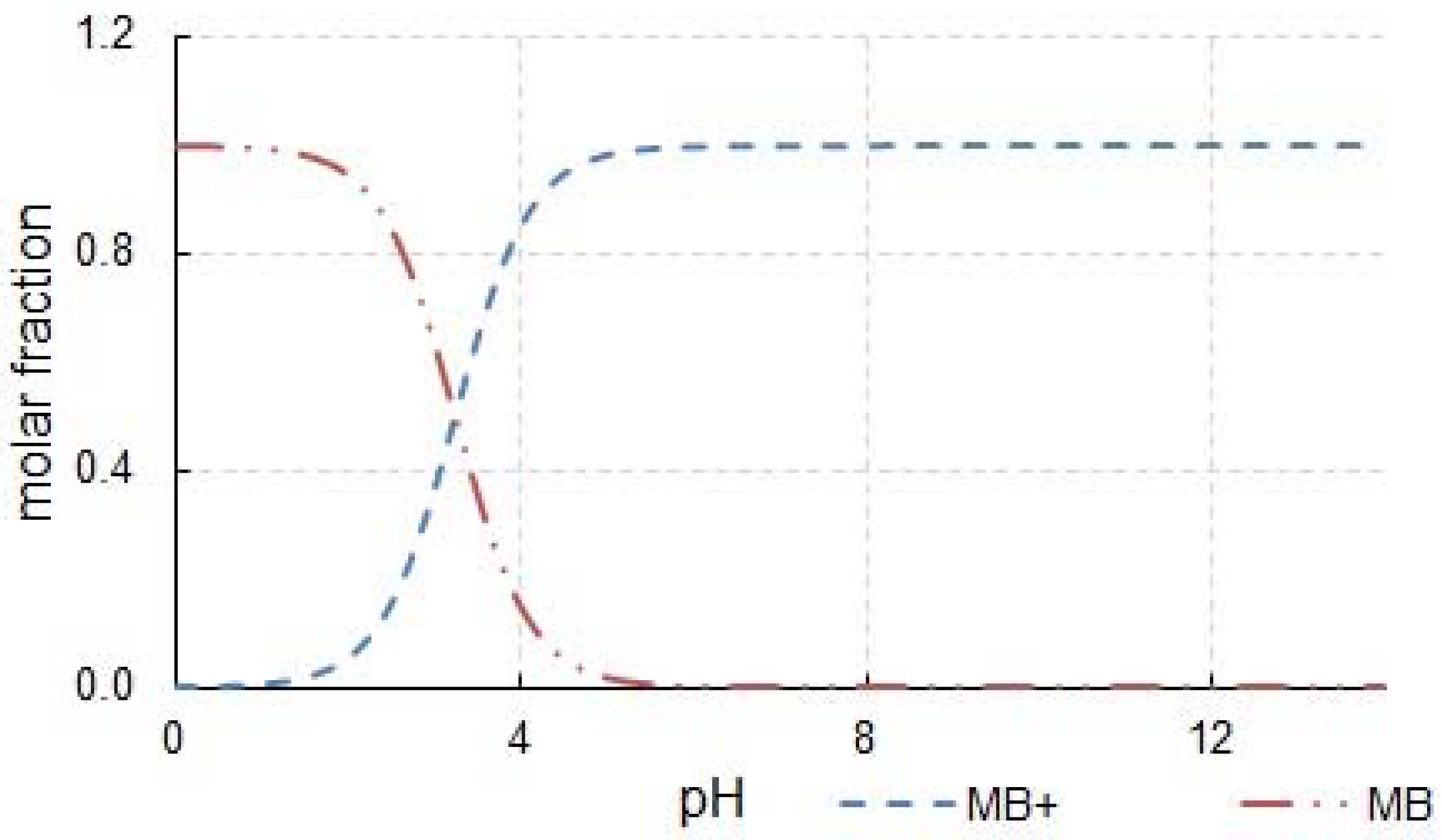
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Environmental Protection Agency. Emerging Technologies for Wastewater Treatment and In-Plant Wet Weather Management; Report EPA 832-R-12-011; Tetra Tech, Inc.: Fairfax, VA, USA, 2013. Available online: http://water.epa.gov/scitech/wastetech/publications.cfm (accessed on 5 September 2021).
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2018. [Google Scholar]
- Žerjav, G.; Zavašnik, J.; Kovač, J.; Pintar, A. The influence of Schottky barrier height onto visible-light triggered photocatalytic activity of TiO2 + Au composites. Appl. Surf. Sci. 2021, 543, 148799. [Google Scholar] [CrossRef]
- Romoli, L.; Rashed, C.; Fiaschi, M. Experimental characterization of the inner surface in micro-drilling of spray holes: A comparison between ultrashort pulsed laser and EDM. Opt. Laser Technol. 2014, 56, 35–42. [Google Scholar] [CrossRef]
- Pham, K.X.; Tanabe, R.; Ito, Y. Laser-induced periodic surface structures formed on the sidewalls of microholes trepanned by a femtosecond laser. Appl. Phys. A 2012, 112, 485–493. [Google Scholar] [CrossRef]
- Gnilitsky, I.; Orazi, L.; Derrien, T.J.-Y.; Bulgakova, N.M.; Mocek, T. Method of Ultrafast Laser Writing of Highly-Regular Periodic Structures on Metallic Materials. Patent of Czech Republic No. PV 2016-424, 27 June 2018. [Google Scholar]
- Bonse, J.; Höhm, S.; Hartelt, M.; Spaltmann, D.; Pentzien, S.; Koter, R.; Marschner, S.; Rosenfeld, A.; Krüger, J. Optically Induced Nanostructures: Biomedical and Technical Applications; De Gruyter: Berlin, Germany, 2015; pp. 141–156. [Google Scholar]
- Gnilitskyi, I.; Rota, A.; Gualtieri, E.; Valeri, S.; Orazi, L. Tribological Properties of High-Speed Uniform Femtosecond Laser Patterning on Stainless Steel. Lubricants 2019, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.; Serro, A.; Oliveira, V.; Almeida, A.; Vilar, R.; Durrieu, M.-C. Wetting behaviour of femtosecond laser textured Ti–6Al–4V surfaces. Appl. Surf. Sci. 2013, 265, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Bizi-Bandoki, P.; Benayoun, S.; Valette, S.; Beaugiraud, B.; Audouard, E. Modifications of roughness and wettability properties of metals induced by femtosecond laser treatment. Appl. Surf. Sci. 2011, 257, 5213–5218. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, M.; Li, J.; Ye, X.; Li, G.; Cai, L. Superhydrophobic surfaces fabricated by microstructuring of stainless steel using a femtosecond laser. Appl. Surf. Sci. 2009, 256, 61–66. [Google Scholar] [CrossRef]
- Rotella, G.; Orazi, L.; Alfano, M.; Candamano, S.; Gnilitskyi, I. Innovative high-speed femtosecond laser nano-patterning for improved adhesive bonding of Ti6Al4V titanium alloy. CIRP J. Manuf. Sci. Technol. 2017, 18, 101–106. [Google Scholar] [CrossRef]
- Fadeeva, E.; Schlie, S.; Koch, J.; Ngezahayo, A.; Chichkov, B.N. The hydrophobic properties of femtosecond laser fabricated spike structures and their effects on cell proliferation. Phys. Status Solidi 2009, 206, 1348–1351. [Google Scholar] [CrossRef]
- Nuutinen, T.; Silvennoinen, M.; Päiväsaari, K.; Vahimaa, P. Control of cultured human cells with femtosecond laser ablated patterns on steel and plastic surfaces. Biomed. Microdevices 2013, 15, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Calderon, M.; Silván, M.M.; Rodriguez, A.; Gomez-Aranzadi, M.; García-Ruiz, J.P.; Olaizola, S.M.; Martin-Palma, R.J. Surface micro- and nano-texturing of stainless steel by femtosecond laser for the control of cell migration. Sci. Rep. 2016, 6, 36296. [Google Scholar] [CrossRef]
- Sipe, J.E.; Young, J.F.; Preston, J.; Van Driel, H.M. Laser-induced periodic surface structure. I. Theory. Phys. Rev. B 1983, 27, 1141–1154. [Google Scholar] [CrossRef]
- Shugaev, M.V.; Gnilitskyi, I.; Bulgakova, N.M.; Zhigilei, L.V. Mechanism of single-pulse ablative generation of laser-induced periodic surface structures. Phys. Rev. B 2017, 96, 205429. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.-Y.; Gnilitskyi, I.; Shugaev, M.V.; Skoulas, E.; Stratakis, E.; Zhigilei, L.V. Effect of a liquid environment on single-pulse generation of laser induced periodic surface structures and nanoparticles. Nanoscale 2020, 12, 7674–7687. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, M.; Kayahara, T.; Nakano, H.; Hashida, M.; Katto, M.; Fujita, M.; Tanaka, M.; Abe, N. Microstructures formation on titanium plate by femtosecond laser ablation. J. Phys. Conf. Ser. 2007, 59, 666–669. [Google Scholar] [CrossRef]
- Heitz, J.; Plamadeala, C.; Muck, M.; Armbruster, O.; Baumgartner, W.; Weth, A.; Steinwender, C.; Blessberger, H.; Kellermair, J.; Kirner, S.V.; et al. Femtosecond laser-induced microstructures on Ti substrates for reduced cell adhesion. Appl. Phys. A 2017, 123, 734. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Li, X.; Jiang, L.; Ran, P.; Wang, H.; Chen, X.; Xu, C.; Tian, M.; Wang, S.; Zhang, J.; et al. Femtosecond laser mediated fabrication of micro/nanostructured TiO2- photoelectrodes: Hierarchical nanotubes array with oxygen vacancies and their photocatalysis properties. Appl. Catal. B Environ. 2020, 277, 119231. [Google Scholar] [CrossRef]
- Huang, T.; Lu, J.; Zhang, X.; Xiao, R.; Yang, W.; Wu, Q. Femtosecond Laser Fabrication of Anatase TiO2 Micro-nanostructures with Chemical Oxidation and Annealing. Sci. Rep. 2017, 7, 2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsibidis, G.D.; Fotakis, C.; Stratakis, E. From ripples to spikes: A hydrodynamical mechanism to interpret femtosecond laser-induced self-assembled structures. Phys. Rev. B 2015, 92, 041405. [Google Scholar] [CrossRef] [Green Version]
- Garcell, E.M.; Lam, B.; Guo, C. Femtosecond laser-induced herringbone patterns. Appl. Phys. A 2018, 124, 405. [Google Scholar] [CrossRef] [Green Version]
- Kabekkodu, S. PDF-4+ 2015 (Database); International Centre for Diffraction Data: Newtown Square, PA, USA, 2015. [Google Scholar]
- Crystallography Open Database. Available online: http://www.crystallography.net/cod/ (accessed on 5 September 2021).
- Atkins, P.; De Paula, J. Atkins’ Physical Chemistry, 8th ed.; W. H. Freeman and Company: New York, NY, USA, 2006; Chapter 22. [Google Scholar]
- Tombacz, E. pH-dependent surface charging of metal oxides. Period. Polytech. Chem. Eng. 2009, 53, 77–86. [Google Scholar] [CrossRef]
- Lawless, D.; Serpone, N.; Meisel, D. Role of hydroxyl radicals and trapped holes in photocatalysis. A pulse radiolysis study. J. Phys. Chem. 1991, 95, 5166–5170. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Banus, M.D.; Reed, T.B.; Strauss, A.J. Electrical and Magnetic Properties of TiO and VO. Phys. Rev. B 1972, 5, 2775–2784. [Google Scholar] [CrossRef]
- Dhumal, S.Y.; Daulton, T.L.; Jiang, J.; Khomami, B.; Biswas, P. Synthesis of visible light-active nanostructured TiOx (x < 2) photocatalysts in a flame aerosol reactor. Appl. Catal. B Environ. 2009, 86, 145–151. [Google Scholar]



| Sample | Energy per Pulse, Ep (μJ) | Repetition Rate, RR (kHz) | Step (µm) | Scanning Speed, Vs (m/s) | Polarization | Pulse Duration, τ (fs) |
|---|---|---|---|---|---|---|
| P_a | 0.9 | 200 | 10.5 | 1.05 | ⊥ | 266 |
| P_b | 5 | 1000 | 9 | 0.12 | ⊥ | 266 |
| Reaction Systems | H2O2 Concentration (%) | |
|---|---|---|
| Signature | Description | |
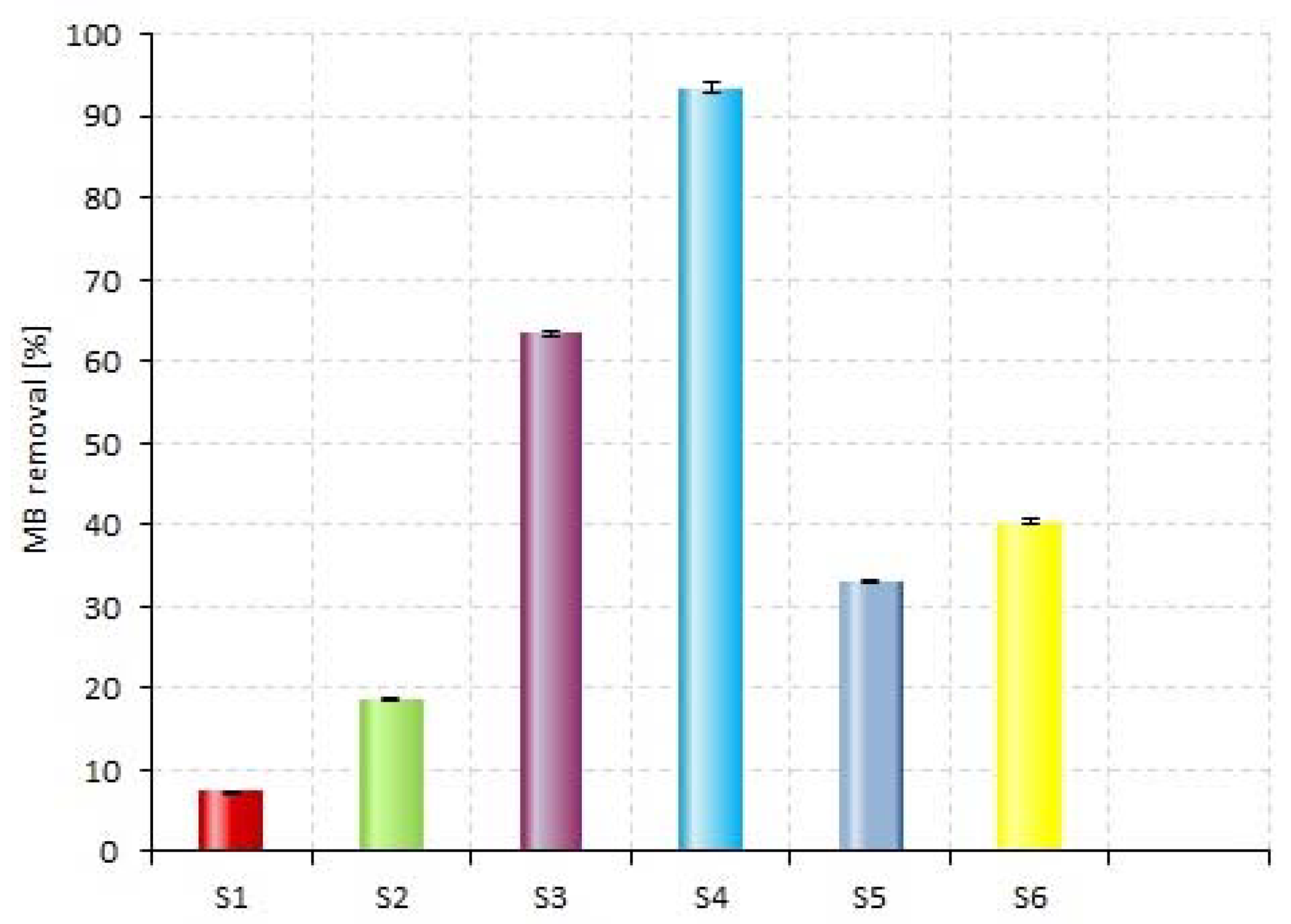
| S1 | MB + light | 0 |
| S2 | MB + P_b + light | 0 |
| S3 | MB + H2O2 + light | 1.14 |
| S4 | MB + H2O2 + P_b + light | 1.14 |
| S5 | MB + P_a + light | 0 |
| S6 | MB + H2O2 + P_a + light | 1.14 |
| Sample | Ti | O |
|---|---|---|
| Ti plate (control) | 84 | 16 |
| P_a | 80 | 20 |
| P_b | 46 | 54 |
| Reaction System | kapp (min−1) | t1/2 (min) |
|---|---|---|
| S1 | 0.9 × 10−3 | 770.1 |
| S2 | 4.2 × 10−3 | 165.0 |
| S3 | 14.6 × 10−3 | 47.8 |
| S4 | 43 × 10−3 | 16.4 |
| S5 | 27.6 × 10−3 | 25.11 |
| S6 | 6.8 × 10−3 | 101.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisała, J.; Gnilitskyi, I.; Cieniek, B.; Krzemiński, P.; Marchewka, M.; Barylyak, A.; Bobitski, Y. Synthesis of Micro-Spikes and Herringbones Structures by Femtosecond Laser Pulses on a Titanium Plate—A New Material for Water Organic Pollutants Degradation. Materials 2021, 14, 5556. https://doi.org/10.3390/ma14195556
Kisała J, Gnilitskyi I, Cieniek B, Krzemiński P, Marchewka M, Barylyak A, Bobitski Y. Synthesis of Micro-Spikes and Herringbones Structures by Femtosecond Laser Pulses on a Titanium Plate—A New Material for Water Organic Pollutants Degradation. Materials. 2021; 14(19):5556. https://doi.org/10.3390/ma14195556
Chicago/Turabian StyleKisała, Joanna, Iaroslav Gnilitskyi, Bogumił Cieniek, Piotr Krzemiński, Michał Marchewka, Adriana Barylyak, and Yaroslav Bobitski. 2021. "Synthesis of Micro-Spikes and Herringbones Structures by Femtosecond Laser Pulses on a Titanium Plate—A New Material for Water Organic Pollutants Degradation" Materials 14, no. 19: 5556. https://doi.org/10.3390/ma14195556