Exploration of the Cs Trapping Phenomenon by Combining Graphene Oxide with α-K6P2W18O62 as Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Synthesis of Graphene Oxide
2.4. Synthesis of α-K6P2W18O62
2.5. Synthesis of GO-POM Composite
2.6. Adsorption Experiment
3. Results and Discussion
3.1. Elemental Analysis
3.2. FT-IR Spectra
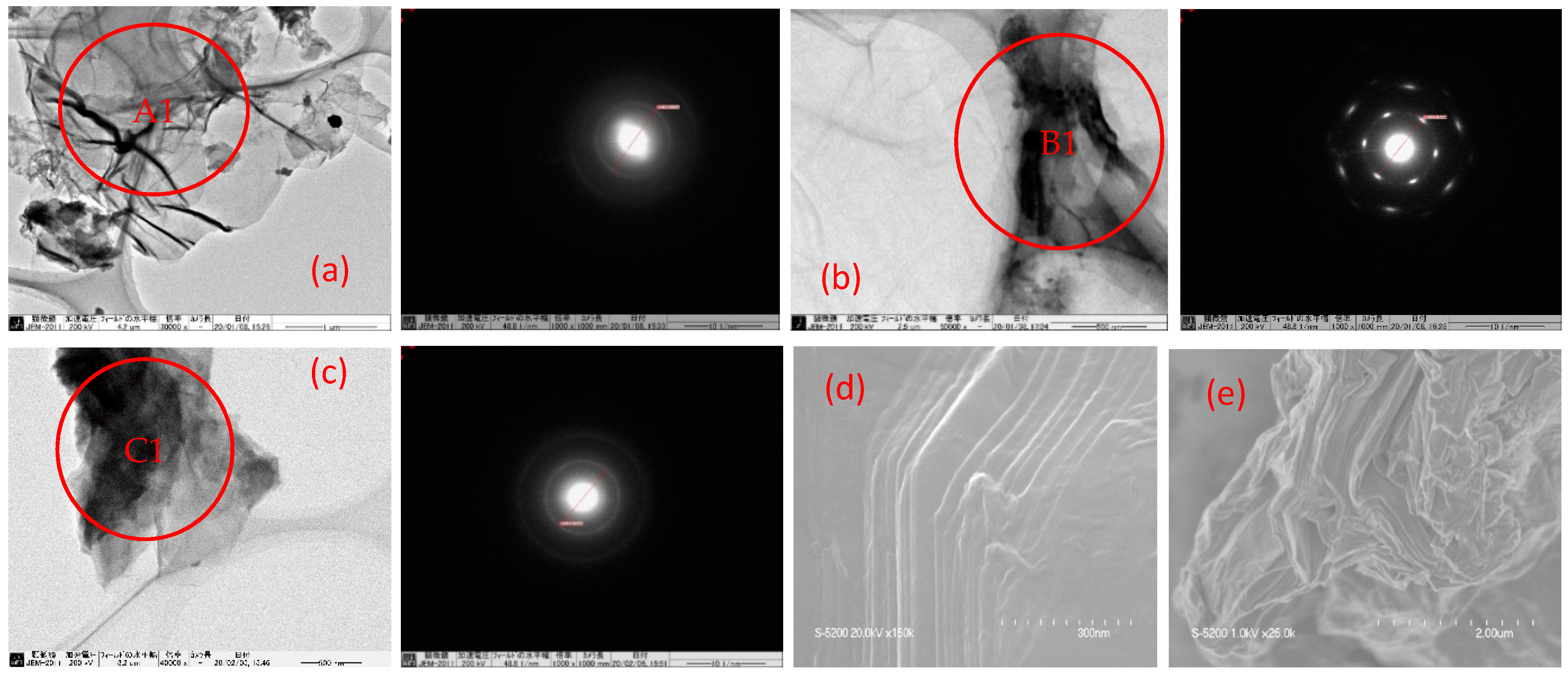
3.3. Morphology of GO
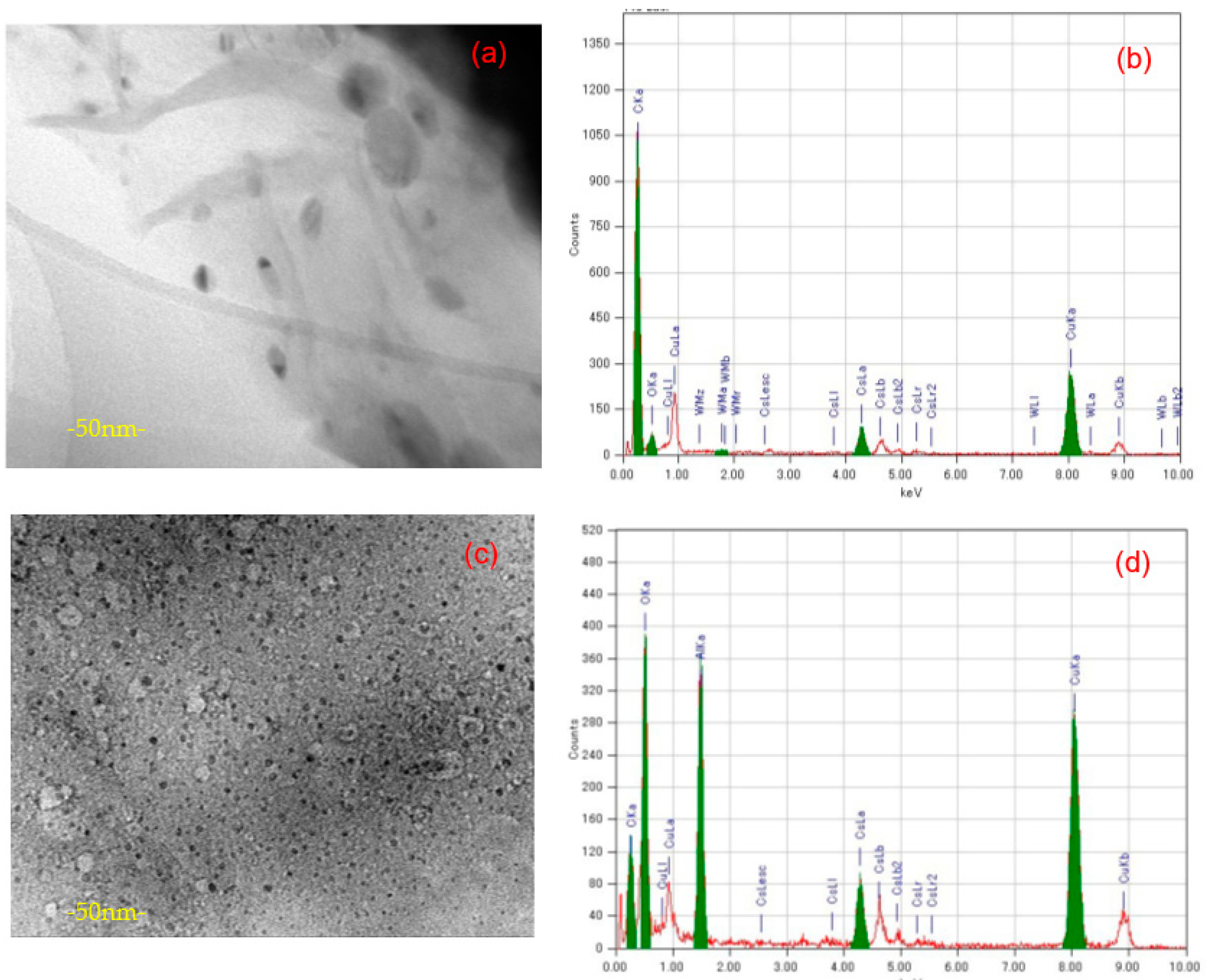
3.4. Morphology of GO-POM Composite and Its EDS Map
3.5. Powder X-ray Diffraction Pattern (PXRD)
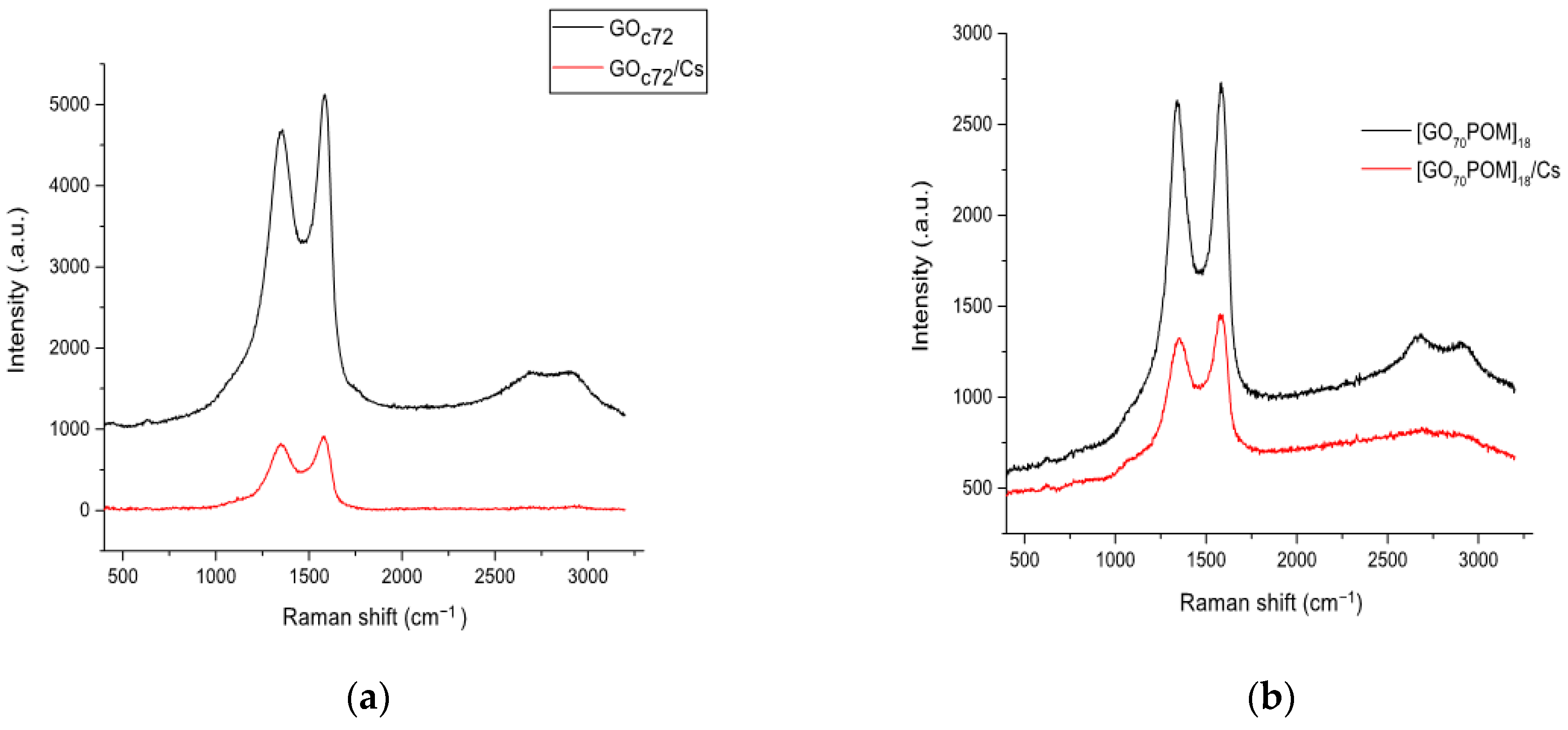
3.6. Raman Spectroscopy
3.7. Cs Adsorption Performance
3.7.1. Adsorption Efficiency and Adsorption Capacity of GO and POM
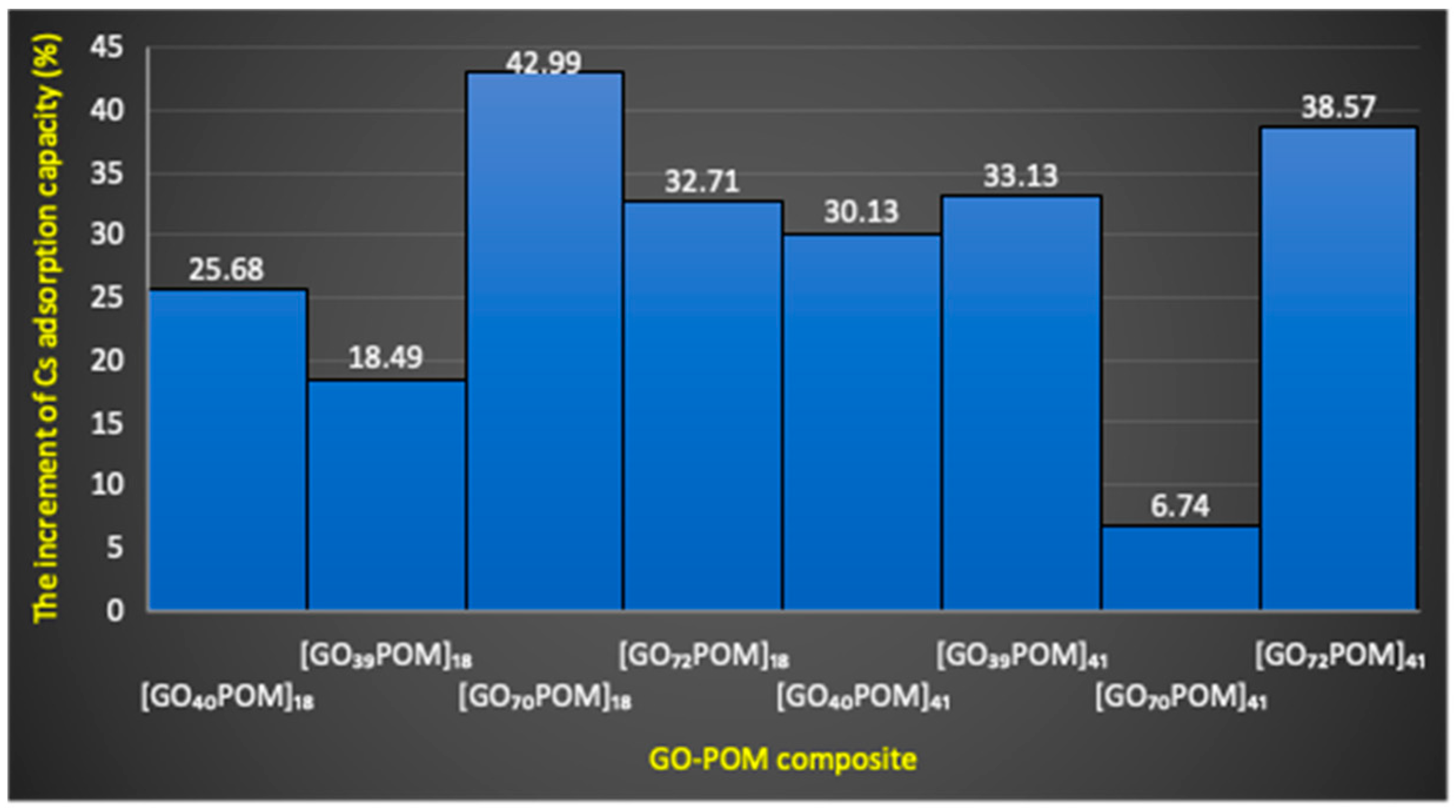
3.7.2. Adsorption Efficiency and Adsorption Capacity of GO-POM Nanocomposite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishihara, K.; Yamagishi, I.; Yasuda, K.; Ishimori, K.; Tanaka, K.; Kuno, T.; Inada, S.; Gotoh, Y. Radionuclide release to stagnant water in the Fukushima-1 nuclear power plant. J. Nucl. Sci. Technol. 2014, 52, 301–307. [Google Scholar] [CrossRef]
- Konings, R.J.M.; Wiss, T.; Beneš, O. Predicting material release during a nuclear reactor accident. Nat. Mater. 2015, 14, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Tagami, K. Soil-to-plant transfer factors of fallout 137Cs and native 133Cs in various crops collected in Japan. J. Radioanal. Nucl. Chem. 2007, 273, 205–210. [Google Scholar] [CrossRef]
- Shizuma, K.; Fujikawa, Y.; Kurihara, M.; Sakurai, Y. Identification and temporal decrease of 137 Cs and 134 Cs in groundwater in Minami-Soma City following the accident at the Fukushima Dai-ichi nuclear power plant. Environ. Pollut. 2018, 234, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koarashi, J.; Atarashi-Andoh, M.; Matsunaga, T.; Sato, T.; Nagao, S.; Nagai, H. Factors affecting vertical distribution of Fukushima accident-derived radiocesium in soil under different land-use conditions. Sci. Total Environ. 2012, 431, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Ozasa, K.; Akiba, S.; Niwa, O.; Kodama, K.; Takamura, N.; Zaharieva, E.K.; Kimura, Y.; Wakeford, R. Long-term effects of radiation exposure on health. Lancet 2015, 386, 469–478. [Google Scholar] [CrossRef]
- Hasegawa, A.; Tanigawa, K.; Ohtsuru, A.; Yabe, H.; Maeda, M.; Shigemura, J.; Ohira, T.; Tominaga, T.; Akashi, M.; Hirohashi, N.; et al. Health effects of radiation and other health problems in the aftermath of nuclear accidents, with an emphasis on Fukushima. Lancet 2015, 386, 479–488. [Google Scholar] [CrossRef]
- Matsuda, N.; Nakashima, S. Radioactive cesium in water and soil and its adsorption to rice plant (interim report). Radiat. Saf. Manag. 2014, 13, 84–91. (in Japanese). [Google Scholar]
- TEPCO. The Evaluation Status of Reactor Core Damage at Fukushima Daiichi Nuclear Power Station Units 1 to 3; TEPCO: Tokyo, Japan, 2011; p. 28. [Google Scholar]
- Yousefi, T.; Torab-Mostaedi, M.; Moosavian, M.A.; Mobtaker, H.G. Potential application of a nanocompo-site:HCNFe@polymer for effective removal of Cs (I) from nuclear waste. Prog. Nucl. Energy 2015, 85, 631–639. [Google Scholar] [CrossRef]
- Jang, S.C.; Kang, S.M.; Haldorai, Y.; Giribabu, K.; Lee, G.W.; Lee, Y.C.; Huh, Y.S. Synergistically strengthened 3D micro-scavenger cage adsorbent for selective removal of radioactive cesium. Sci. Rep. 2016, 6, 1–12. [Google Scholar]
- Tamura, K.; Kogure, T.; Watanabe, Y.; Nagai, C.; Yamada, H. Uptake of Cesium and Strontium Ions by Artificially Altered Phlogopite. Environ. Sci. Technol. 2014, 48, 5808–5815. [Google Scholar] [CrossRef]
- Eigler, S.; Grimm, S.; Hof, F.; Hirsch, A. Graphene oxide: A stable carbon framework for functionalization. J. Mater. Chem. A. 2013, 1, 1159. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Rios-Carvajal, T.; Andersson, M.P.; Ceccato, M.; Stipp, S.L.S.; Hassenkam, T. Ion effects on molecular interaction between graphene oxide and organic molecules. Environ. Sci. Nano 2019, 6, 2281–2291. [Google Scholar] [CrossRef]
- Neklyudov, V.V.; Khafizov, N.R.; Sedov, I.; Dimiev, A.M. New insights into the solubility of graphene oxide in water and alcohols. Phys. Chem. Chem. Phys. 2017, 19, 17000–17008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konkena, B.; Vasudevan, S. Understanding aqueous dispersibility of graphene oxide and reduced graphene oxide through pKa measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef]
- Ritchie, C.; Streb, C.; Thiel, J.; Mitchell, S.G.; Miras, H.; Long, D.-L.; Boyd, T.; Peacock, R.D.; McGlone, T.; Cronin, L. Reversible Redox Reactions in an Extended Polyoxometalate Framework Solid. Angew. Chem. Int. Ed. 2008, 47, 6881–6884. [Google Scholar] [CrossRef]
- Kawahara, R.; Uchida, S.; Mizuno, N. Redox-Induced Reversible Uptake–Release of Cations in Porous Ionic Crystals Based on Polyoxometalate: Cooperative Migration of Electrons with Alkali Metal Ions. Chem. Mater. 2015, 27, 2092–2099. [Google Scholar] [CrossRef]
- Uchida, S. Frontiers and progress in cation-uptake and exchange chemistry of polyoxometalate-based compounds. Chem. Sci. 2019, 10, 7670–7679. [Google Scholar] [CrossRef] [PubMed]
- Hitose, S.; Uchida, S. Rapid Uptake/Release of Cs+ in Isostructural Redox-Active Porous Ionic Crystals with Large-Molecular-Size and Easily Reducible Dawson-Type Polyoxometalates as Building Blocks. Inorg. Chem. 2018, 57, 4833–4836. [Google Scholar] [CrossRef] [PubMed]
- Gall, R.D.; Hill, C.L.; Walker, J.E. Carbon powder and fiber-supported polyoxometalate catalytic materials. Preparation, characterization, and catalytic oxidation of dialkyl sulfides as mustard (HD) analogues. Chem. Mater. 1996, 8, 2523–2527. [Google Scholar] [CrossRef]
- Li, H.; Pang, S.; Feng, X.; Müllen, K.; Bubeck, C. Polyoxometalate assisted photoreduction of graphene oxide and its nanocomposite formation. Chem. Commun. 2010, 46, 6243. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, B.S.; Wihadi, M.N.K.; Grote, F.; Eigler, S.; Nakashima, S. Potentiality of Graphene Oxide and Polyoxometalate as Radionuclides Adsorbent to Restore the Environment after Fukushima Disaster: A Mini Review. Indones. J. Chem. 2021, 21, 776–786. [Google Scholar] [CrossRef]
- Eigler, S.; Hirsch, A. Chemistry with Graphene and Graphene Oxide-Challenges for Synthetic Chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ranjan, P.; Agrawal, S.; Sinha, A.; Rao, T.R.; Balakrishnan, J.; Thakur, A.D. A Low-Cost Non-explosive Synthesis of Graphene Oxide for Scalable Applications. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Mbomekalle, I.M.; Lu, Y.W.; Keita, B.; Nadjo, L. Simple, high yield and reagent-saving synthesis of pure α-K6P2W18O62 · 14H2O. Inorg. Chem. Commun. 2004, 7, 86–90. [Google Scholar] [CrossRef]
- Contant, R.; Thouvenot, R. A reinvestigation of isomerism in the Dawson structure: Syntheses and 183W NMR structural characterization of three new polyoxotungstates [X2W18O62]6− (X=PV, AsV). Inorganica Chim. Acta 1993, 212, 41–50. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Bouša, D.; Luxa, J.; Mazánek, V.; Jankovsky, O.; Sedmidubský, D.; Klímová, K.; Pumera, M.; Sofer, Z. Toward graphene chloride: Chlorination of graphene and graphene oxide. RSC Adv. 2016, 6, 66884–66892. [Google Scholar] [CrossRef] [Green Version]
- Cote, L.J.; Kim, J.; Zhang, Z.; Sun, C.; Huang, J. Tunable assembly of graphene oxide surfactant sheets: Wrinkles, over-laps and impacts on thin film properties. Soft Matter 2010, 6, 6096–6101. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, S. Spectroscopic investigation of confinement effects on optical properties of graphene oxide. Appl. Phys. Lett. 2011, 98, 073104. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-Layer Assembly of Ultrathin Composite Films from Micron-Sized Graphite Oxide Sheets and Polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Seehra, M.S.; Narang, V.; Geddam, U.K.; Stefaniak, A.B. Correlation between X-ray diffraction and Raman spectra of 16 commercial graphene–based materials and their resulting classification. Carbon 2017, 111, 380–385. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Dang, L.; Liu, Y.; Jiao, W. Preparation, characterization and photocatalytic activity of Dawson type phospho-tungstate/graphene oxide composites. Adv. Powder Technol. 2019, 30, 1400–1408. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Graphite Oxide/Polyoxometalate Nanocomposites as Adsorbents of Ammonia. J. Phys. Chem. C 2009, 113, 3800–3809. [Google Scholar] [CrossRef]
- Sharma, R.; Chadha, N.; Saini, P. Determination of defect density, crystallite size and number of graphene layers in graphene analogues using X-ray diffraction and Raman spectroscopy. Indian J. Pure Appl. Phys. 2017, 55, 625–629. [Google Scholar]
- Dresselhaus, M.S.; Jorio, A.; Filho, A.G.S.; Saito, R. Defect characterization in graphene and carbon nanotubes us-ing Raman spectroscopy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 5355–5377. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Eigler, S. Graphene Oxide: Fundamentals and Applications; John Wiley & Son, Ltd.: London, UK, 2017. [Google Scholar]
- King, A.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.; Minett, A.I. A New Raman Metric for the Characterisation of Graphene oxide and its Derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.Y.; Xu, Y.; Zhang, W.; Zhao, J.; Liu, A.; Tang, X.; Tsai, C.-H.; Huang, Y.; Li, L.-J. Highly Efficient Restoration of Graphitic Structure in Graphene Oxide Using Alcohol Vapors. ACS Nano 2010, 4, 5285–5292. [Google Scholar] [CrossRef] [PubMed]
- Mkhoyan, K.A.; Countryman, A.W.; Sylcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and elec-tronic structure of graphene oxide. Nano Lett. 2009, 9, 1058–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Qi, Y.; Liu, J.; Qi, Z.; Chen, W.; Wiesner, M.R. Cation-inhibited transport of graphene oxide nanomaterials in saturated porous media: The hofmeister effects. Environ. Sci. Technol. 2017, 51, 828–837. [Google Scholar] [CrossRef]
- Romanchuk, A.; Slesarev, A.S.; Kalmykov, S.N.; Kosynkin, D.V.; Tour, J. Graphene oxide for effective radionuclide removal. Phys. Chem. Chem. Phys. 2013, 15, 2321–2327. [Google Scholar] [CrossRef]
- Parfitt, G.D.; Rochester, C.H. Adsorption from Solution at the Solid/Liquid Interface; Academic Press: London, UK, 1983. [Google Scholar]
- Radovic, L.R. Active Sites in Graphene and the Mechanism of CO2 Formation in Carbon Oxidation. J. Am. Chem. Soc. 2009, 131, 17166–17175. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight-graphene –based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.A.; Striolo, A. Polarizability effects in molecular dynamics simulations of the graphene-water interface. J. Chem. Phys. 2013, 138, 054117. [Google Scholar] [CrossRef]
- Guardia, J.S.E.; Marti, J. Specific ion effects in aqueous electrolyte solutions confined within graphene sheets at the na-nometric scale. Phys. Chem. Chem. Phys. 2012, 14, 10799–10808. [Google Scholar]
- Dimiev, A.; Alemany, L.B.; Tour, J. Graphene Oxide. Origin of Acidity, Its Instability in Water, and a New Dynamic Structural Model. ACS Nano 2013, 7, 576–588. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M. Heterogeneous catalysis. Chem. Rev. 1998, 98, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, T.; Nishimura, T.; Watanabe, H.; Misono, M. Insoluble heteropoly compounds as highly active catalysts for liquid-phase reactions. J. Mol. Catal. 1992, 74, 247–256. [Google Scholar] [CrossRef]
- Sánchez-García, I.; Núñez, A.; Bonales, L.J.; Perlado, J.M.; Cobos, J. Study of the adsorption capacity of graphene oxide under gamma radiation in different media. Radiat. Phys. Chem. 2019, 165, 108395. [Google Scholar] [CrossRef]
- Kaewmee, P.; Manyam, J.; Opaprakasit, P.; Le, G.T.T.; Chanlek, N.; Sreearunothai, P. Effective removal of cesium by pristine graphene oxide: Performance, characterizations and mechanisms. RSC Adv. 2017, 7, 38747–38756. [Google Scholar] [CrossRef] [Green Version]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Schwegler, M.A.; Vinke, P.; van der Eijk, M.; van Bekkum, H. Activated carbon as a support for heteropolyanion catalysts. Appl. Catal. A Gen. 1992, 80, 41–57. [Google Scholar] [CrossRef]
- Dizaji, A.K.; Mortaheb, H.R.; Mokhtarani, B. Preparation of supported catalyst by adsorption of polyoxometalate on graphene oxide/reduced graphene oxide. Mater. Chem. Phys. 2017, 199, 424–434. [Google Scholar] [CrossRef]
- Zhou, D.; Han, B.-H. Graphene-Based Nanoporous Materials Assembled by Mediation of Polyoxometalate Nanoparticles. Adv. Funct. Mater. 2010, 20, 2717–2722. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Lai, X.; Miao, J.; He, D.; Li, N.; Liu, S. Polyoxometalate-Modified Sponge-Like Graphene Oxide Monolith with High Proton-Conducting Performance. Adv. Funct. Mater. 2015, 25, 4480–4485. [Google Scholar] [CrossRef]
- Tu, Q.; Pang, L.; Chen, Y.; Zhang, Y.; Zhang, R.; Lu, B.; Wang, J. Effects of surface charges of graphene oxide on neuronal outgrowth and branching. Analyst 2014, 139, 105–115. [Google Scholar] [CrossRef]
- Misra, A.; Kozma, K.; Streb, C.; Nymann, M. Beyond Charge Balance: Counter-cation in Polyoxometalate Chemistry. Angew. Chem. Int. Ed. 2020, 59, 596–612. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Chen, B.; Zhu, X.; Xing, B. Aggregation, Adsorption, and Morphological Transformation of Graphene Oxide in Aqueous Solutions Containing Different Metal Cations. Environ. Sci. Technol. 2016, 50, 11066–11075. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xiong, C.; Li, Y.; Tang, H.; Meng, X.; Zhu, W. The critical contribution of oxidation debris on the acidic proper-ties of graphene oxide in an aqueous solution. J. Hazard. Mater. 2021, 402, 123552. [Google Scholar] [CrossRef]
- Szabo, T.; Maroni, P.; Szilagyi, I. Size-dependent aggregation of graphene oxide. Carbon 2020, 160, 145–155. [Google Scholar] [CrossRef]
- Ma, J.C.; Dougherty, D.A. The cation-π interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, J.; Zhu, W.; Luo, X.; Chen, K.; Jiang, H. Influence of the water molecule on cation-π interaction: Ab initio second order møller-plesset perturbation theory (MP2) calculations. J. Phys. Chem. B 2005, 109, 5945–5949. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic radii in aqueous solutions. Chem. Rev. 1988, 88, 1475–1498. [Google Scholar] [CrossRef]
- Taniguchi, T.; Kurihara, S.; Tateishi, H.; Hatakeyama, K.; Koinuma, M.; Yokoi, H.; Hara, M.; Ishikawa, H.; Matsumoto, Y. pH-driven, reversible epoxy ring opening/closing in graphene oxide. Carbon 2015, 84, 560–566. [Google Scholar] [CrossRef]
- Acik, M.; Mattevi, C.; Gong, C.; Lee, G.; Cho, K.; Chhowalla, M.; Chabal, Y.J. The Role of Intercalated Water in Multilayered Graphene Oxide. ACS Nano 2010, 4, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- Amirov, R.R.; Shayimova, J.; Nasirova, Z.; Dimiev, A.M. Chemistry of graphene oxide. Reactions with transition metal cations. Carbon 2017, 116, 356–365. [Google Scholar]
- Lian, B.; de Luca, S.; You, Y.; Alwarappan, S.; Yoshimura, M.; Sahajwalla, V.; Smith, S.C.; Leslie, G.; Joshi, R.K. Extraordinary water adsorption characteristics of graphene oxide. Chem. Sci. 2018, 9, 5106. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Wu, H.; Li, K.; Wang, Y.; Tao, X.; Yang, H.; Li, A.; Cheng, R. Influence of the Surface Structure of Graphene Oxide on the Adsorption of Aromatic Organic Compounds from Water. ACS Appl. Mater. Interfaces 2015, 7, 6690–6697. [Google Scholar] [CrossRef]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the Local Chemical Structure of Graphene Oxide and Reduced Graphene Oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef] [PubMed]
- Szabo, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, A.D.; Dékány, I. Evolution of Surface Functional Groups in a Series of Progressively Oxidized Graphite Oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Ebajo, V.D.; Santos, C.R.L.; Alea, G.V.; Lin, Y.A.; Chen, C.-H. Regenerable Acidity of Graphene Oxide in Promoting Multicomponent Organic Synthesis. Sci. Rep. 2019, 9, 15579. [Google Scholar] [CrossRef] [PubMed]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Kim, Y.; Shanmugam, S. Polyoxometalate-reduced graphene oxide hybrid catalyst: Synthesis, structure, and electro-chemical properties. ACS Appl. Mater. Interfaces 2013, 5, 12197–12204. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Y.; He, C.; Gao, Y.; Jia, N.; Chen, Z.; Song, W. The role of sp2/sp3 hybrid carbon regulation in the nonlinear optical properties of graphene oxide materials. RSC Adv. 2017, 7, 53643–53652. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.I. Changing the sp2 Carbon Clusters in Graphene Oxide During Exfoliation. Trans. Electr. Electron. Mater. 2015, 16, 49–52. [Google Scholar] [CrossRef] [Green Version]




| Sample | C% | H% | N% | O% | C/O |
|---|---|---|---|---|---|
| GOc70 | 70.87 | 7.95 | - | 21.18 | 3.34 |
| GOc72 | 72.04 | 8.07 | - | 19.89 | 3.61 |
| GOc39 | 39.28 | 2.76 | 0.27 | 57.69 | 0.68 |
| GOc40 | 39.61 | 2.58 | 0.25 | 57.56 | 0.69 |
| Materials | Adsorption Efficiency | Adsorption Capacity | Stirring | Sonification Time |
|---|---|---|---|---|
| (%) | (mmol/g) | Time | (minute) | |
| POM-Cs | 58.3 | 32.8 | 24 h | 5 |
| GOc70-Cs | 58.8 | 33.1 | 24 h | 5 |
| GOc72-Cs | 56.8 | 31.9 | 24 h | 5 |
| GOc39-Cs | 61.3 | 34.5 | 24 h | 5 |
| GOc40-Cs | 56.3 | 31.7 | 24 h | 5 |
| Materials | Composition | Concentration Ratio | Adsorption | Adsorption Capacity | Stirring | Sonification Time |
|---|---|---|---|---|---|---|
| GO:POM | Efficiency (%) | (mmol/g) | Time | (minute) | ||
| [GO70POM]18 | (GOc70/POM) | 1:08 | 84 | 47.3 | 24 h | 5 |
| [GO70POM]41 | (GOc70/POM) | 4:01 | 62.7 | 35.3 | 24 h | 5 |
| [GO72POM]18 | (GOc72/POM) | 1:08 | 75.4 | 42.4 | 24 h | 5 |
| [GO72POM]41 | (GOc72/POM) | 4:01 | 78.7 | 44.3 | 24 h | 5 |
| [GO39POM]18 | (GOc39/POM) | 1:08 | 72.7 | 40.9 | 24 h | 5 |
| [GO39POM]41 | (GOc39/POM) | 4:01 | 81.7 | 45.9 | 24 h | 5 |
| [GO40POM]18 | (GOc40/POM) | 1:08 | 70.7 | 39.8 | 24 h | 5 |
| [GO40POM]41 | (GOc40/POM) | 4:01 | 73.5 | 41.3 | 24 h | 5 |
| Material | Composition | Graphitic Zone (G%) | Oxidized Zone (%) |
|---|---|---|---|
| GOc70 | - | 69.7 | 30.3 |
| GOc72 | - | 75.4 | 24.6 |
| GOc39 | - | 74.9 | 25.1 |
| GOc40 | - | 74.5 | 25.5 |
| [GO70POM]18 | GOc70/POM, 1:8 | 68.4 | 31.6 |
| [GO70POM]41 | GOc70/POM, 4:1 | 71.4 | 28.6 |
| [GO72POM]18 | GOc72/POM, 1:8 | 67.5 | 32.5 |
| [GO72POM]41 | GOc72/POM, 4:1 | 69.0 | 30.9 |
| [GO39POM]18 | GOc39/POM, 1:8 | 69.0 | 31.0 |
| [GO39POM]41 | GOc39/POM, 4:1 | 69.3 | 30.7 |
| [GO40POM]18 | GOc40/POM, 1:8 | 69.1 | 30.0 |
| [GO40POM]41 | GOc40/POM, 4:1 | 69.5 | 30.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugroho, B.S.; Kato, A.; Kowa, C.; Nakashima, T.; Wada, A.; Wihadi, M.N.K.; Nakashima, S. Exploration of the Cs Trapping Phenomenon by Combining Graphene Oxide with α-K6P2W18O62 as Nanocomposite. Materials 2021, 14, 5577. https://doi.org/10.3390/ma14195577
Nugroho BS, Kato A, Kowa C, Nakashima T, Wada A, Wihadi MNK, Nakashima S. Exploration of the Cs Trapping Phenomenon by Combining Graphene Oxide with α-K6P2W18O62 as Nanocomposite. Materials. 2021; 14(19):5577. https://doi.org/10.3390/ma14195577
Chicago/Turabian StyleNugroho, Bangun Satrio, Akane Kato, Chie Kowa, Tomoya Nakashima, Atsushi Wada, Muh. Nur Khoiru Wihadi, and Satoru Nakashima. 2021. "Exploration of the Cs Trapping Phenomenon by Combining Graphene Oxide with α-K6P2W18O62 as Nanocomposite" Materials 14, no. 19: 5577. https://doi.org/10.3390/ma14195577






