Innovative Method for Longer Effective Corrosion Inhibition Time: Controlled Release Oil Palm Empty Fruit Bunch Hemicellulose Inhibitor Tablet
Abstract
:1. Introduction
- To characterize the functional groups and chemical constituents of EFB-H.
- To investigate the mechanism and kinetics of EFB-H anticorrosion action using potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS).
- To optimize the inhibition efficiency of EFB-H using response surface methodology-central composite design (RSM/CCD) at different dosages and immersion times.
- To formulate EFB-H tablet using D-optimal mixture design, determine the tablet properties and compare its anticorrosion action with EFB-H powder inhibitor at extended immersion times.
2. Materials and Methods
2.1. Materials
2.2. Preparation of EFB-H Inhibitor
2.3. Characterization of EFB-H
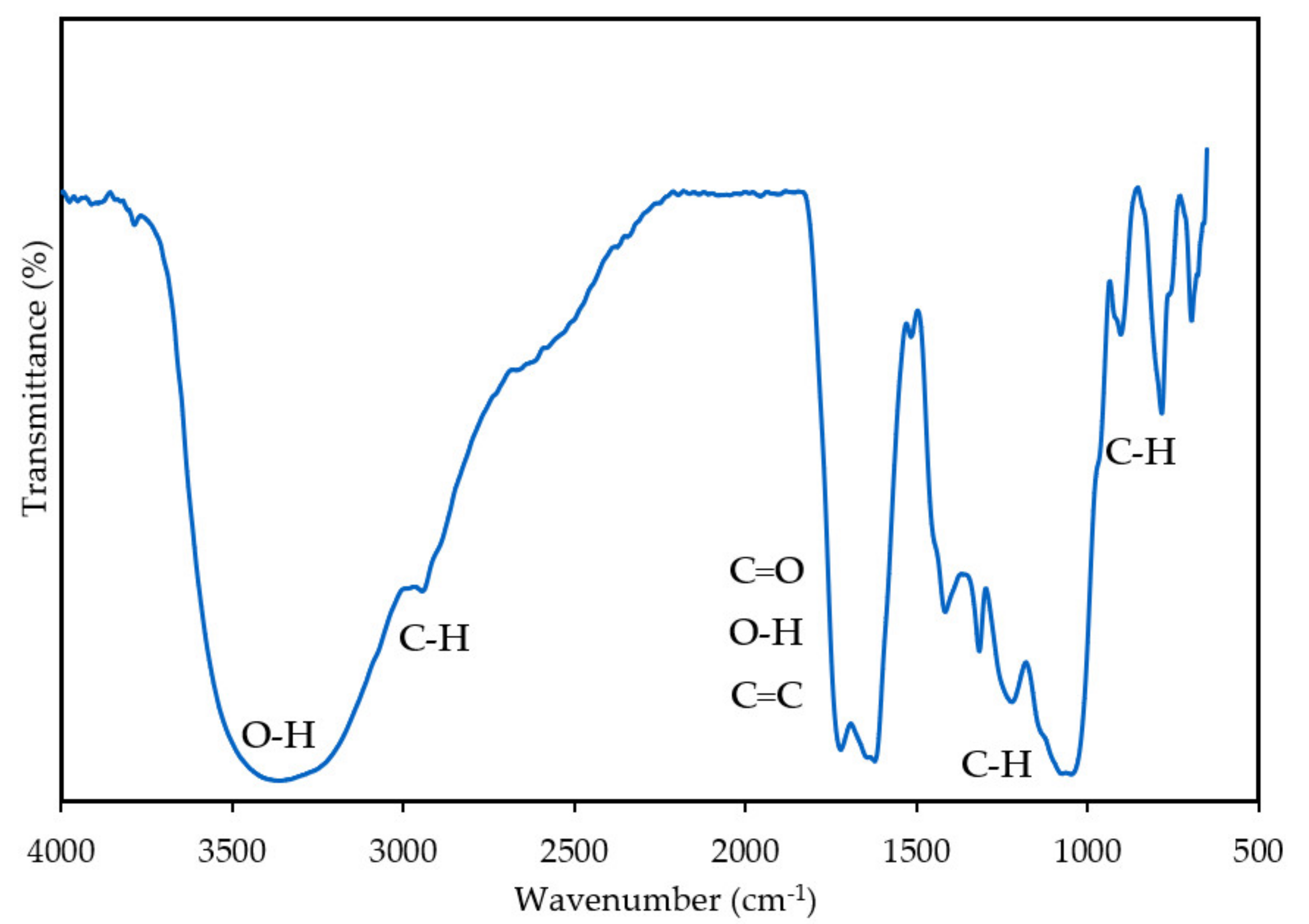
2.3.1. Fourier-transform Infrared Spectroscopy Analysis
2.3.2. Chemical Composition (Cellulose, Hemicellulose, Lignin and Extractives)
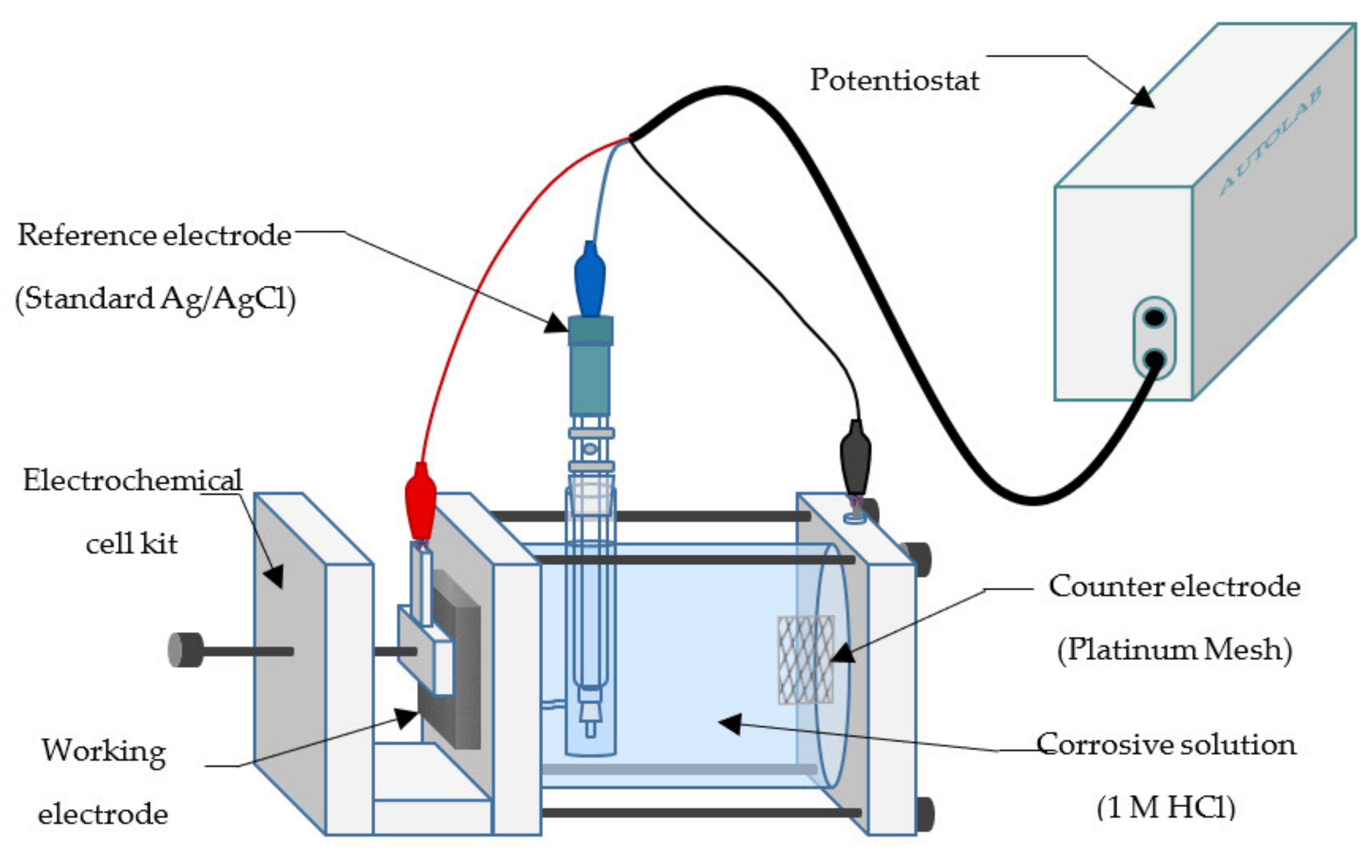
2.4. Electrochemical Tests
2.4.1. Potentiodynamic Polarization
2.4.2. Electrochemical Impedance Spectroscopy
2.5. Optimization—Response Surface Methodology
- Varying dosages of 0.1, 0.3 and 0.5 g at a fixed immersion time at 120 h,
- Varying immersion times of 24, 120 and 216 h at a constant dosage.
2.6. Preparation and Corrosion Inhibition Study of EFB-H Tablet
2.6.1. EFB-H Tablet Formulation—Mixture Design
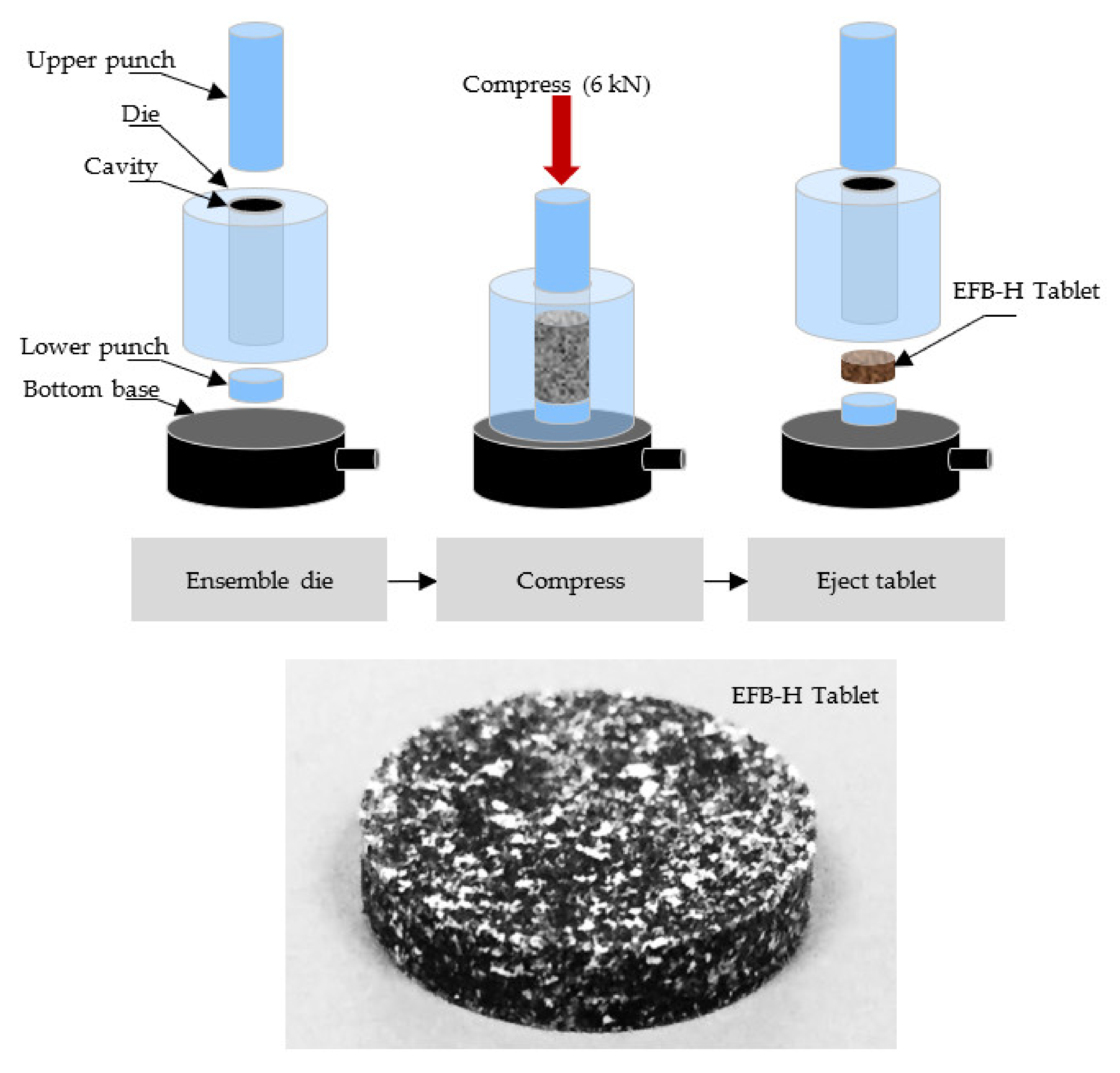
2.6.2. EFB-H Tablet Compression
2.6.3. Tablet Properties
2.6.4. Extended Weight Loss Study
3. Results
3.1. EFB-H Characterization
3.1.1. Fourier-Transform Infrared Spectroscopy Analysis
3.1.2. Chemical Composition (Cellulose, Hemicellulose, Lignin and Extractives)
3.2. Electrochemical Tests
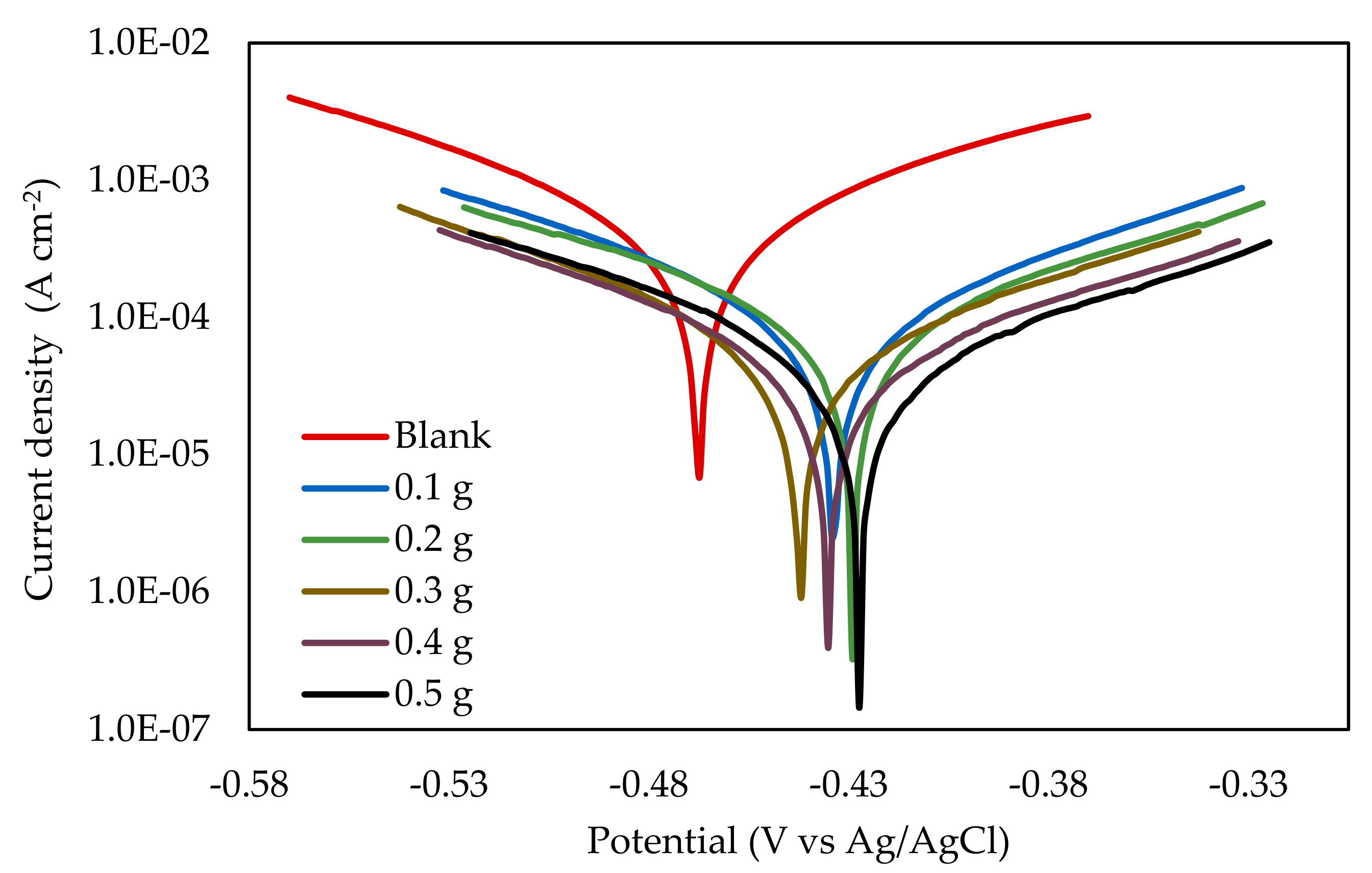
3.2.1. Potentiodynamic Polarization
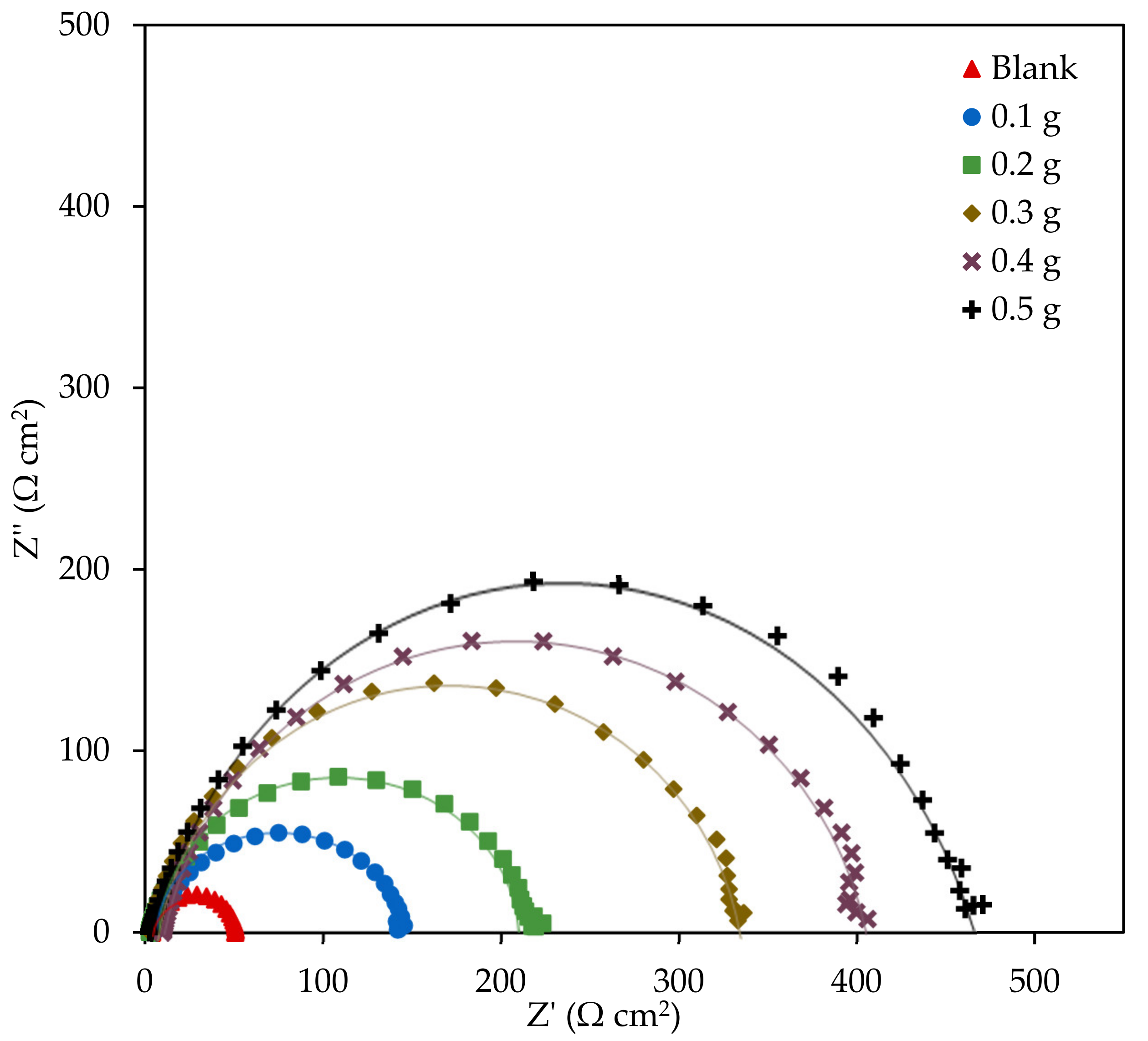
3.2.2. Electrochemical Impedance Spectroscopy
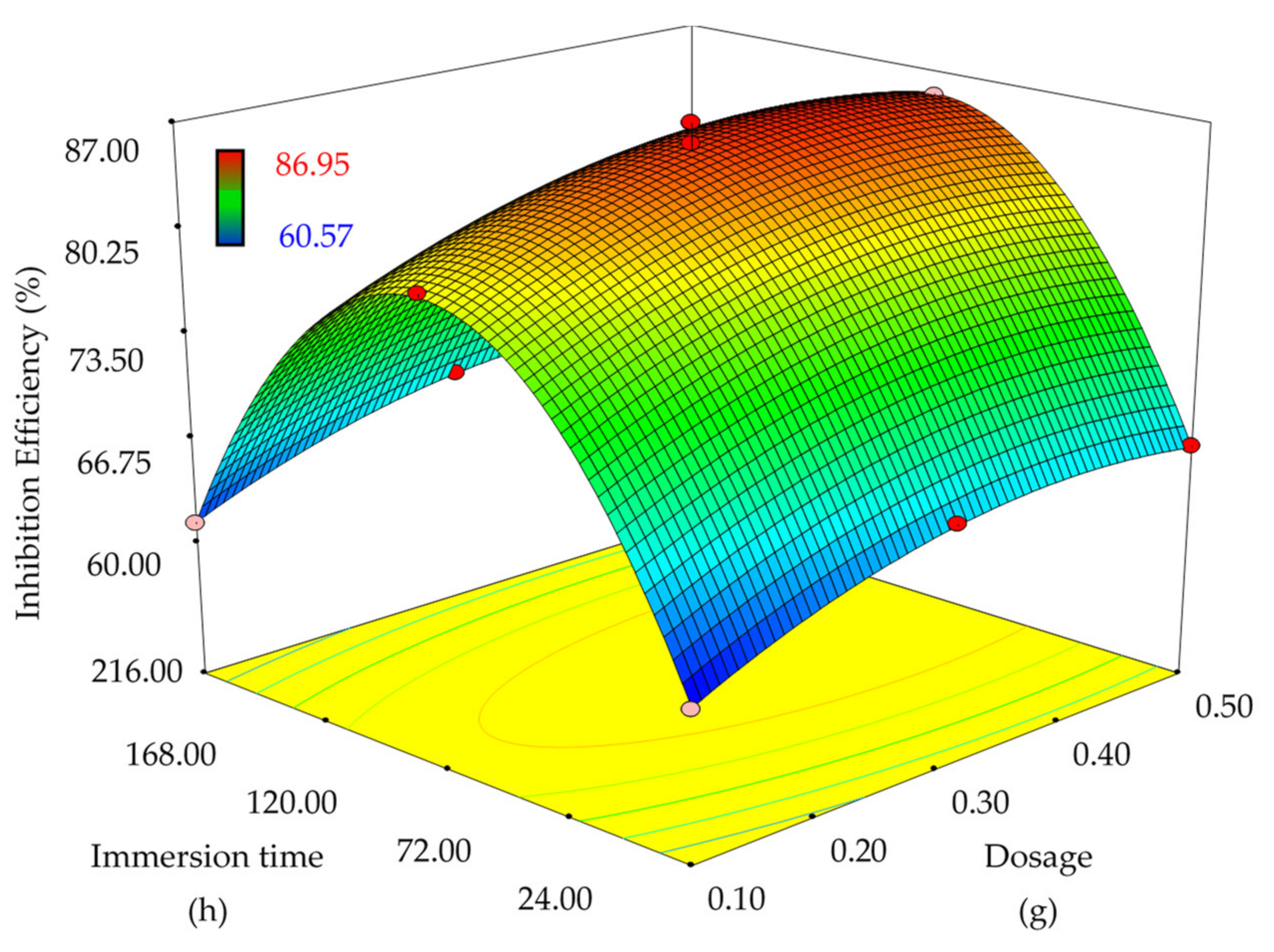
3.3. Optimization—Response Surface Methodology
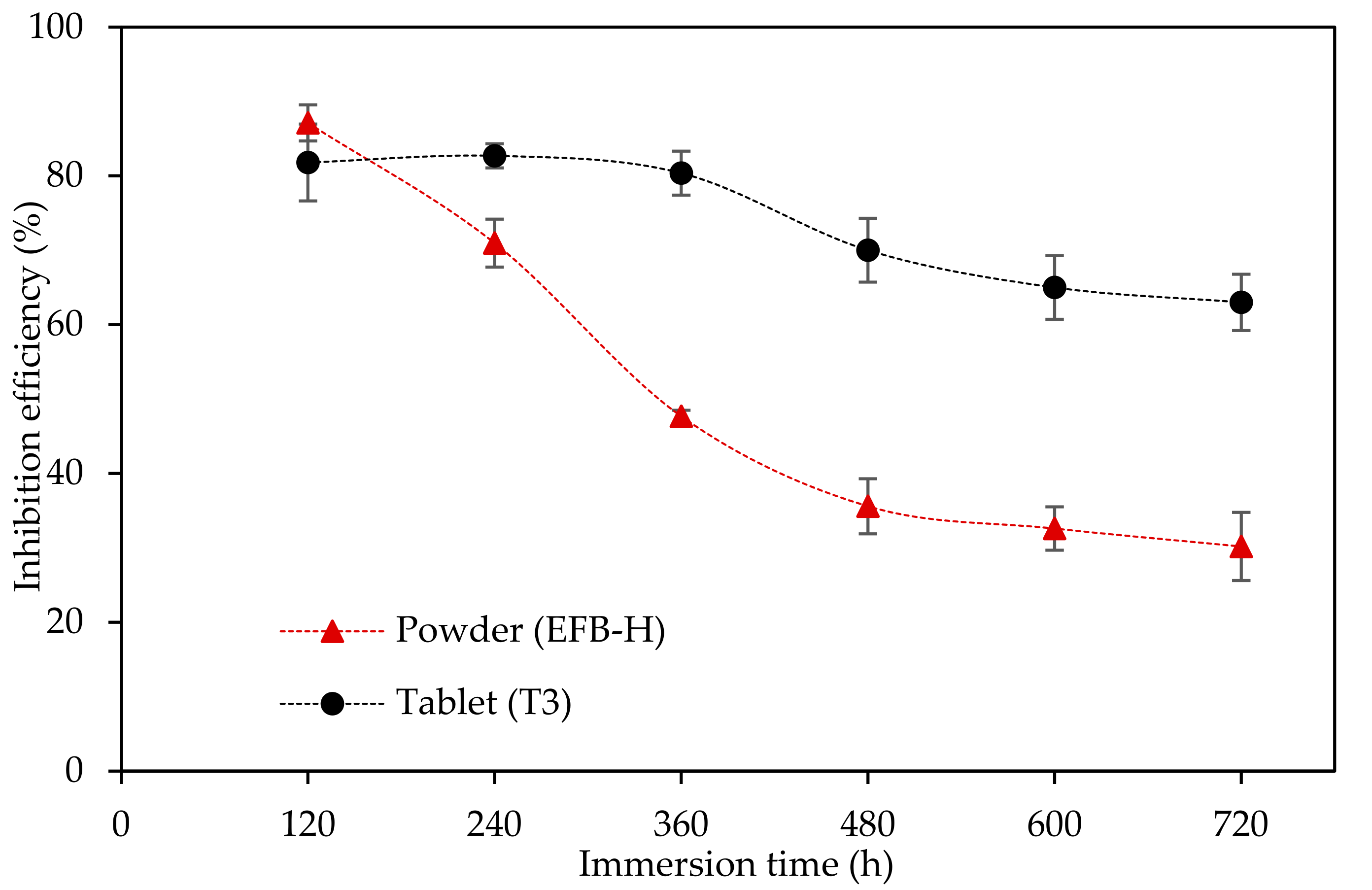
3.4. Corrosion Inhibition Study of Oil Palm Empty Fruit Bunch Tablet Inhibitor
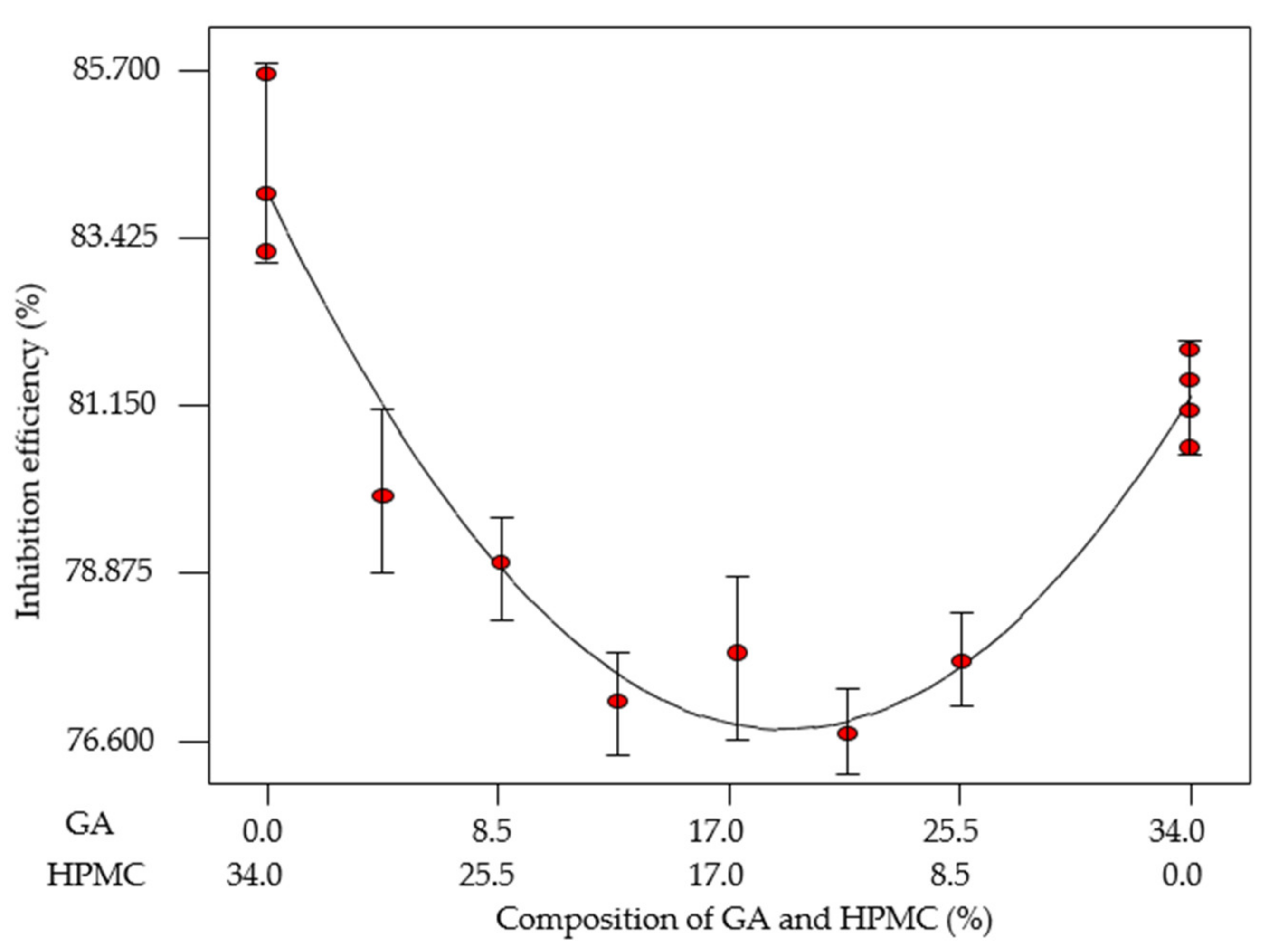
3.4.1. Mixture Design Formulation
3.4.2. Tensile Strength, Disintegration Time and Dissolution Profile
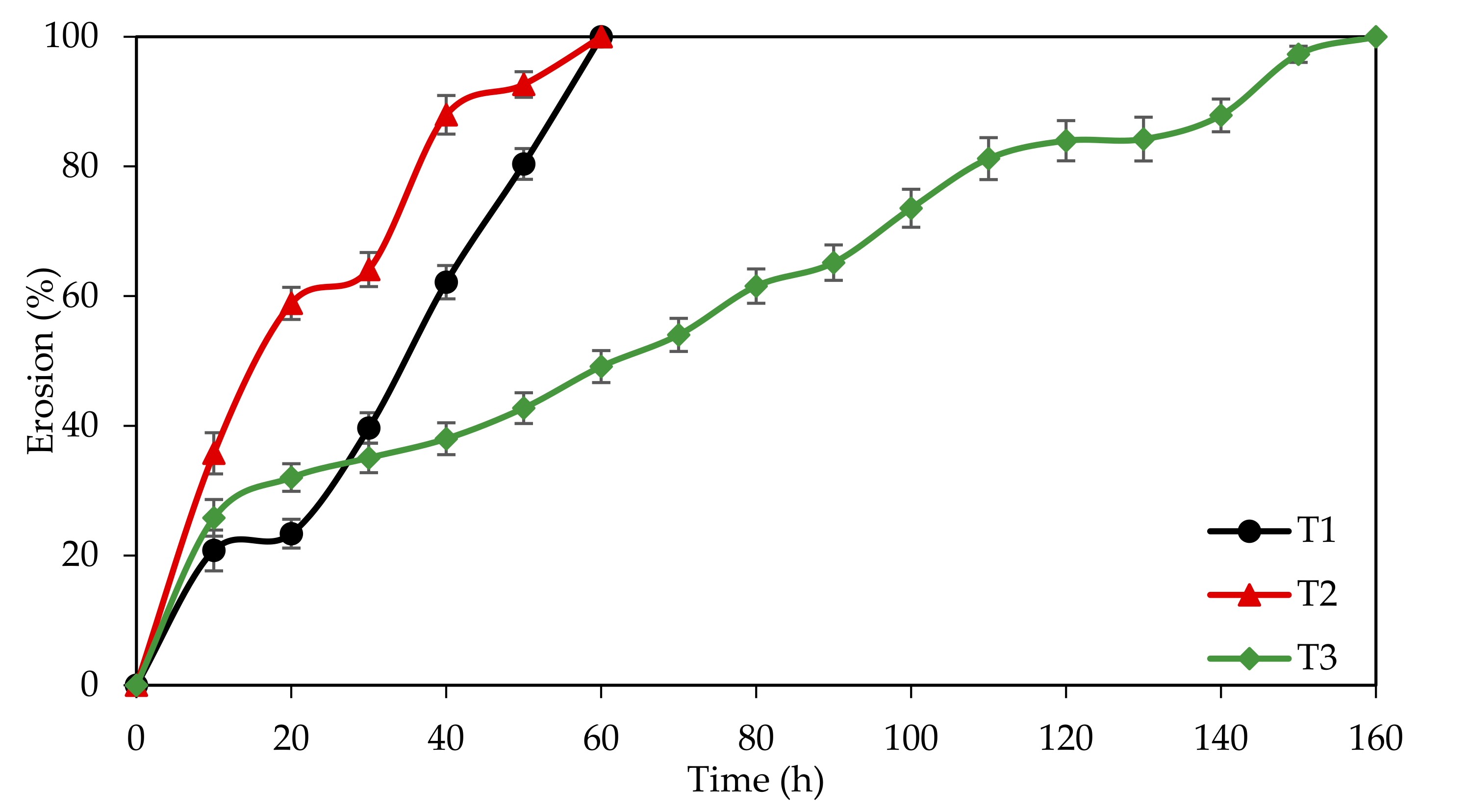
3.4.3. Extended Weight Loss Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chauhan, D.S.; Mazumder, M.J.; Quraishi, M.; Ansari, K. Chitosan-cinnamaldehyde Schiff base: A bioinspired macromolecule as corrosion inhibitor for oil and gas industry. Int. J. Biol. Macromol. 2020, 158, 127–138. [Google Scholar] [CrossRef]
- Monticelli, C. Corrosion Inhibitors. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Oxford, UK, 2018; pp. 164–171. [Google Scholar]
- Tamalmani, K.; Husin, H. Review on Corrosion Inhibitors for Oil and Gas Corrosion Issues. Appl. Sci. 2020, 10, 3389. [Google Scholar] [CrossRef]
- Gouamid, M.; Zenkhri, L.; Benhamida, S.; Chebouat, E.; Charradi, K. Inhibitive and Adsorption behaviour of date Palm Hemicellulose on Mild Steel Corrosion in Sulfuric Acid Media. Asian J. Res. Chem. 2019, 12, 303. [Google Scholar] [CrossRef]
- Guedes, D.; Martins, G.R.; Jaramillo, L.Y.A.; Dias, D.S.B.; da Silva, A.J.R.; Lutterbach, M.T.S.; Reznik, L.Y.; Sérvulo, E.F.C.; Alviano, C.S.; Alviano, D.S. Proanthocyanidins with Corrosion Inhibition Activity for AISI 1020 Carbon Steel under Neutral pH Conditions of Coconut (Cocos nucifera L.) Husk Fibers. ACS Omega 2021, 6, 6893–6901. [Google Scholar] [CrossRef]
- Komalasari; Zultiniar; Karim, A.R.M.; Susanto, R.; Azhari, I.; Utami, S.P.; Heltina, D. Biomass Extraction as Green Corrosion Inhibitor for Low Carbon Steel in Hydrochloric Acid Solution. J. Phys. Conf. Ser. 2020, 1655. [Google Scholar] [CrossRef]
- De Lima, K.C.D.S.; Paiva, V.M.; Perrone, D.; Ripper, B.; Simoes, G.; Rocco, M.L.M.; da Veiga, A.G.; D’Elia, E. Glycine max meal extracts as corrosion inhibitor for mild steel in sulphuric acid solution. J. Mater. Res. Technol. 2020, 9, 12756–12772. Available online: https://www.sciencedirect.com/science/article/pii/S2238785420317427 (accessed on 15 June 2021). [CrossRef]
- El-Hashemy, M.A.; Sallam, A. The inhibitive action of Calendula officinalis flower heads extract for mild steel corrosion in 1 M HCl solution. J. Mater. Res. Technol. 2020, 9, 13509–13523. [Google Scholar] [CrossRef]
- Chahul, H.F.; Danat, T.B.; Wuana, R.A. Corrosion inhibition studies on the influence of colocasia esculenta leaves extract on mild steel in 1.0 M HCl. J. Mater. Environ. Sci. 2019, 10, 266–273. [Google Scholar]
- Asfia, M.P.; Rezaei, M.; Bahlakeh, G. Corrosion prevention of AISI 304 stainless steel in hydrochloric acid medium using garlic extract as a green corrosion inhibitor: Electrochemical and theoretical studies. J. Mol. Liq. 2020, 315, 113679. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; Hu, B.; Liu, Z.; Wang, C.; Han, J.; Su, M.; Li, Y.; Li, C. Modified chitosan-oligosaccharide and sodium silicate as efficient sustainable inhibitor for carbon steel against chloride-induced corrosion. J. Clean. Prod. 2019, 238, 117823. [Google Scholar] [CrossRef]
- Ong, G.; Kasi, R.; Subramaniam, R. A review on plant extracts as natural additives in coating applications. Prog. Org. Coat. 2021, 151, 106091. [Google Scholar] [CrossRef]
- Pineda Hernández, D.A.; Restrepo Parra, E.; Arango Arango, P.J.; Segura Giraldo, B.; Acosta Medina, C.D. Innovative Method for Coating of Natural Corrosion Inhibitor Based on Artemisia vulgaris. Materials 2021, 14, 2234. [Google Scholar] [CrossRef]
- Haag, A.P.; Relenyi, A.G. Slow Release Tablet Composition for Treating Industrial Water Systems. US Patent 9,090,495 B2, 28 July 2015. [Google Scholar]
- Wondi, M.H.; Shamsudin, R.; Yunus, R.; Alsultan, A.; Iswardi, A.H. Centrifugal separation-assisted and extraction of crude palm oil from separated mesocarp fiber: Central composite design optimization. J. Food Process Eng. 2020, 43, e13426. [Google Scholar] [CrossRef]
- Haris, N.I.N.; Sobri, S.; Kassim, N. Oil palm empty fruit bunch extract as green corrosion inhibitor for mild steel in hydrochloric acid solution: Central composite design optimization. Mater. Corros. 2019, 70, 1111–1119. [Google Scholar] [CrossRef]
- Haris, N.I.N.; Sobri, S.; Yusof, Y.A.; Kassim, N. Oil palm empty fruit bunch extract and powder as an eco-friendly corrosion inhibitor for mild steel: A comparison study. Mater. Corros. 2019, 70, 2326–2333. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. Available online: https://www.sciencedirect.com/science/article/pii/S0960852420301176 (accessed on 30 June 2021). [CrossRef]
- Palamae, S.; Dechatiwongse, P.; Choorit, W.; Chisti, Y.; Prasertsan, P. Cellulose and hemicellulose recovery from oil palm empty fruit bunch (EFB) fibers and production of sugars from the fibers. Carbohydr. Polym. 2017, 155, 491–497. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, B.H. Process Variable Optimization of Kenaf Two-Stage Fractionation Based on Dilute Hydrochloric Acid Followed by Ethanol Organosolv for Component Separation. BioEnergy Res. 2020, 13, 249–259. [Google Scholar] [CrossRef]
- Ioelovich, M. Methods for determination of chemical composition of plant biomass. Sci. Isreal Technol. Advant. 2015, 17, 208–214. [Google Scholar]
- Ech-Chihbi, E.; Nahlé, A.; Salim, R.; Benhiba, F.; Moussaif, A.; El-Hajjaji, F.; Oudda, H.; Guenbour, A.; Taleb, M.; Warad, I.; et al. Computational, MD simulation, SEM/EDX and experimental studies for understanding adsorption of benzimidazole derivatives as corrosion inhibitors in 1.0 M HCl solution. J. Alloys Compd. 2020, 844, 155842. [Google Scholar] [CrossRef]
- Djenane, M.; Chafaa, S.; Chafai, N.; Kerkour, R.; Hellal, A. Synthesis, spectral properties and corrosion inhibition efficiency of new ethyl hydrogen [(methoxyphenyl) (methylamino) methyl] phosphonate derivatives: Experimental and theoretical investigation. J. Mol. Struct. 2019, 1175, 398–413. [Google Scholar] [CrossRef]
- Gamry Instruments. Getting Started with Electrochemical Corrosion Measurement Rev 2; Gamry Instruments Inc.: Warminster, PA, USA, 2017. [Google Scholar]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2019, 69, 18–31. [Google Scholar] [CrossRef]
- Quraishi, M.A.; Chauhan, D.S.; Saji, V.S. Heterocyclic Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–298. [Google Scholar]
- Lgaz, H.; Masroor, S.; Chafiq, M.; Damej, M.; Brahmia, A.; Salghi, R.; Benmessaoud, M.; Ali, I.H.; Alghamdi, M.M.; Chaouiki, A.; et al. Evaluation of 2-Mercaptobenzimidazole Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid. Metals 2020, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Khalid, N.I.; Sulaiman, N.S.; Ab Aziz, N.; Taip, F.S.; Sobri, S.; Ab Rashid, N.-K.M. Optimization of Electrolysis Parameters for Green Sanitation Chemicals Production Using Response Surface Methodology. Processes 2020, 8, 792. [Google Scholar] [CrossRef]
- Yusof, Y.; Salleh, F.M.; Chin, N.; Talib, R.A. The drying and tabletting of pitaya powder. J. Food Process Eng. 2012, 35, 763–771. [Google Scholar] [CrossRef]
- Bisharat, L.; AlKhatib, H.; Al Muhaissen, S.; Quodbach, J.; Blaibleh, A.; Cespi, M.; Berardi, A. The influence of ethanol on superdisintegrants and on tablets disintegration. Eur. J. Pharm. Sci. 2019, 129, 140–147. Available online: https://www.sciencedirect.com/science/article/pii/S0928098719300120 (accessed on 11 July 2021). [CrossRef]
- Bykov, I. Characterization of Natural and Technical Lignins Using FTIR Spectroscopy; Lulea University of Technology: Luleå, Swiss, 2008. [Google Scholar]
- Zianor Azrina, Z.A.; Beg, M.D.H.; Rosli, M.Y.; Ramli, R.; Junadi, N.; Alam, A.K.M.M. Spherical nanocrystalline cellulose (NCC) from oil palm empty fruit bunch pulp via ultrasound assisted hydrolysis. Carbohydr. Polym. 2017, 162, 115–120. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Sulaiman, O.; Leh, C.P.; Sugimoto, T.; Nordin, N.A. Cellulose nanocrystals isolated from oil palm trunk. Carbohydr. Polym. 2015, 127, 202–208. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, J.H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef] [Green Version]
- Aranguren, M.I.; Mosiewicki, M.A.; Marcovich, N.E. Spectroscopic Characterization of Renewable Nanoparticles and Their Composites. In Biopolymer Nanocomposites: Processing, Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 509–540. [Google Scholar]
- Le Troedec, M.; Sedan, D.; Peyratout, C.; Bonnet, J.P.; Smith, A.; Guinebretiere, R.; Gloaguen, V.; Krausz, P. Influence of various chemical treatments on the composition and structure of hemp fibres. Compos. Part A Appl. Sci. Manuf. 2008, 39, 514–522. [Google Scholar] [CrossRef]
- Bekiaris, G.; Koutrotsios, G.; Tarantilis, P.A.; Pappas, C.S.; Zervakis, G.I. FTIR assessment of compositional changes in lignocellulosic wastes during cultivation of Cyclocybe cylindracea mushrooms and use of chemometric models to predict production performance. J. Mater. Cycles Waste Manag. 2020, 22, 1027–1035. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, T.; Wu, Z.; Zeng, G.; Huang, D.; Shen, Y.; He, X.; Lai, M.; He, Y. Study on biodegradation process of lignin by FTIR and DSC. Environ. Sci. Pollut. Res. 2014, 21, 14004–14013. [Google Scholar] [CrossRef] [PubMed]
- Boukir, A.; Fellak, S.; Doumenq, P. Structural characterization of Argania spinosa Moroccan wooden artifacts during natural degradation progress using infrared spectroscopy (ATR-FTIR) and X-Ray diffraction (XRD). Heliyon 2019, 5, e02477. [Google Scholar] [CrossRef] [Green Version]
- Hussin, M.H.; Samad, N.A.; Latif, N.H.A.; Rozuli, N.A.; Yusoff, S.B.; Gambier, F.; Brosse, N. Production of oil palm (Elaeis guineensis) fronds lignin-derived non-toxic aldehyde for eco-friendly wood adhesive. Int. J. Biol. Macromol. 2018, 113, 1266–1272. [Google Scholar] [CrossRef]
- Lyu, G.; Wu, Q.; Li, T.; Jiang, W.; Ji, X.; Yang, G. Thermochemical properties of lignin extracted from willow by deep eutectic solvents (DES). Cellulose 2019, 26, 8501–8511. [Google Scholar] [CrossRef]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. Characterization and modeling of the moisture diffusion behavior of natural fibers. J. Appl. Polym. Sci. 2013, 130, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-F.; Jing, Z.; Fowler, P.; Wu, Y.; Rajaratnam, M. Structural characterization and isolation of lignin and hemicelluloses from barley straw. Ind. Crops Prod. 2011, 33, 588–598. [Google Scholar] [CrossRef]
- Kumneadklang, S.; O-Thong, S.; Larpkiattaworn, S. Characterization of cellulose fiber isolated from oil palm frond biomass. Mater. Today Proc. 2019, 17, 1995–2001. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Y.; Ma, H.; Liu, L.; Hu, Y.; Xu, J.; Wang, Z.; Fan, Y. Contribution of hemicellulose to cellulose nanofiber-based nanocomposite films with enhanced strength, flexibility and UV-blocking properties. Cellulose 2019, 26, 6023–6034. [Google Scholar] [CrossRef]
- Alaneme, K.; Olusegun, S.; Alo, A. Corrosion inhibitory properties of elephant grass (Pennisetum purpureum) extract: Effect on mild steel corrosion in 1M HCl solution. Alex. Eng. J. 2016, 55, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Alaneme, K.K.; Daramola, Y.S.; Olusegun, S.J.; Afolabi, A.S. Corrosion inhibition and adsorption characteristics of rice husk extracts on mild steel immersed in 1M H2SO4 and HCl solutions. Int. J. Electrochem. Sci. 2015, 10, 3553–3567. [Google Scholar]
- Schmatz, A.A.; Tyhoda, L.; Brienzo, M. Sugarcane biomass conversion influenced by lignin. Biofuels Bioprod. Biorefin. 2020, 14, 469–480. [Google Scholar] [CrossRef]
- Chen, X.-F.; Zhang, L.-Q.; Huang, C.; Xiong, L.; Li, H.-L.; Wang, C.; Zhao, C.; Huang, Q.-L.; Chen, X.-D. Adsorption Study of Acid Soluble Lignin Removal from Sugarcane Bagasse Hydrolysate by a Self-Synthesized Resin for Lipid Production. Appl. Biochem. Biotechnol. 2019, 188, 585–601. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Ferdosi Heragh, M.; Tavakoli, H. Electrochemical properties of a new green corrosion inhibitor derived from prosopis farcta for St37 steel in 1 M hydrochloric acid. Met. Mater. Int. 2020, 26, 1654–1663. [Google Scholar] [CrossRef]
- Nwankwo, H.U.; Akpan, E.D.; Olasunkanmi, L.O.; Verma, C.; Al-Mohaimeed, A.M.; Al Farraj, D.A.; Ebenso, E.E. N-substituted carbazoles as corrosion inhibitors in microbiologically influenced and acidic corrosion of mild steel: Gravimetric, electrochemical, surface and computational studies. J. Mol. Struct. 2021, 1223, 129328. [Google Scholar] [CrossRef]
- El Aatiaoui, A.; Koudad, M.; Chelfi, T.; Erkan, S.; Azzouzi, M.; Aouniti, A.; Savaş, K.; Kaddouri, M.; Benchat, N.; Oussaid, A. Experimental and theoretical study of new Schiff bases based on imidazo(1,2-a)pyridine as corrosion inhibitor of mild steel in 1M HCl. J. Mol. Struct. 2021, 1226, 129372. [Google Scholar] [CrossRef]
- Belakhdar, A.; Ferkous, H.; Djellali, S.; Sahraoui, R.; Lahbib, H.; Ben Amor, Y.; Erto, A.; Balsamo, M.; Benguerba, Y. Computational and experimental studies on the efficiency of Rosmarinus officinalis polyphenols as green corrosion inhibitors for XC48 steel in acidic medium. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125458. Available online: https://www.sciencedirect.com/science/article/pii/S0927775720310517 (accessed on 3 May 2021). [CrossRef]
- Hsissou, R.; Abbout, S.; Seghiri, R.; Rehioui, M.; Berisha, A.; Erramli, H.; Assouag, M.; Elharfi, A. Evaluation of corrosion inhibition performance of phosphorus polymer for carbon steel in [1 M] HCl: Computational studies (DFT, MC and MD simulations). J. Mater. Res. Technol. 2020, 9, 2691–2703. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Jiang, H.; Gu, Y.; Li, X.; Xu, L.-L. Pueraria lobata leaf extract as green corrosion inhibitor for low carbon steel in 1.0 M HCl solution. Res. Chem. Intermed. 2021, 47, 1051–1069. [Google Scholar] [CrossRef]
- Pal, A.; Das, C. A novel use of solid waste extract from tea factory as corrosion inhibitor in acidic media on boiler quality steel. Ind. Crops Prod. 2020, 151, 112468. [Google Scholar] [CrossRef]
- Kakaei, K.; Esrafili, M.D.; Ehsani, A. Graphene and Anticorrosive Properties. In Graphene Surfaces; Kakaei, K., Esrafili, M.D., Ehsani, A.B.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–337. Available online: https://www.sciencedirect.com/science/article/pii/B9780128145234000083 (accessed on 2 August 2021).
- Fernández-Solis, C.D.; Vimalanandan, A.; Altin, A.; Mondragón-Ochoa, J.S.; Kreth, K.; Keil, P.; Erbe, A. Fundamentals of electrochemistry, corrosion and corrosion protection. In Soft Matter at Aqueous Interfaces; Springer: Berlin/Heidelberg, Germany, 2016; pp. 26–70. [Google Scholar]
- Kumari, P.; Yadav, M.; Obot, I.B. Investigation on corrosion protection behavior and adsorption of carbohydrazide-pyrazole compounds on mild steel in 15% HCl solution: Electrochemical and computational approach. J. Mol. Liq. 2020, 314, 113513. [Google Scholar] [CrossRef]
- Ellis, C.L.; Smith, E.; Javaid, H.; Berns, G.; Venkataraman, D. Ion Migration in Hybrid Perovskites. In Perovskite Photovoltaics: Basic to Advanced Concepts and Implementation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–196. [Google Scholar]
- Guo, L.; Tan, J.; Kaya, S.; Leng, S.; Li, Q.; Zhang, F. Multidimensional insights into the corrosion inhibition of 3,3-dithiodipropionic acid on Q235 steel in H2SO4 medium: A combined experimental and in silico investigation. J. Colloid Interface Sci. 2020, 570, 116–124. [Google Scholar] [CrossRef]
- Mohd, N.K.; Yeong, S.K.; Ibrahim, N.A.; Nor, S.M.M.; Chan, C.H.; Tang, S.W.; Lim, W.H.; Idris, Z. Corrosion inhibition, adsorption behaviour and thermodynamic properties of n-cinnamalidene palmitohydrazide on mild steel in hydrochloric acid solution. J. Oil Palm Res. 2020, 32, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Aslam, R.; Mobin, M.; Shoeb, M.; Murmu, M.; Banerjee, P. Proline nitrate ionic liquid as high temperature acid corrosion inhibitor for mild steel: Experimental and molecular-level insights. J. Ind. Eng. Chem. 2021, 100, 333–350. Available online: https://www.sciencedirect.com/science/article/pii/S1226086X21002677 (accessed on 8 June 2021). [CrossRef]
- Feliu, S. Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: Brief review and challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M.; Alavi, A. A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corros. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Pina, V.G.; Alvarez, L.X.; de Albuquerque, A.C.F.; Júnior, F.M.D.S.; Barrios, A.M.; Velasco, J.A.; Ponzio, E.A. Use of a theoretical prediction method and quantum chemical calculations for the design, synthesis and experimental evaluation of three green corrosion inhibitors for mild steel. Colloids Surf. A 2020, 599, 124857. [Google Scholar] [CrossRef]
- Breynaert, E.; Houlleberghs, M.; Radhakrishnan, S.; Grübel, G.; Taulelle, F.; Martens, J.A. Water as a tuneable solvent: A perspective. Chem. Soc. Rev. 2020, 49, 2557–2569. [Google Scholar] [CrossRef]
- Rousseva, S.; Besten, H.D.; Van Kooij, F.S.; Doting, E.L.; Doumon, N.Y.; Douvogianni, E.; Koster, L.J.A.; Hummelen, J.C. Reaching a Double-Digit Dielectric Constant with Fullerene Derivatives. J. Phys. Chem. C 2020, 124, 8633–8638. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.R.; Cisneros, M.G.V.; Chavarín, J.U.; Arteaga, C.C.; Rodríguez, M.A.V. The use of Brugmansia arborea as a green corrosion inhibitor for AISI 1018 carbon steel in acid media. Green Chem. Lett. Rev. 2021, 14, 107–117. [Google Scholar] [CrossRef]
- Arukalam, I.O.; Madu, I.O.; Ijomah, N.T.; Ewulonu, C.M.; Onyeagoro, G.N. Acid Corrosion Inhibition and Adsorption Behaviour of Ethyl Hydroxyethyl Cellulose on Mild Steel Corrosion. J. Mater. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Edoziuno, F.O.; Adediran, A.A.; Odoni, B.; Akinwekomi, A.; Adesina, O.; Oki, M. Optimization and development of predictive models for the corrosion inhibition of mild steel in sulphuric acid by methyl-5-benzoyl-2-benzimidazole carbamate (mebendazole). Cogent Eng. 2020, 7, 1714100. [Google Scholar] [CrossRef]
- Abbout, S.; Zouarhi, M.; Chebabe, D.; Damej, M.; Berisha, A.; Hajjaji, N. Galactomannan as a new bio-sourced corrosion inhibitor for iron in acidic media. Heliyon 2020, 6, e03574. [Google Scholar] [CrossRef]
- Onyeachu, I.B.; Solomon, M.M.; Umoren, S.A.; Obot, I.B.; Sorour, A.A. Corrosion inhibition effect of a benzimidazole derivative on heat exchanger tubing materials during acid cleaning of multistage flash desalination plants. Desalination 2020, 479, 114283. [Google Scholar] [CrossRef]
- Ye, Y.; Zou, Y.; Jiang, Z.; Yang, Q.; Chen, L.; Guo, S.; Chen, H. An effective corrosion inhibitor of N doped carbon dots for Q235 steel in 1 M HCl solution. J. Alloys Compd. 2020, 815, 152338. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of stereochemical conformation into the corrosion inhibitive behaviour of double azomethine based Schiff bases on mild steel surface in 1 mol L−1 HCl medium: An experimental, density functional theory and molecular dynamics simulation study. Corros. Sci. 2019, 146, 134–151. [Google Scholar] [CrossRef]
- Barmatov, E.; Hughes, T. Degradation of a schiff-base corrosion inhibitor by hydrolysis, and its effects on the inhibition efficiency for steel in hydrochloric acid. Mater. Chem. Phys. 2021, 257, 123758. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J.; Bezener, M.A. Getting Your Toe into Mixtures. In Formulation Simplified; Routledge: London, UK, 2018; pp. 1–30. [Google Scholar]
- Lee, W.B.; Widjaja, E.; Heng, P.W.S.; Chan, L.W. Effect of excipient particle size distribution variability on compact tensile strength; and its in-line prediction by force-displacement and force-time profiling. Eur. J. Pharm. Sci. 2021, 159, 105703. [Google Scholar] [CrossRef]
- Ogunjimi, A.; Alebiowu, G. Material and tableting properties of Azadirachta indica gum with reference to official acacia gum. Acta Pol. Pharm. 2014, 71, 107–118. [Google Scholar]
- Siow, C.R.S.; Heng, P.W.S.; Chan, L.W. A study on the impact of HPMC viscosity grade and proportion on the functional properties of co-freeze-dried mannitol-HPMC cushioning excipients for compacted MUPS. Eur. J. Pharm. Biopharm. 2020, 151, 98–107. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Huang, Z.; Cui, Y.; Zhao, Z.; Yue, X.; Wang, G.; Liang, R.; Huang, Y.; Tan, W.; et al. Development and pharmacokinetics evaluation of quetiapine fumarate sustained-release tablets based on hydrophilic matrix. J. Drug Deliv. Sci. Technol. 2019, 54, 101322. [Google Scholar] [CrossRef]
- Liu, S.; Jing, Y.; Zhang, T.; Zhang, J.; Xu, F.; Song, Q.; Ye, Q.; Liu, S.; Liu, W. Excellent tribological and anti-corrosion performances enabled by novel hollow graphite carbon nanosphere with controlled release of corrosion inhibitor. Chem. Eng. J. 2021, 412, 128648. Available online: https://www.sciencedirect.com/science/article/pii/S1385894721002461 (accessed on 5 May 2021). [CrossRef]
- Geethamani, P. Corrosion Inhibitors; IntechOpen: London, UK, 2019. [Google Scholar]
- Rajakumari, R.; Volova, T.; Oluwafemi, O.S.; Thomas, S.; Kalarikkal, N. Transformation of essential minerals into tablet formulation with enhanced stability. Adv. Powder Technol. 2020, 31, 2806–2819. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Sun, C.C. Interfacial bonding in formulated bilayer tablets. Eur. J. Pharm. Biopharm. 2020, 147, 69–75. [Google Scholar] [CrossRef]
- Zafari, S.; Shahrak, M.N.; Ghahramaninezhad, M. New MOF-Based Corrosion Inhibitor for Carbon Steel in Acidic Media. Met. Mater. Int. 2020, 26, 25–38. [Google Scholar] [CrossRef]
- Popoola, L.T. Organic green corrosion inhibitors (OGCIs): A critical review. Corros. Rev. 2019, 37, 71–102. [Google Scholar] [CrossRef]

| Element | Chemical Composition (wt.%) |
|---|---|
| Iron | 98.874 |
| Carbon | 0.140 |
| Silicon | 0.170 |
| Manganese | 0.550 |
| Copper | 0.230 |
| Sulfur | 0.027 |
| Phosphorus | 0.009 |
| Tablet | Tablet Formulation (% w/w) | ||
|---|---|---|---|
| EFB-H | GA | HPMC | |
| T1 | 100.0 | 0.0 | 0.0 |
| T2 | 66.0 | 34.0 | 0.0 |
| T3 | 66.0 | 0.0 | 34.0 |
| Components | Composition (% w/w) | |
|---|---|---|
| Raw OPEFB | EFB-H | |
| Holocellulose | 83.56 | 90.34 |
| Cellulose | 29.85 | 0.85 |
| Hemicellulose | 53.71 | 89.51 |
| Lignin | 15.64 | 9.28 |
| Acid soluble | 8.78 | 5.39 |
| Acid insoluble | 6.86 | 3.89 |
| Extractives | 0.81 | 0.38 |
| EFB-H Concentration (g 300 mL−1) | Ecorr (mV) | βa | −βc | Icorr (µA cm−2) | CR (mm y−1) | IEPDP (%) |
|---|---|---|---|---|---|---|
| (mVdecade−1) | ||||||
| Blank | −463.51 | 122.14 | 135.25 | 598.65 | 8.79 | - |
| 0.1 | −420.14 | 129.45 | 128.31 | 163.15 | 2.40 | 72.75 |
| 0.2 | −418.84 | 136.97 | 145.08 | 128.28 | 1.88 | 78.57 |
| 0.3 | −431.57 | 109.04 | 139.38 | 78.04 | 1.15 | 86.96 |
| 0.4 | −421.95 | 109.59 | 133.09 | 58.52 | 0.86 | 90.22 |
| 0.5 | −414.49 | 111.89 | 140.28 | 60.31 | 0.89 | 89.92 |
| EFB-H Concentration (g 300 mL−1) | Rs (Ω cm2) | Rct (Ω cm2) | IEEIS (%) | n | Cdl (µF cm−2) |
|---|---|---|---|---|---|
| Blank | 3.78 | 47.28 | - | 0.9987 | 145.36 |
| 0.1 | 4.06 | 142.08 | 66.72 | 0.9971 | 93.20 |
| 0.2 | 3.18 | 216.14 | 78.12 | 0.9975 | 83.56 |
| 0.3 | 1.68 | 336.13 | 85.93 | 0.9978 | 72.81 |
| 0.4 | 1.20 | 397.11 | 88.09 | 0.9978 | 69.07 |
| 0.5 | 4.09 | 462.77 | 89.78 | 0.9979 | 72.86 |
| Independent Parameter | IEWL (%) | |
|---|---|---|
| EFB-H Dosage (g) | Immersion Time (h) | |
| 0.1 | 80.37 ± 0.72 | |
| 0.3 | 120 | 85.66 ± 1.28 |
| 0.5 | 85.37 ± 1.44 | |
| 24 | 66.35 ± 0.69 | |
| 0.3 | 120 | 85.66 ± 1.28 |
| 216 | 66.69 ± 1.43 | |
| Standard Order | Run Order | EFB-H Dosage (g) | Immersion Time (h) | IEWL (%) |
|---|---|---|---|---|
| 1 | 10 | 0.1 | 120.0 | 80.37 |
| 2 | 4 | 0.5 | 120.0 | 85.00 |
| 3 | 8 | 0.1 | 360.0 | 59.02 |
| 4 | 6 | 0.5 | 360.0 | 77.61 |
| 5 | 2 | 0.1 | 240.0 | 57.25 |
| 6 | 11 | 0.5 | 240.0 | 66.55 |
| 7 | 9 | 0.3 | 120.0 | 85.66 |
| 8 | 1 | 0.3 | 360.0 | 77.41 |
| 9 | 7 | 0.3 | 240.0 | 65.23 |
| 10 | 13 | 0.3 | 240.0 | 67.89 |
| 11 | 5 | 0.3 | 240.0 | 67.84 |
| 12 | 3 | 0.3 | 240.0 | 66.49 |
| 13 | 12 | 0.3 | 240.0 | 64.57 |
| Standard Order | Run Order | EFB-H (%) | GA (%) | HPMC (%) | IEWL (%) |
|---|---|---|---|---|---|
| 1 | 1 | 66.00 | 34.00 | 0.00 | 81.90 |
| 2 | 3 | 66.00 | 0.00 | 34.00 | 85.64 |
| 3 | 7 | 66.00 | 17.34 | 16.66 | 80.79 |
| 4 | 13 | 66.00 | 8.62 | 25.38 | 79.01 |
| 5 | 11 | 66.00 | 25.62 | 8.38 | 77.67 |
| 6 | 10 | 66.00 | 12.94 | 21.06 | 77.12 |
| 7 | 6 | 66.00 | 4.29 | 29.71 | 79.92 |
| 8 | 4 | 66.00 | 21.42 | 12.58 | 76.69 |
| 9 | 5 | 66.00 | 34.00 | 0.00 | 81.07 |
| 10 | 2 | 66.00 | 0.00 | 34.00 | 84.02 |
| 11 | 9 | 66.00 | 34.00 | 0.00 | 80.57 |
| 12 | 12 | 66.00 | 0.00 | 34.00 | 83.23 |
| 13 | 8 | 66.00 | 34.00 | 0.00 | 81.49 |
| Tablet | Formulation (% w/w) | IEWL (%) | Tensile Strength (MPa) | Disintegration Time (min) | ||
|---|---|---|---|---|---|---|
| EFB-H | GA | HPMC | ||||
| T1 | 100.0 | 0.0 | 0.0 | 87.12 | 0.130 ± 0.027 | 63.00 ± 1.00 |
| T2 | 66.0 | 34.0 | 0.0 | 81.28 | 0.099 ± 0.017 | 53.00 ± 3.00 |
| T3 | 66.0 | 0.0 | 34.0 | 84.30 | 0.243 ± 0.006 | 152.00 ± 2.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haris, N.I.N.; Sobri, S.; Yusof, Y.A.; Kassim, N.K. Innovative Method for Longer Effective Corrosion Inhibition Time: Controlled Release Oil Palm Empty Fruit Bunch Hemicellulose Inhibitor Tablet. Materials 2021, 14, 5657. https://doi.org/10.3390/ma14195657
Haris NIN, Sobri S, Yusof YA, Kassim NK. Innovative Method for Longer Effective Corrosion Inhibition Time: Controlled Release Oil Palm Empty Fruit Bunch Hemicellulose Inhibitor Tablet. Materials. 2021; 14(19):5657. https://doi.org/10.3390/ma14195657
Chicago/Turabian StyleHaris, Nur Izzah Nabilah, Shafreeza Sobri, Yus Aniza Yusof, and Nur Kartinee Kassim. 2021. "Innovative Method for Longer Effective Corrosion Inhibition Time: Controlled Release Oil Palm Empty Fruit Bunch Hemicellulose Inhibitor Tablet" Materials 14, no. 19: 5657. https://doi.org/10.3390/ma14195657
APA StyleHaris, N. I. N., Sobri, S., Yusof, Y. A., & Kassim, N. K. (2021). Innovative Method for Longer Effective Corrosion Inhibition Time: Controlled Release Oil Palm Empty Fruit Bunch Hemicellulose Inhibitor Tablet. Materials, 14(19), 5657. https://doi.org/10.3390/ma14195657






