Use of Design of Experiments (DoE) to Model the Sulphate Agent Amount of (Ultra)Finely Ground and Fast Hardening Portland Cement Clinker
Abstract
:1. Introduction
1.1. Fast-Hardening Cements
- (1)
- Silicate reaction of the clinker phases C3S (alite) and C2S (belite): Calcium silicate hydrates (C-S-H phases) and additional portlandite (Ca(OH)2) are formed. The hydration of the alite proceeds comparatively quickly. Although the tabular portlandite can be identified by X-ray, the C-S-H crystals on the contrary are apparently X-ray amorphous and thus can only be quantified to a limited extent by XRD analysis. High conversion of the silicate reaction stands for a high degree of hydration and high early strength.
- (2)
- Aluminate reaction at the early hydration age of the C3A (tricalcium aluminate) with the sulphate agent: Prismatic ettringite and/or tabular monosulphate crystals are formed. In the absence of a sulphate agent, C-A-H phases are formed simultaneously. However, due to the mostly low crystallinity, the variable chemism and instabilities, a qualitative determination of the tabular monosulphate and the C-A-H phases is difficult [2].
- Increase in the C3S-amount
- Increase in the C3A-amount
- Change of sulphate agent and sulphate amount
- Increasing the grinding fineness of the cement
- Use of inorganic and organic additives.
1.2. Models to Determine the Optimised SO3 Content in Dependency of Different Clinker Types
1.3. Design of Experiments (DoE)
- ❖
- A-optimality: optimisation of the mean-variance of the regression coefficients
- ❖
- G-optimality: optimisation of the max. occurring variance of the prediction values
- ❖
- V-optimality: optimisation of the mean prediction quality in the factor space.
2. Materials and Methods
2.1. Basic Mixture
- Clinker 3000 cm²/g (C3): w/b-ratio = 0.3
- Clinker 7000 cm²/g (C7): w/b-ratio = 0.35
- Clinker 12,000 cm²/g (C12): w/b-ratio = 0.45.
- Hemihydrate (7080 cm²/g Blaine)
- Anhydrite (7835 cm²/g Blaine)
- C-S-H-seeds (Circosil® 0,1 (tobermorite), Circolit® (xonotlite))
- Silica fume.
- ➢
- (clinker-fineness/1000)_(SO3[%])_(hemihydrate/anhydrite)
2.2. Analytics
2.2.1. XRD
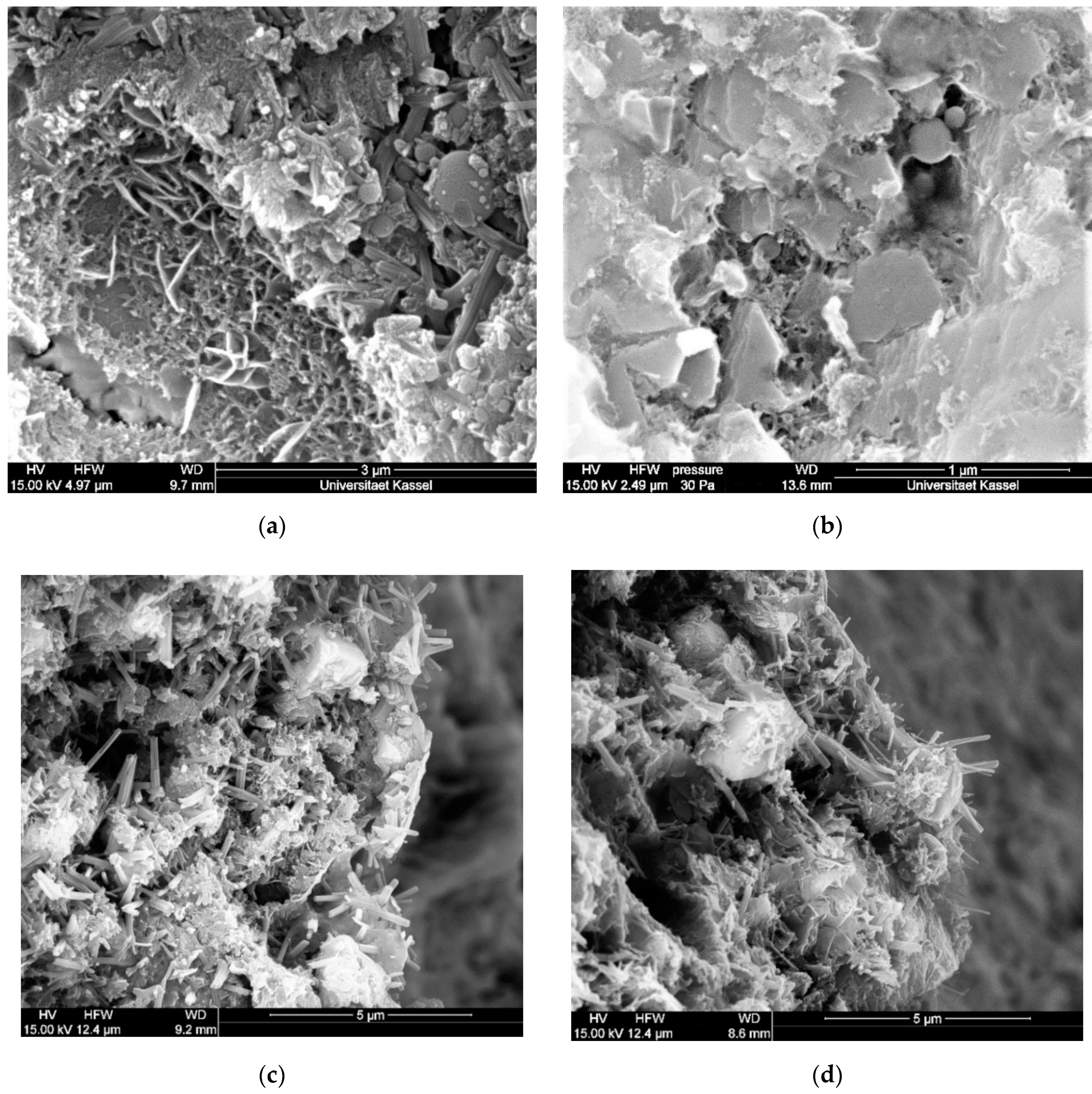
2.2.2. ESEM
2.2.3. Ultrasonic Test
2.3. Design of Experiments
- The sulphate agent ratio was varied from 0/100–100/0 (hemihydrate/anhydrite).
- The sulphate agent amount was varied in defined steps by the choice of a prefactor concerning Equation (3). Without using a sulphate agent the mixtures did not allow processability.
- The amount of C-S-H-seeds was varied in relation to the cement content.
- The clinker meal was varied in the grinding finenesses 3000, 7000 and 12,000 cm²/g.
- The C-S-H-seeds type is divided into tobermorite- (Circosil® 0.1) and xonotlite-structure (Circolit®).
3. Results
3.1. Development of a D-Optimal Test Matrix by DoE
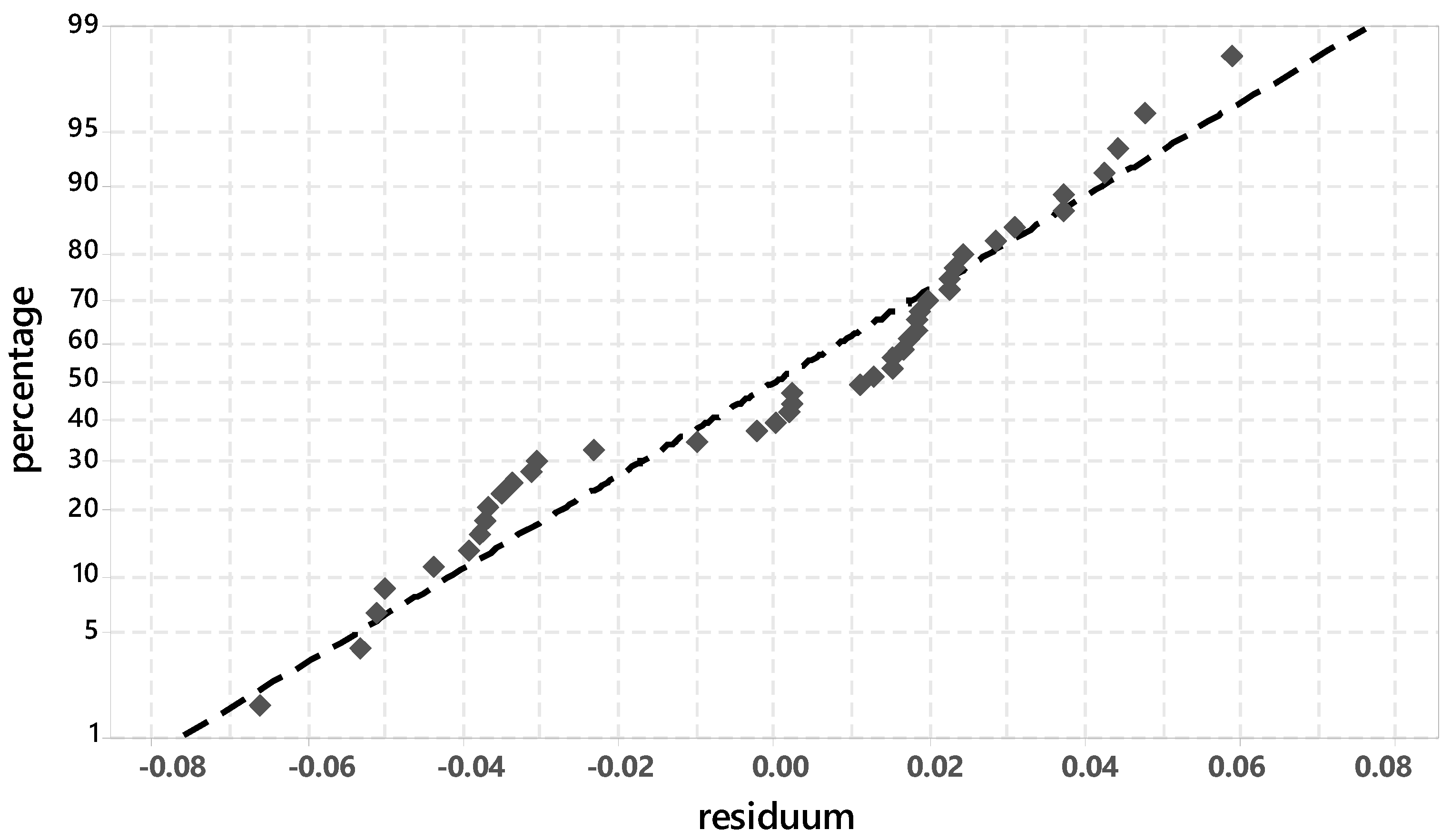
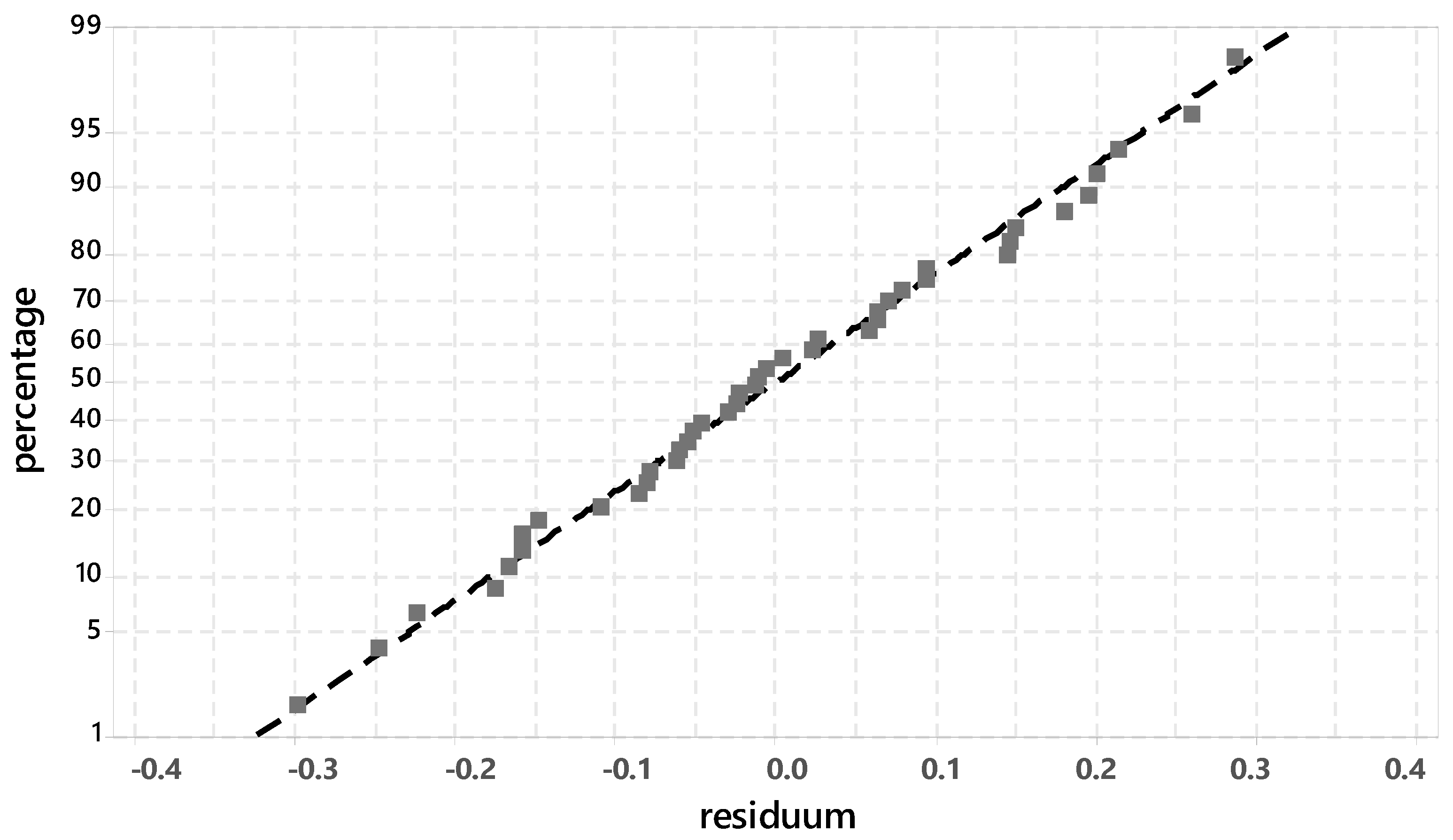
3.2. Analysis of the Degree of Hydration with Regard to the Silicate Phase
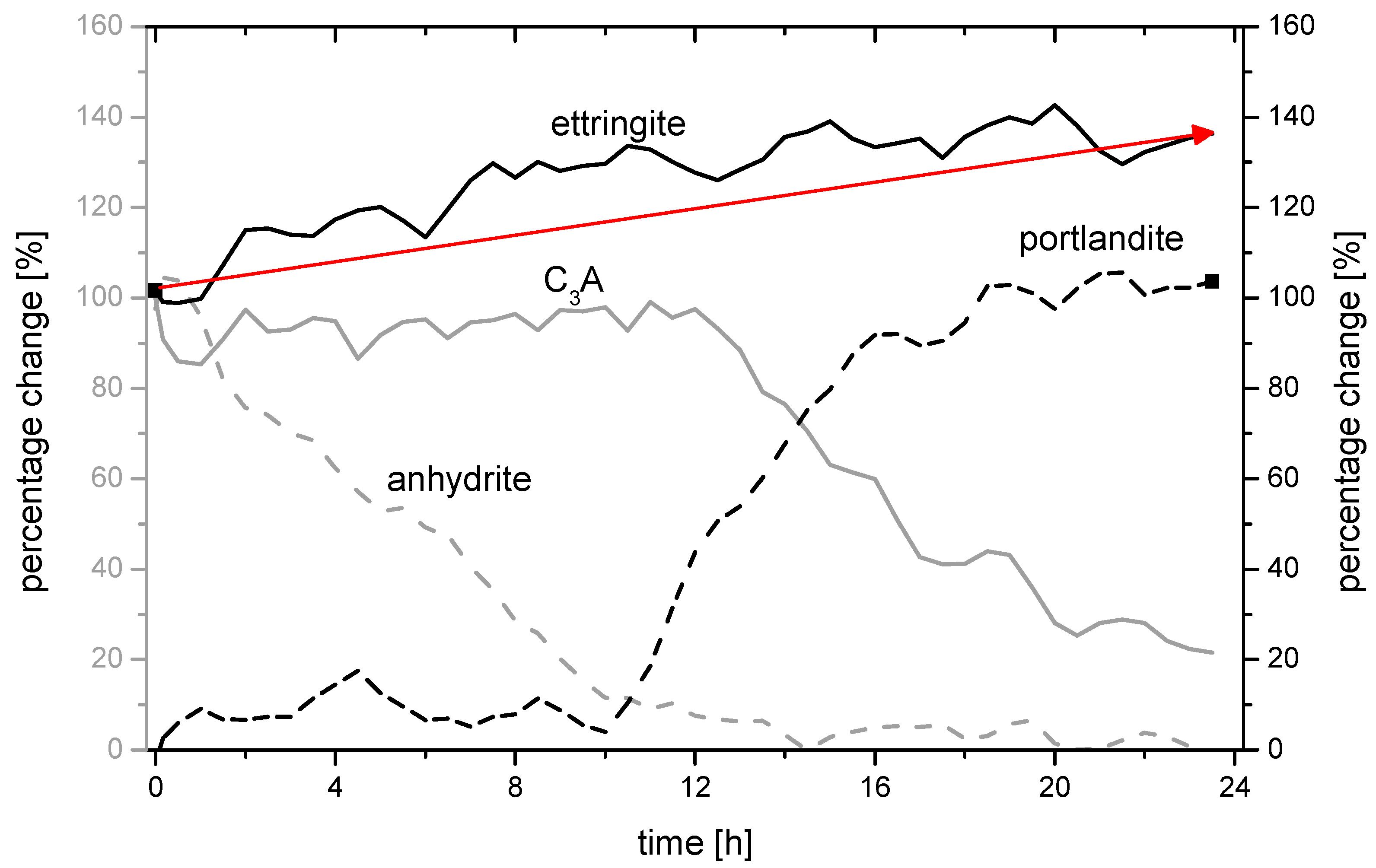
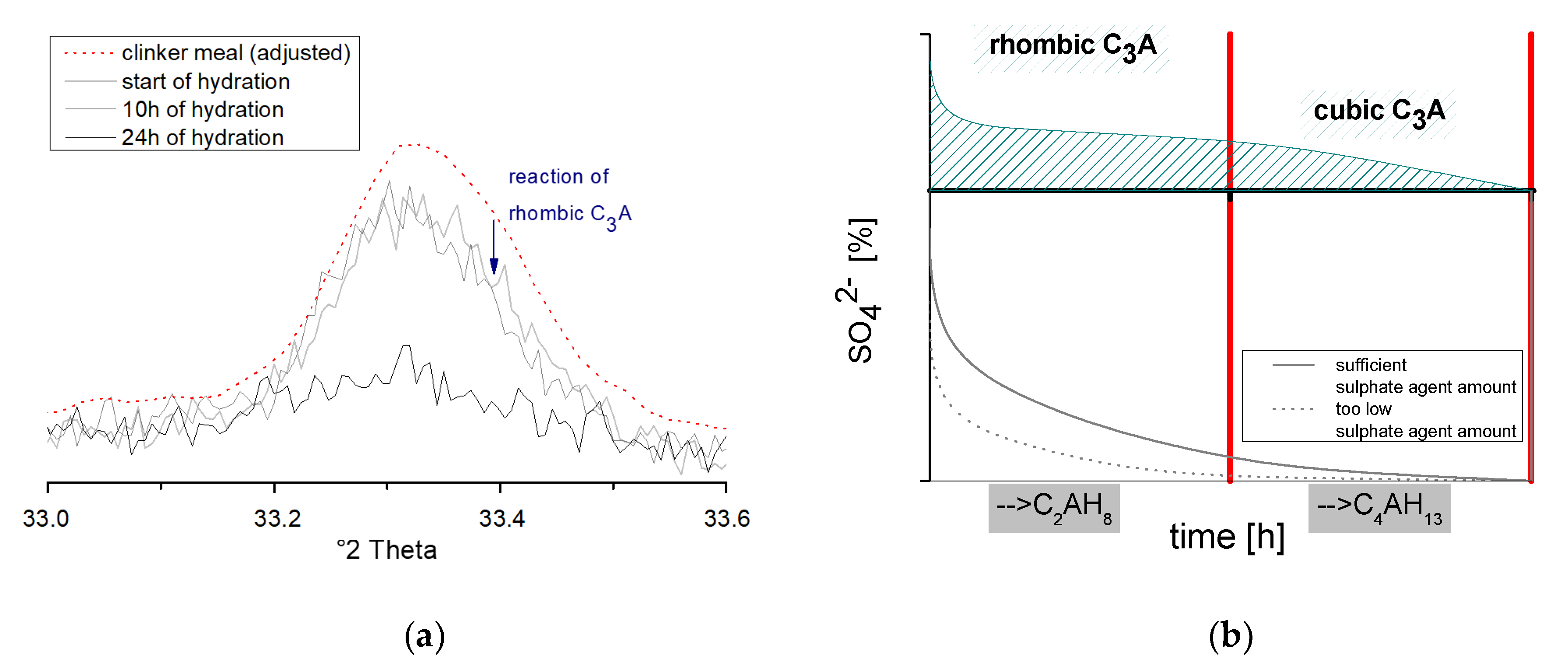
3.3. Analysis of the Ettringite Formation with Regard to the Aluminum Phase
3.3.1. Evaluation of Significant Factors Influencing the Ettringite Formation by DoE
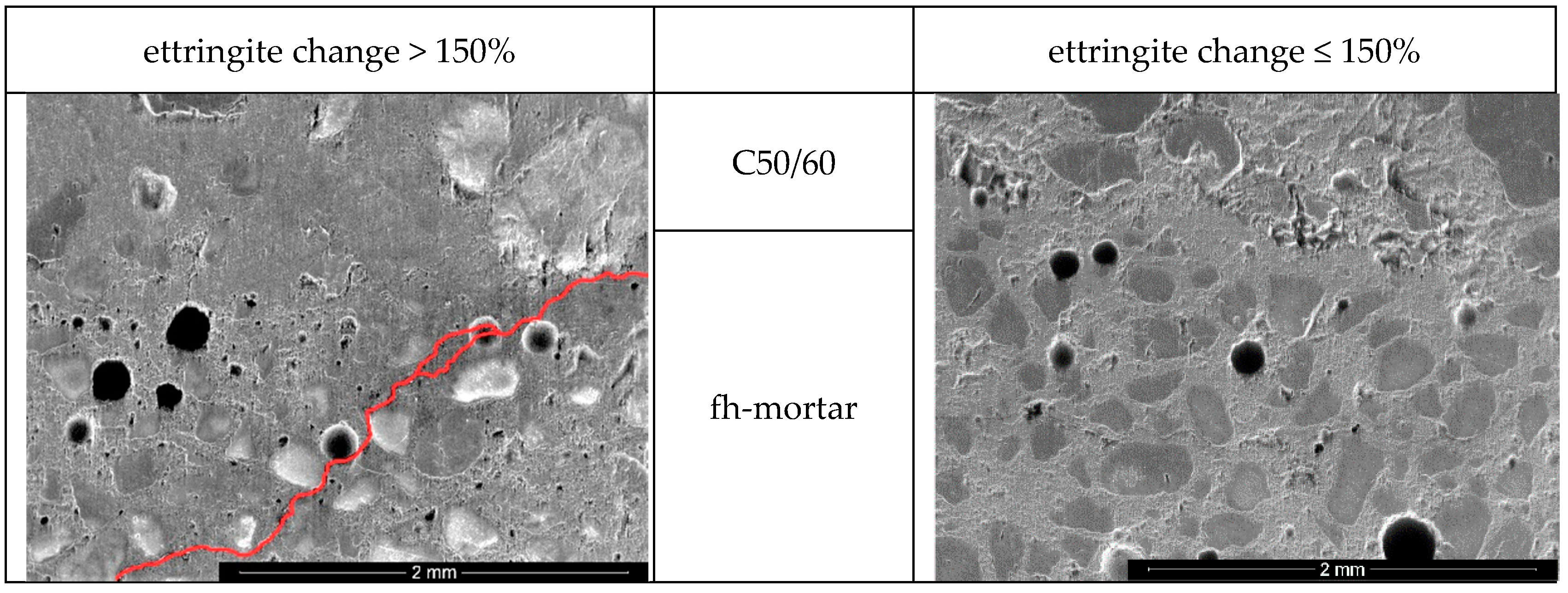
3.3.2. Chemical-Mineralogical Analysis of Phase Development
- C7_4.50_25/75 (ettringite change: 138.4%; 77.3 MPa)
- C7_4.50_75/25 (ettringite change: 132.9%; 72.5 MPa).
4. Discussion and Evaluation
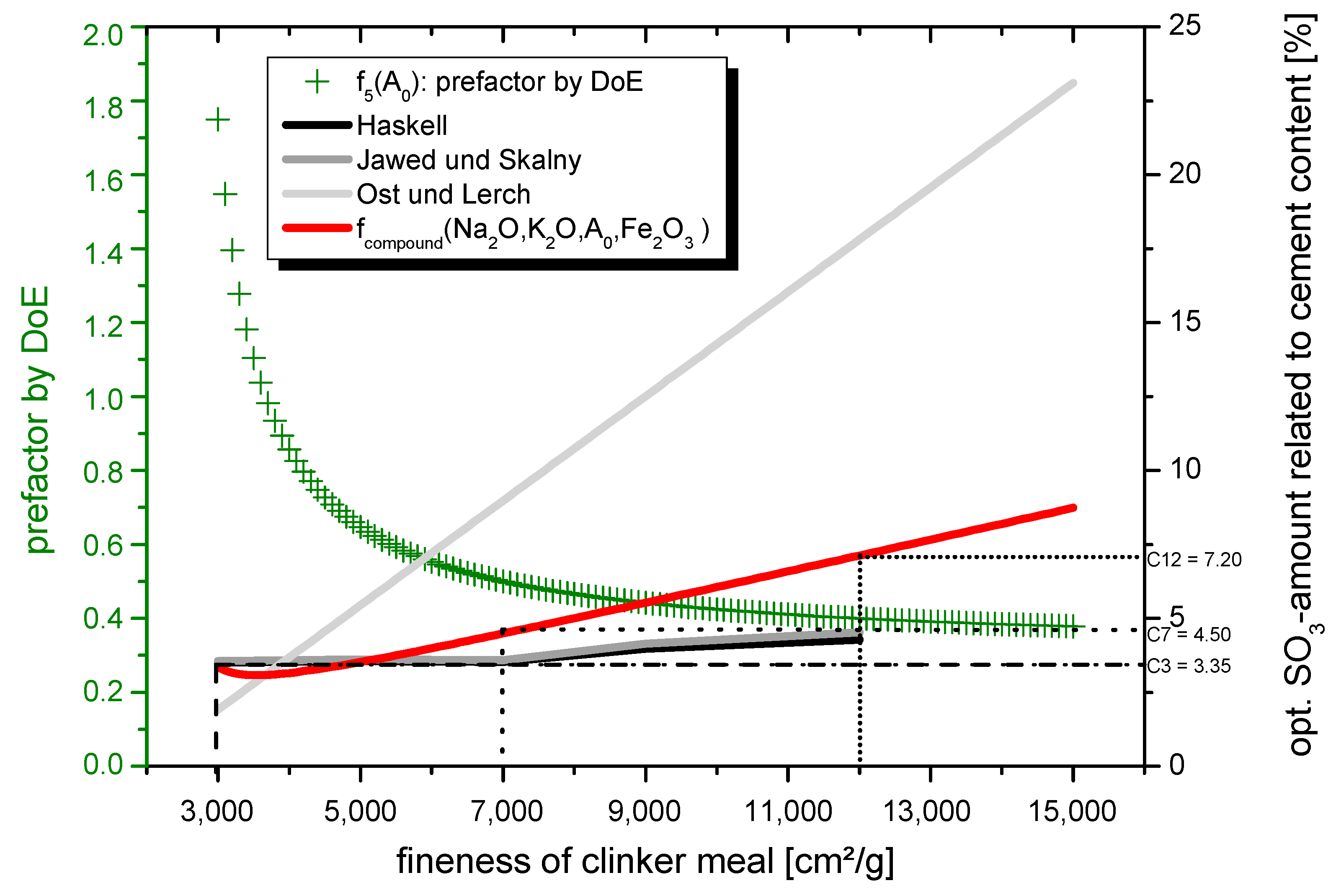
4.1. Modeling the Optimal SO3-Content for Clinker Meals with a High Grinding Fineness
- C3: XScontent = 1.75
- C7: XScontent = 0.50
- C12: XScontent = 0.39.
- ❖
- runs through the measured values determined using DoE
- ❖
- is monotonically decreasing in the interval I1 = [3; ∞)
- ❖
- only slowly tends towards 0 for x → +∞
- ❖
- has exclusively positive y-values in the interval I1 = [3; ∞).
- ❖
- exponential function of the form f1(x) = a∙ebx, f2(x) = b∙akx
- ❖
- power function of the form f3(x) = a∙xn (n ∈ R), which guarantees monotonicity as written above
- ❖
- fractional rational function of the form f4(x) = und f5(x) =.
- fk(3) = 1.75
- fk(7) > k(7) = 0.50
- fk(12) k(12) = 0.40 (rounded up).
- a = 0.372208
- b = 2.38462
- c = 0.064516.
4.2. Evaluation
- fcompound (Na2O, K2O, 3000, Fe2O3) = 3.35% SO3
- fcompound (Na2O, K2O, 12,000, Fe2O3) = 7.20% SO3.
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, H. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Seufert, S. Einfluss des Sulfatträgers auf ein Portlandzement/Calciumaluminatzement-Schnellzementsystem. PhD Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg, Naturwissenschaftliche Fakultät, Nürnberg, Germany, 2011. [Google Scholar]
- Stark, J.; Wicht, B. Zement und Kalk–Der Baustoff als Werkstoff; Birkhäuser Verlag: Basel, Switzerland, 2000. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials–State-of-the-Art Report, RILEM TC 224-AAM; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Chindaprasirt, P.; De Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Jiao, Z. Influence of curing on the strength development of calcium-containing geopolymer mortar. Materials 2013, 6, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.; Wang, Y.; Li, Z.; Li, Y.; Ren, Y. Binary reaction behaviors of red mud based cementitious material: Hydration characteristics and Na+ utilization. J. Hazard. Mater. 2021, 410, 124592. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J. A study on the setting characteristics of sodium silicate-activated slag pastes. Cem. Concr. Res. 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Jansson, H.; Tang, L. Parameters influencing the initial setting time of alkali-activated ground granulated blastfurnace slag materials. Nordic Concr. Res. 2015, 52, 63–76. [Google Scholar]
- Fernández-Jiménez, A.; Puertas, F. Effect of activator mix on the hydration and strength behaviour of alkali-activated slag cements. Adv. Cem. Res. 2003, 15, 129–136. [Google Scholar] [CrossRef]
- Adams, P.; Ideker, J.H. Influence of aggregate type on conversion and strength in calcium aluminate cement concrete. Cem. Concr. Res. 2017, 100, 284–296. [Google Scholar] [CrossRef]
- Midgley, H.G.; Midgley, A. The conversion of highalumina cement. Mag. Concr. Res. 1975, 27, 59–77. [Google Scholar] [CrossRef]
- Bizzozerro, J.; Gosselin, C.; Scrivener, K.L. Expansion Mechanisms in Calcium Aluminate and Sulfoaluminate Systems with Calcium Sulfate. Cem. Concr. Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Wang, L.; Glasser, F.P. Hydration of calcium sulphoaluminate cements. Adv. Cem. Res. 1996, 8, 127–134. [Google Scholar] [CrossRef]
- Fang, X.; Garcia, A.; Winnefeld, F.; Partl, M.N. Impact of rapid-hardening cements on mechanical properties of cement bitumen emulsion asphalt. Mater. Struct. 2016, 49, 487–498. [Google Scholar] [CrossRef]
- Odler, I. Special Inorganic Cements; E & FN Spon: London, UK; New York, NY, USA, 2000. [Google Scholar]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Mobili, A.; Belli, A.; Giosuè, C.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium Sulfoaluminate, Geopolymeric, and Cementitious Mortars for Structural Applications. Environments 2017, 4, 64. [Google Scholar] [CrossRef] [Green Version]
- Bentz, D.P.; Sant, G.; Weiss, W.J. Early-Age Properties of Cement-Based Materials. I: Influence of Cement Fineness. J. Mater. Civ. Eng. 2008, 20, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Erdoğan, S.; Koçak, T.C. Influence of slag fineness on the strength and heat evolution of multiple-clinker blended cements. Constr. Build. Mater. 2017, 155, 800–810. [Google Scholar] [CrossRef]
- Owens, K.; Russell, M.I.; Donnelly, G.; Kirk, A.; Basheer, P.A.M. Use of nanocrystal seeding chemical admixture in improving Portland cement strength development: Application for precast concrete industry. Adv. Appl. Ceram. 2014, 113, 478–484. [Google Scholar] [CrossRef]
- Das, S.; Ray, S.; Sarkar, S. Early strength development in concrete using preformed CSH nano crystals. Constr. Build. Mater. 2020, 233, 117214. [Google Scholar] [CrossRef]
- Nicoleau, L. The acceleration of cement hydration by seeding: Influence of the cement mineralogy. ZKG Int. 2013, 1, 40–49. [Google Scholar]
- Kontoleontos, F.; Tsakiridis, P.; Marinos, A.; Katsiotis, N.; Kaloidas, V.; Katsioti, M. Dry-grinded ultrafine cements hydration. physicochemical and microstructural characterization. Mater. Res. 2013, 16, 404–416. [Google Scholar] [CrossRef]
- Cornell, J.A. Experiments with Mixtures. Designs, Models, and the Analysis of Mixture Data, 3rd ed.; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Lazic, Z.R. Design of Experiments in Chemical Engineering; WILEY-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Subulan, K.; Cakmakci, M. A feasibility study using simulation-based optimization and Taguchi experimental design method for materialhandling—Transfer system in the automobile industry. Int. J. Adv. Manuf. Tech. 2011, 59, 433–443. [Google Scholar] [CrossRef]
- Montevechi, J.A.B.; de Pinho, A.F.; Leal, F.; Marins, F.A.S. Application of design of experiments on the simulation of a process in automotive industry. In Proceedings of the Winter Simulation Conference, Washington, DC, USA, 9–12 December 2007; pp. 1601–1609. [Google Scholar]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Murray, P.M.; Bellany, F.; Benhamou, L.; Bučar, D.-K.; Tabor, A.B.; Sheppard, T.D. The application of design of experiments (DoE) reaction optimisation and solvent selection in the development of new synthetic chemistry. Org. Biomol. Chem. 2016, 14, 2373–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schade, T.; Middendorf, B.; Eden, W.; Kuhnt, S.; Becker-Emden, E.-C. Raising the quality of calcium silicate units with simultaneous reduction of production costs using Statistical Design of Experiments (DoE). ZKG Int. 2020, 3, 35–42. [Google Scholar]
- Kuhnt, S.; Becker-Emden, E.-C.; Schade, T.; Eden, W.; Middendorf, B. Use of optimal mixture-process designs and response surface models to study properties of calcium silicate units. Qual. Reliab. Eng. Int. 2021, 37, 391–408. [Google Scholar] [CrossRef]
- Coetzer, R.; Haines, L.M. The construction of D- and I-optimal designs for mixture experiments with linear constraints on the components. Chemom. Intell. Lab. Sys. 2017, 171, 112–124. [Google Scholar] [CrossRef]
- Goos, P.; Jones, B.; Syafitri, U. I-Optimal Design of Mixture Experiments. J. Am. Stat. Assoc. 2016, 111, 899–911. [Google Scholar] [CrossRef]
- Syafitri, U.; Sartono, B.; Goos, P. D- and I-optimal design of mixture experiments in the presence of ingredient availability constraints. J. Qual. Technol. 2015, 3, 220–234. [Google Scholar] [CrossRef]
- Siebertz, K.; van Bebber, D.; Hochkirchen, T. Statistische Versuchsplanung–Design of Experiments (DoE); Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Schmidt, M. Sustainable building with ultra-high performance concrete (UHPC)—coordinated research program in Germany. In Research and Applications in Structural Engineering, Mechanics and Computation, 1st ed.; Zingoni, A., Ed.; CRC Press: London, United Kingdom, 2013; pp. 587–588. [Google Scholar]
- Hesse, C. Der Reaktionsverlauf der frühen Hydratation von Portlandzementen in Relation zur Temperatur. PhD Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg, Naturwissenschaftliche Fakultät, Erlangen, Germany, 2009. [Google Scholar]
- Dubina, E.; Plank, J.; Wadsö, L.; Black, L. Impact of environmental moisture on C3A polymorphs in the absence and presence of CaSO4 0.5 H2O. Adv. Cem. Res. 2014, 26, 29–40. [Google Scholar] [CrossRef]






| Oxide | C3 (Clinker 3000 cm²/g) | C7 (Clinker 7000 cm²/g) | C12 (Clinker 12,000 cm²/g) |
|---|---|---|---|
| [wt%] | [wt%] | [wt%] | |
| SiO2 | 22.00 | 20.88 | 19.77 |
| Al2O3 | 5.90 | 5.59 | 5.73 |
| Fe2O3 | 2.14 | 2.13 | 2.37 |
| CaO | 66.94 | 65.74 | 63.85 |
| MgO | 0.97 | 0.92 | 0.97 |
| SO3 | 0.82 | 0.80 | 1.43 |
| Na2O | 0.37 | 0.34 | 0.45 |
| K2O | 0.65 | 0.66 | 1.16 |
| Cl | 0.01 | 0.01 | 0.02 |
| P2O5 | 0.14 | 0.14 | 0.13 |
| sulphide | 0.09 | 0.10 | 0.14 |
| Parameter | Value |
|---|---|
| Radiation | CuKα |
| Voltage/Current | 40 kV/30 mA |
| Angle range | 5° 2 Theta–65° 2Theta |
| Step width | 0.0057 °2Theta |
| Measuring time per step | 0.05 s |
| Total number of steps | 10,522 |
| Delay time | 47 s |
| Measuring time per range | 10 min |
| Divergence gap | 0.05 mm |
| Factor | Type | Bottom Star Point | Lower Limit | Center Point | Higher Limit | Upper Star Point |
|---|---|---|---|---|---|---|
| Sulphate agent ratio | steady | 0/100 | 25/75 | 50/50 | 75/25 | 100/0 |
| SO3-amount (pre-factor Ost & Lerch) | steady | 0.25 * | 0.5 * | 0.75 * | 1.0 * | 1.25 * |
| C-S-H-seeds amount | steady | 0 wt% | 0.5 wt% | 1 wt% | 1.5 wt% | 2 wt% |
| Fineness of clinker | categorical | 3000 cm²/g | 7000 cm²/g | 12,000 cm²/g | ||
| C-S-H-seeds type | categorical | Circosil® 0.1 | Circolit® |
| Clinker 3000 cm²/g | Clinker 12,000 cm²/g | ||
|---|---|---|---|
| C3_2.90_25/75 | 67.3 MPa | C12_6.75_25/75 | 70.7 MPa |
| C3_3.35_25/75 | 71.4 MPa | C12_7.20_25/75 | 76.2 MPa |
| C3_3.80_25/75 | 63.1 MPa | C12_7.65_25/75 | 72.1 MPa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schade, T.; Middendorf, B. Use of Design of Experiments (DoE) to Model the Sulphate Agent Amount of (Ultra)Finely Ground and Fast Hardening Portland Cement Clinker. Materials 2021, 14, 5573. https://doi.org/10.3390/ma14195573
Schade T, Middendorf B. Use of Design of Experiments (DoE) to Model the Sulphate Agent Amount of (Ultra)Finely Ground and Fast Hardening Portland Cement Clinker. Materials. 2021; 14(19):5573. https://doi.org/10.3390/ma14195573
Chicago/Turabian StyleSchade, Tim, and Bernhard Middendorf. 2021. "Use of Design of Experiments (DoE) to Model the Sulphate Agent Amount of (Ultra)Finely Ground and Fast Hardening Portland Cement Clinker" Materials 14, no. 19: 5573. https://doi.org/10.3390/ma14195573






