Investigation of Regeneration Mechanisms of Aged Solar Salt
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
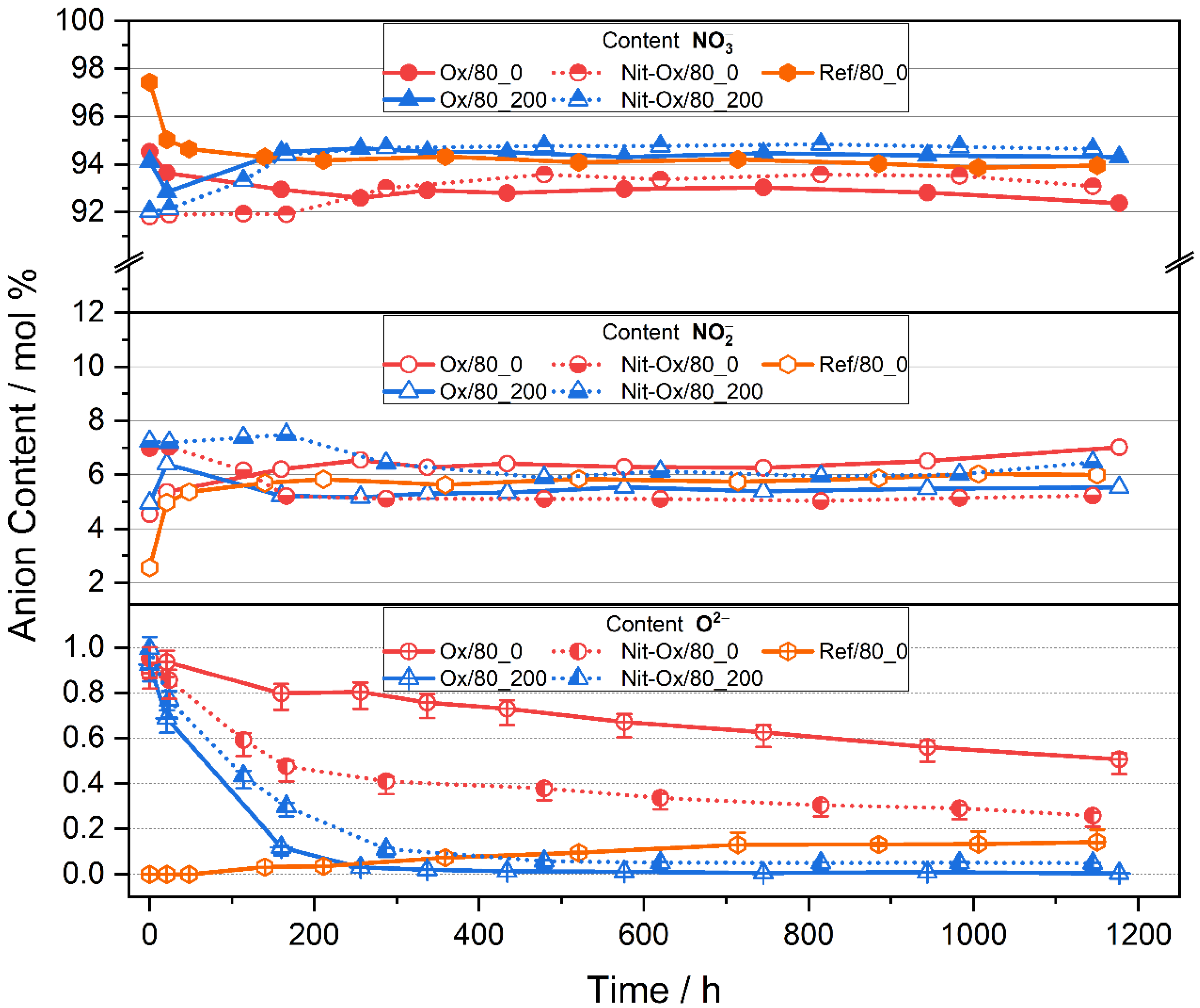
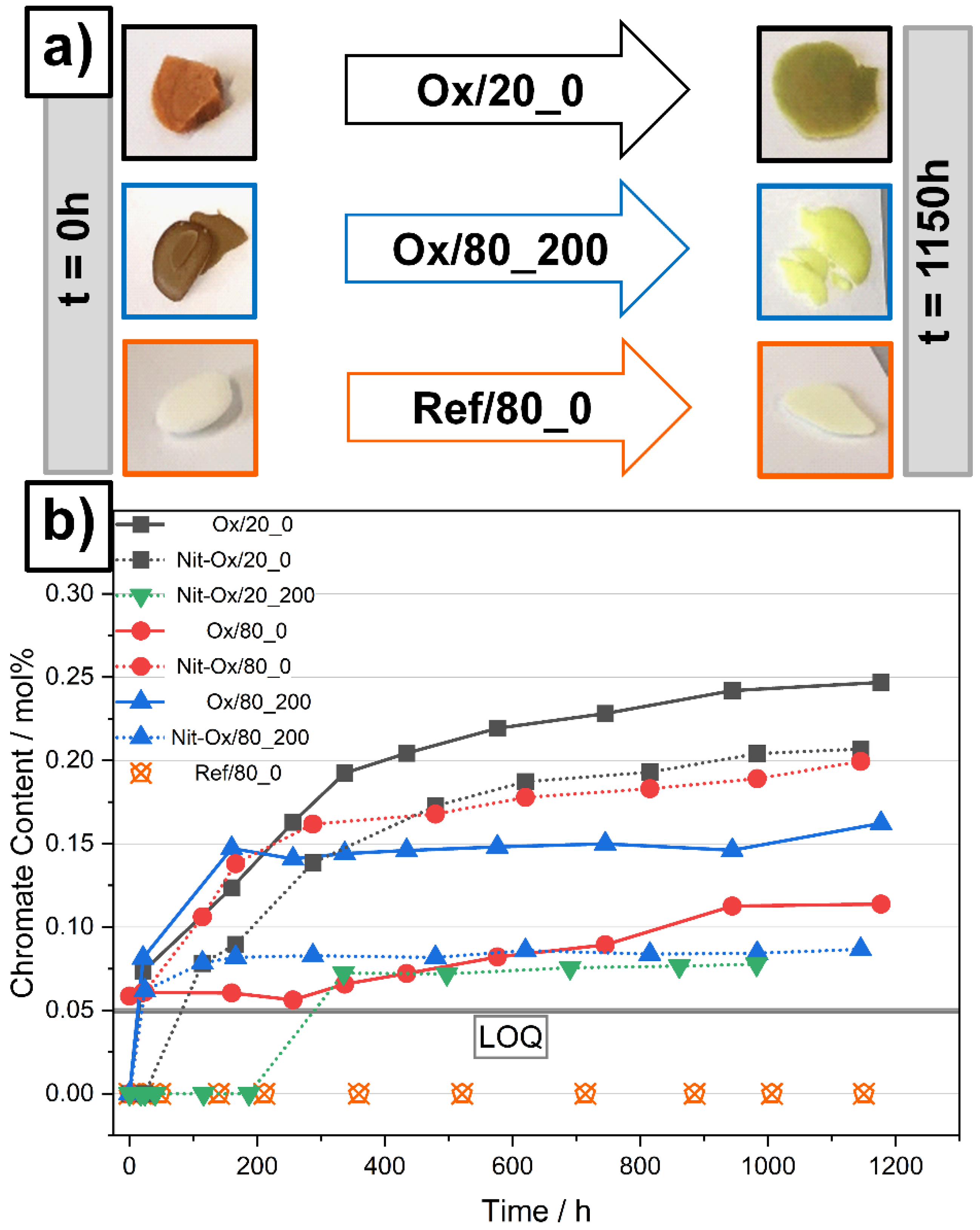
3.1. Behavior of an Oxide Rich Nitrate Melt under Various Conditions
3.2. Indicators for Corrosivity in an Oxide Rich Nitrate Melt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durth, M.; Prieto, C.; Rodriguez-Sanchez, A.; Patino-Rodriguez, D.; Cabeza, L.F. Effects of sodium nitrate concentration on thermophysical properties of solar salts and on the thermal energy storage cost. Sol. Energy 2019, 182, 57–63. [Google Scholar] [CrossRef]
- Bauer, T.; Pfleger, N.; Laing, D.; Steinmann, W.-D.; Eck, M.; Kaesche, S. High-temperature molten salts for solar power application. In Molten Salts Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 415–438. [Google Scholar]
- Stern, K.H. High temperature properties and decomposition of inorganic salts part 3, nitrates and nitrites. J. Phys. Chem. Ref. Data 1972, 1, 747–772. [Google Scholar] [CrossRef] [Green Version]
- Nissen, D.A.; Meeker, D.E. Nitrate/nitrite chemistry in sodium nitrate-potassium nitrate melts. Inorg. Chem. 1983, 22, 716–721. [Google Scholar] [CrossRef]
- Wright, S.; Tran, T.; Chen, C.; Olivares, R.I.; Sun, S. Thermal Stability of Potassium and Sodium Nitrate Molten Salt Mixtures Above 500 °C. In Proceedings of the Ninth International Conference on Molten Slags, Fluxes and Salts, Beijing, China, 27–30 May 2012. [Google Scholar]
- Mohammad, M.B.; Brooks, G.A.; Rhamdhani, M.A. Premelting, Melting, and Degradation Properties of Molten Alkali Nitrates: LiNO3, NaNO3, KNO3, and Binary NaNO3-KNO3. Metall. Mater. Trans. B 2018, 49, 1482–1498. [Google Scholar] [CrossRef]
- Sotz, V.A.; Bonk, A.; Steinbrecher, J.; Bauer, T. Defined purge gas composition stabilizes molten nitrate salt—Experimental prove and thermodynamic calculations. Sol. Energy 2020, 211, 453–462. [Google Scholar] [CrossRef]
- Fei, Z.; Zhang, Y.; Ge, M.; Wang, Y.; Li, Y.; Cheng, J.; Wei, B.; Hou, H.; Liu, H. Probing thermal decomposition mechanism of molten nitrite/nitrates salt by time of flight mass spectrometry. Sol. Energy 2019, 183, 823–828. [Google Scholar] [CrossRef]
- Freeman, E.S. The kinetics of the thermal decomposition of sodium nitrate and of the reaction between sodium nitrite and oxygen. J. Phys. Chem. 1956, 60, 1487–1493. [Google Scholar] [CrossRef]
- Bonk, A.; Martin, C.; Braun, M.; Bauer, T. Material Investigations on the Thermal Stability of Solar Salt and Potential Filler Materials for Molten Salt Storage. AIP Conf. Proc. 2017, 1850, 080008. [Google Scholar] [CrossRef] [Green Version]
- Sötz, V.A.; Bonk, A.; Bauer, T. With a view to elevated operating temperatures in thermal energy storage—Reaction chemistry of Solar Salt up to 630 °C. Sol. Energy Mater. Sol. Cells 2020, 212, 110577. [Google Scholar] [CrossRef]
- Fernandez, A.G.; Cabeza, L.F. Molten salt corrosion mechanisms of nitrate based thermal energy storage materials for concentrated solar power plants: A review. Sol. Energy Mater. Sol. Cells 2019, 194, 160–165. [Google Scholar] [CrossRef]
- Nikulshina, V.; Ayesa, N.; Galvez, M.E.; Steinfeld, A. Feasibility of Na-based thermochemical cycles for the capture of CO2 from air—Thermodynamic and thermogravimetric analyses. Chem. Eng. J. 2008, 140, 62–70. [Google Scholar] [CrossRef]
- Bonk, A.; Braun, M.; Hanke, A.; Forstner, J.; Rückle, D.; Kaesche, S.; Sötz, V.A.; Bauer, T. Influence of different atmospheres on molten salt chemistry and its effect on steel corrosion. AIP Conf. Proc. 2018, 2033, 090003. [Google Scholar] [CrossRef]
- Sötz, V.A.; Bonk, A.; Forstner, J.; Bauer, T. Microkinetics of the reaction NO3−⇌NO2−+0.5 O2 in molten sodium nitrate and potassium nitrate salt. Thermochim. Acta 2019, 678, 178301. [Google Scholar] [CrossRef]
- Tracey, T.R. Conceptual Design of Advanced Central Receiver Power System; Final Report; Martin Marietta Corp.: Denver, CO, USA, 1978. [Google Scholar]
- Paniccia, F.; Zambonin, P.G. Redox mechanism in an ionic matrix. IV. Catalytic effects of peroxide and superoxide on the oxidation of nitrite by molecular oxygen in molten alkali nitrates. J. Phys. Chem. 1974, 78, 1693–1698. [Google Scholar] [CrossRef]
- Federsel, K.; Wortmann, J.; Ladenberger, M. High-temperature and corrosion behavior of nitrate nitrite molten salt mixtures regarding their application in concentrating solar power plants. Enrgy Proced 2015, 69, 618–625. [Google Scholar] [CrossRef] [Green Version]
- Villada, C.; Bonk, A.; Bauer, T.; Bolivar, F. High-temperature stability of nitrate/nitrite molten salt mixtures under different atmospheres. Appl. Energy 2018, 226, 107–115. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.; Wu, Y.; Lu, Y. Comparative study on high temperature thermal stability of quaternary nitrate-nitrite mixed salt and Solar salt. Sol. Energy Mater. Sol. Cells 2021, 230, 111197. [Google Scholar] [CrossRef]


| Label | Anion Content (mol %) | Purge Gas (100 mL/min) | ||||
|---|---|---|---|---|---|---|
| Nitrate | Nitrite | Peroxide | N2 | O2 | NO | |
| Ref/20_0 [a] | 100 | 0 | 0 | 80 vol% | 20 vol% | 0 |
| Ref/20_200 [b] | 100 | 0 | 0 | 80 vol% | 20 vol% | 200 ppm |
| Ox/20_0 | 99 | 0 | 1 | 80 vol% | 20 vol% | 0 |
| Nit-Ox/20_0 | 89 | 10 | 1 | 80 vol% | 20 vol% | 0 |
| Nit-Ox/20_200 | 89 | 10 | 1 | 80 vol% | 20 vol% | 200 ppm |
| Ref/80_0 | 100 | 0 | 0 | 20 vol% | 80 vol% | 0 |
| Ox/80_0 | 99 | 0 | 1 | 20 vol% | 80 vol% | 0 |
| Nit-Ox/80_0 | 89 | 10 | 1 | 20 vol% | 80 vol% | 0 |
| Ox/80_200 | 99 | 0 | 1 | 20 vol% | 80 vol% | 200 ppm |
| Nit-Ox/80_200 | 89 | 10 | 1 | 20 vol% | 80 vol% | 200 ppm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinbrecher, J.; Bonk, A.; Sötz, V.A.; Bauer, T. Investigation of Regeneration Mechanisms of Aged Solar Salt. Materials 2021, 14, 5664. https://doi.org/10.3390/ma14195664
Steinbrecher J, Bonk A, Sötz VA, Bauer T. Investigation of Regeneration Mechanisms of Aged Solar Salt. Materials. 2021; 14(19):5664. https://doi.org/10.3390/ma14195664
Chicago/Turabian StyleSteinbrecher, Julian, Alexander Bonk, Veronika Anna Sötz, and Thomas Bauer. 2021. "Investigation of Regeneration Mechanisms of Aged Solar Salt" Materials 14, no. 19: 5664. https://doi.org/10.3390/ma14195664






