Effect of Thermo-Mechanical Treatment on the Microstructure and Tensile Properties of the Fe-22Cr-5Al-0.1Y Alloy
Abstract
:1. Introduction
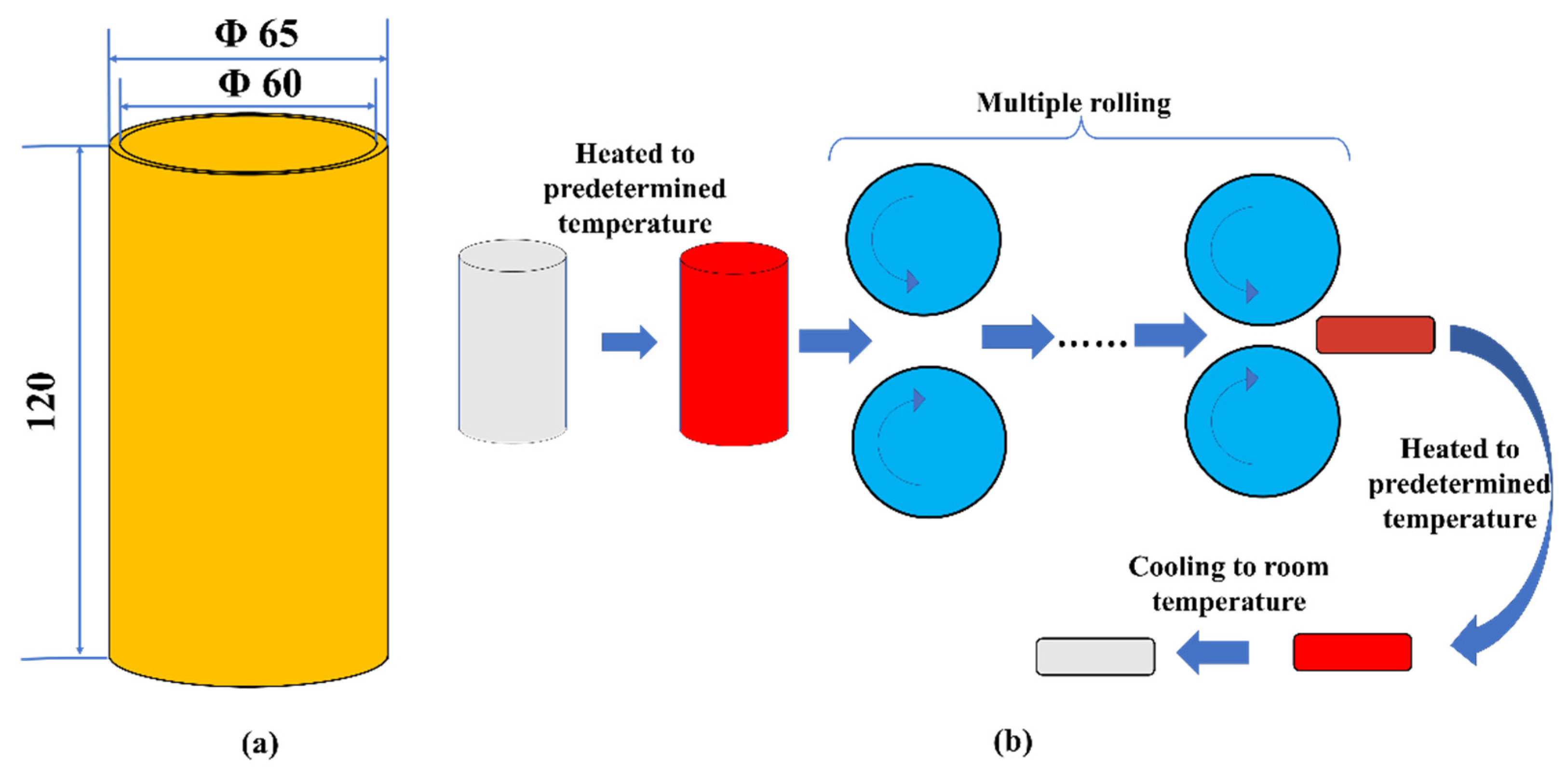
2. Materials and Methods
2.1. Materials
2.2. Microstructure Characterization
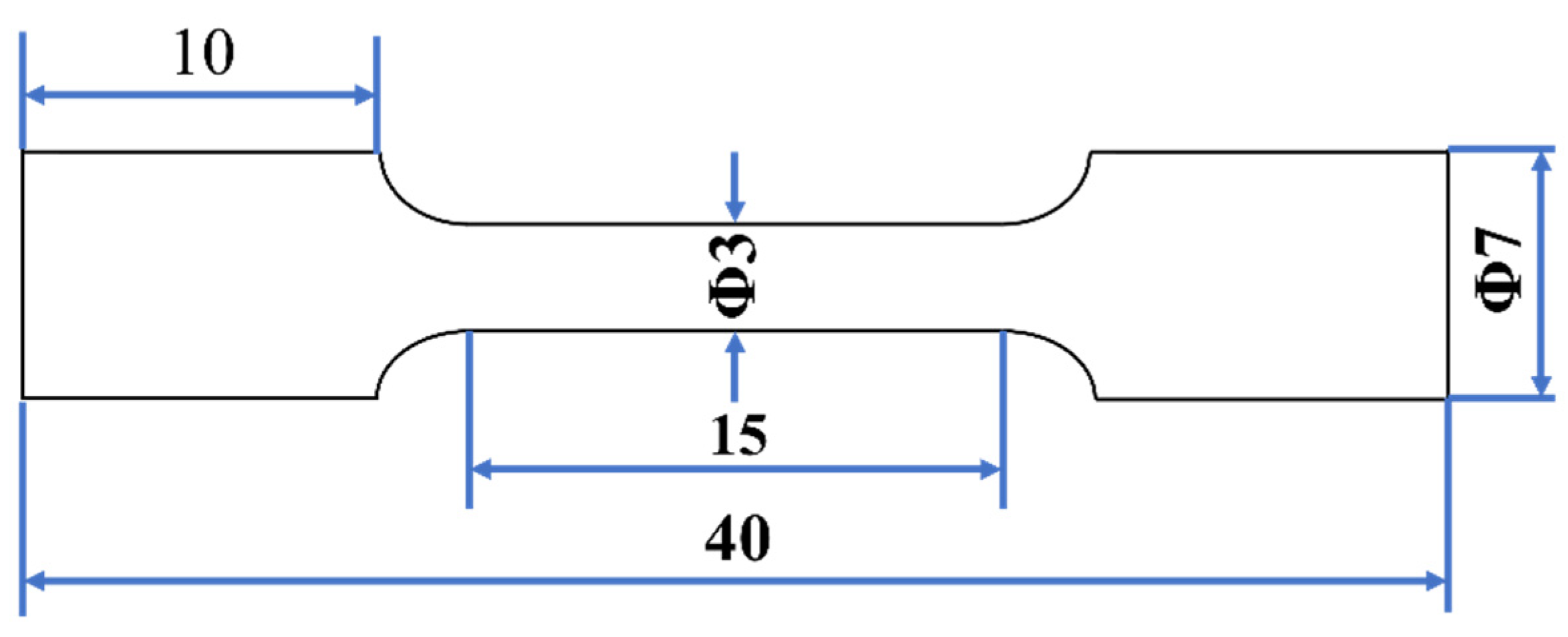
2.3. Tensile Test
3. Results
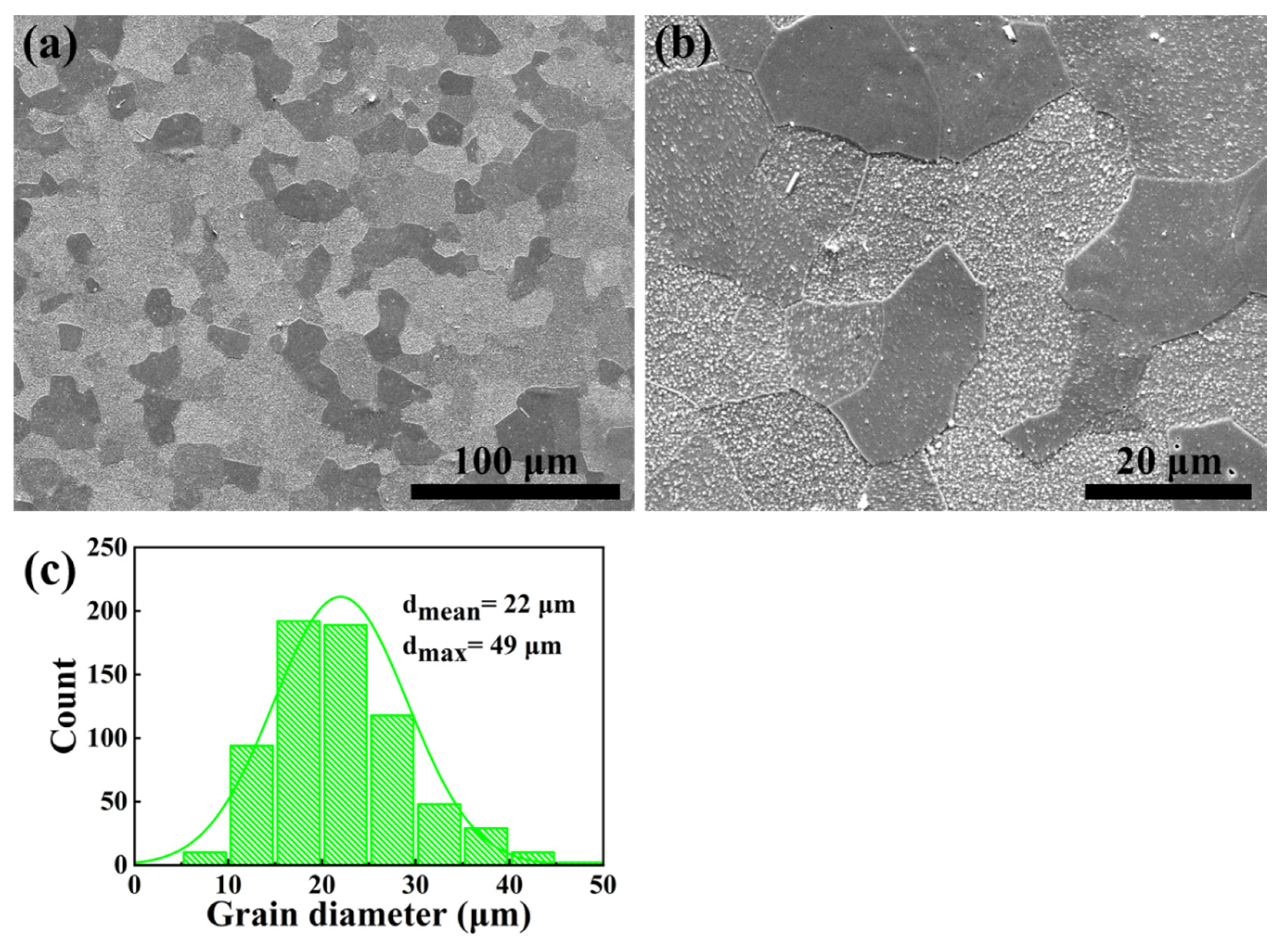
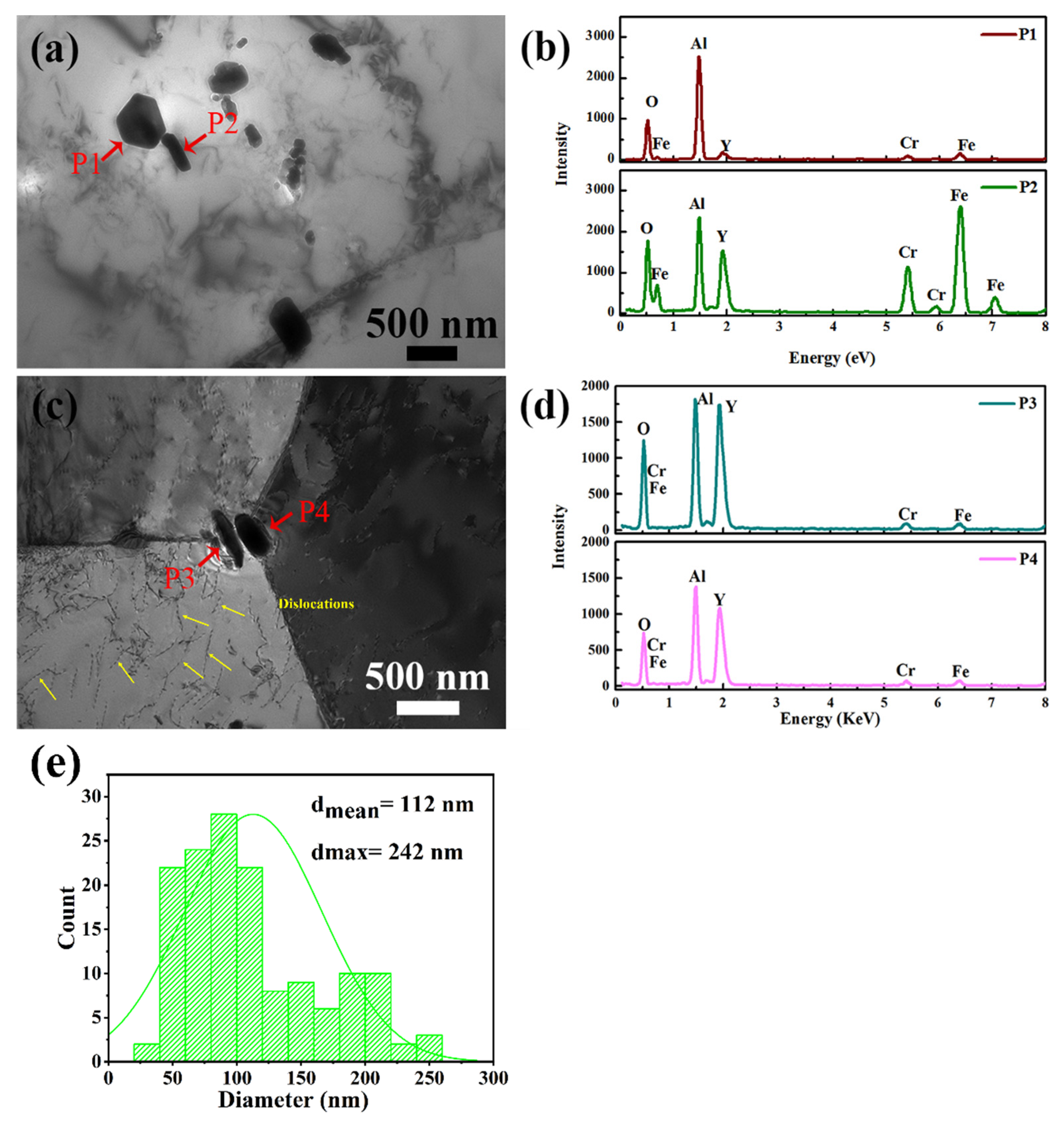
3.1. Microstructure
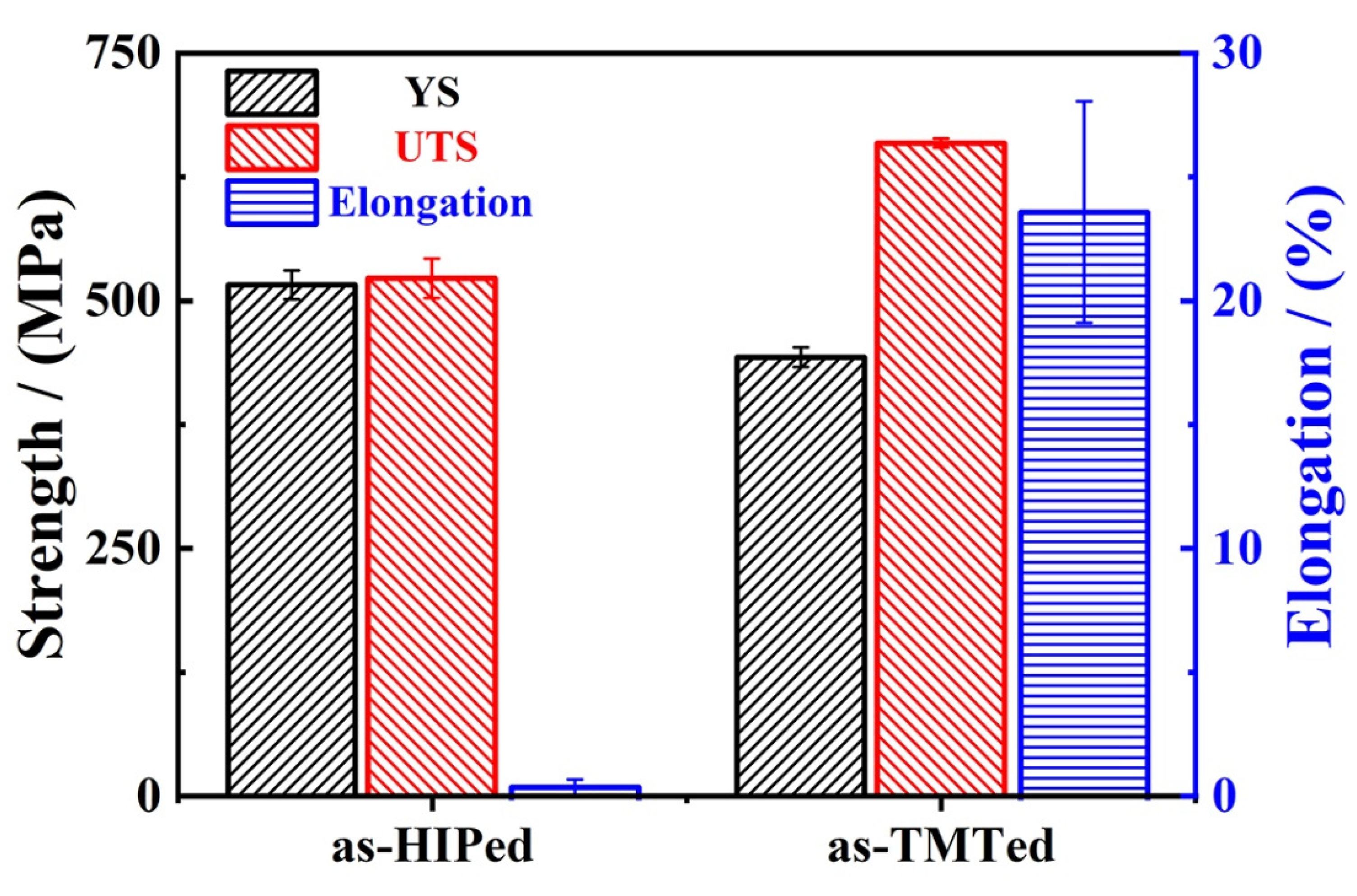
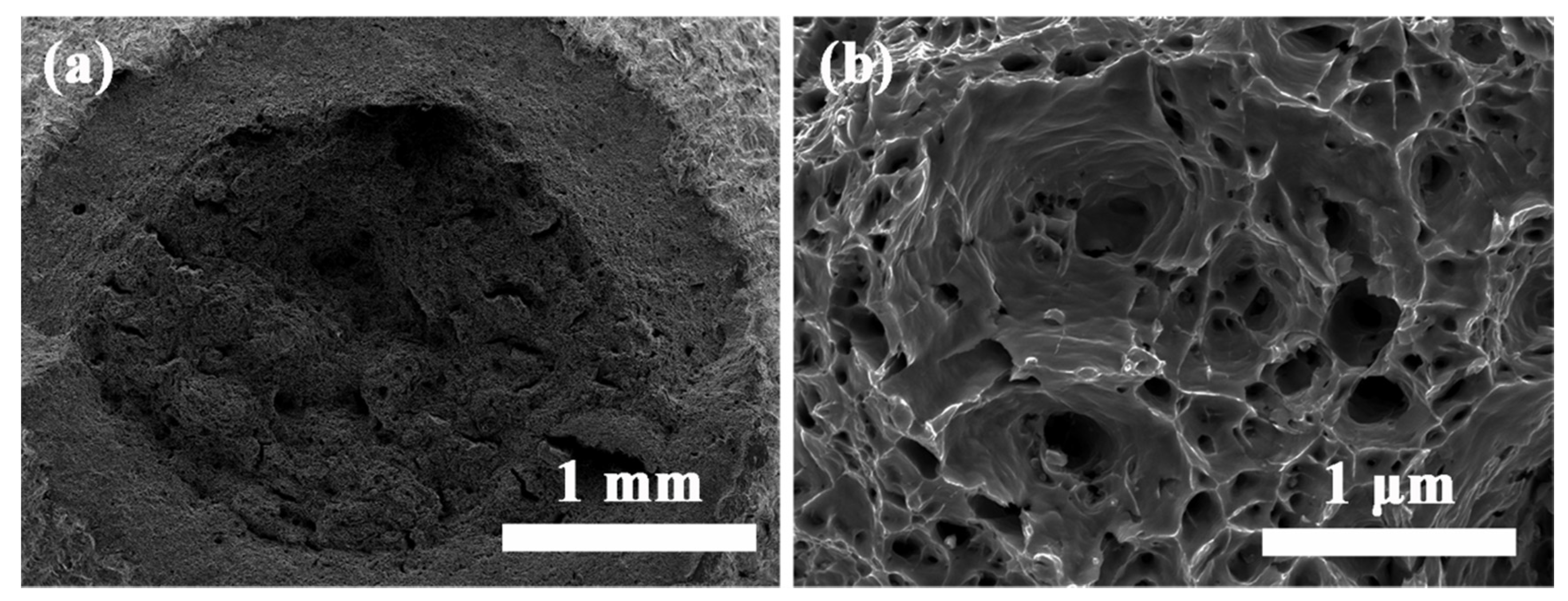
3.2. Tensile Properties and Fracture Surface
4. Discussion
4.1. Formation of Al-Containing Precipitates
4.2. Effect of TMT Process on Microstructure
4.3. Possible Strengthening Mechanism
5. Conclusions
- Both continuous and dispersed Al2O3 particles formed at the grain boundaries within as-HIPed alloys, which led to low strength and intergranular fracture of the as-HIPed alloy at 25 °C;
- Microstructure of the as-HIPed alloy was optimized after TMT. The mean grain diameter decreased from 127 μm to 22 μm. In addition, large and continuous Al2O3 particles were refined, and the distribution of the oxide became dispersed. Part of the Al2O3 reacted with Y2O3 to form more stable Y-Al-O oxides at the grain boundaries and within the grains;
- The optimization of the microstructure promoted the improvement in mechanical properties. The UTS increased from 522 MPa to 658 MPa, and the elongation increased from 0.5% to 23%. The fracture of alloy transformed from a cleavage/intergranular fracture into a ductile fracture.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Odette, G.R.; Alinger, M.J.; Wirth, B.D. Recent Developments in Irradiation-Resistant Steels. Annu. Rev. Mater. Res. 2008, 38, 471–503. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Y.; Peng, Y.; Yang, W.; Ma, X.; Wang, B. High-strength joint of nuclear-grade FeCrAl alloys achieved by friction stir welding and its strengthening mechanism. J. Manuf. Process. 2021, 65, 1–11. [Google Scholar] [CrossRef]
- Engkvist, J.; Canovic, S.; Hellström, K.; Järdnäs, A.; Svensson, J.E.; Johansson, L.G.; Olsson, M.; Halvarsson, M. Alumina Scale Formation on a Powder Metallurgical FeCrAl Alloy (Kanthal APMT) at 900–1,100 °C in Dry O2 and in O2 + H2O. Oxid. Met. 2009, 73, 233–253. [Google Scholar] [CrossRef]
- Aydogan, E.; El-Atwani, O.; Takajo, S.; Vogel, S.C.; Maloy, S.A. High temperature microstructural stability and recrystallization mechanisms in 14YWT alloys. Acta Mater. 2018, 148, 467–481. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Influence of Zr addition on the microstructures and mechanical properties of 14Cr ODS steels. Mater. Sci. Eng. A 2017, 695, 66–73. [Google Scholar] [CrossRef]
- Siska, F.; Stratil, L.; Hadraba, H.; Fintova, S.; Kubena, I.; Hornik, V.; Husak, R.; Bartkova, D.; Zalezak, T. Strengthening mechanisms of different oxide particles in 9Cr ODS steel at high temperatures. Mater. Sci. Eng. A 2018, 732, 112–119. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Li, F.; Yang, H.; Zhao, Z.; Kano, S.; Matsukawa, Y.; Satoh, Y.; Abe, H. Microstructural characterization and strengthening mechanisms of a 12Cr-ODS steel. Mater. Sci. Eng. A 2016, 673, 624–632. [Google Scholar] [CrossRef]
- Kumar, D.; Prakash, U.; Dabhade, V.V.; Laha, K.; Sakthivel, T. High yttria ferritic ODS steels through powder forging. J. Nucl. Mater. 2017, 488, 75–82. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Z.; Xie, R.; Lu, C.; Shi, Y.; Liu, C. Effects of Y2O3, La2O3 and CeO2 additions on microstructure and mechanical properties of 14Cr-ODS ferrite alloys produced by spark plasma sintering. Fusion Eng. Des. 2017, 121, 159–166. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.; Liu, Y.; Huang, Y.; Guo, Q.; Li, H.; Wu, J. Evolution of Al-containing phases in ODS steel by hot pressing and annealing. Powder Technol. 2017, 311, 449–455. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.; Liu, Y.; Huang, Y.; Ma, Z.; Li, H.; Wu, J. Microstructure and tensile properties of a 14Cr ODS ferritic steel. Mater. Sci. Eng. A 2017, 680, 347–350. [Google Scholar] [CrossRef]
- Chauhan, A.; Walter, M.; Aktaa, J. Towards improved ODS steels: A comparative high-temperature low-cycle fatigue study. Fatigue Fract. Eng. Mater. Struct. 2017, 40, 2128–2140. [Google Scholar] [CrossRef]
- Ukai, S.; Ohtsuka, S. Low cycle fatigue properties of ODS ferritic–martensitic steels at high temperature. J. Nucl. Mater. 2007, 367–370, 234–238. [Google Scholar] [CrossRef]
- Chauhan, A.; Hoffmann, J.; Litvinov, D.; Aktaa, J. High-temperature low-cycle fatigue behavior of a 9Cr-ODS steel: Part 1—Pure fatigue, microstructure evolution and damage characteristics. Mater. Sci. Eng. A 2017, 707, 207–220. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Z.; Wang, D.; Zhang, Z.; Han, Y.; Liu, C. Structure and composition of oxides in FeCrAl ODS alloy with Zr addition. Mater. Sci. Technol. 2017, 33, 1790–1795. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Long, F.; Jia, H.; Guo, N.; Yao, Z.; Daymond, M.R. Combination of back stress strengthening and Orowan strengthening in bimodal structured Fe–9Cr–Al ODS steel with high Al addition. Mater. Sci. Eng. A 2019, 739, 45–52. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Z.; Jia, H.; Yao, Z. Microstructure Characterization and Mechanical Properties of Al Alloyed 9Cr ODS Steels with Different Al Contents. Steel Res. Int. 2019, 90, 1800594. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, Z.; Mo, K.; Wang, P.; Miao, Y.; Li, S.; Wang, M.; Liu, X.; Gong, M.; Almer, J.; et al. The microstructure and mechanical properties of Al-containing 9Cr ODS ferritic alloy. J. Alloys Compd. 2015, 648, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Kaur, G.; Panigrahi, B.K.; David, C.; Amarendra, G. Effect of Zr and Al addition on nanocluster formation in oxide dispersion strengthened steel—An ab initio study. J. Alloys Compd. 2018, 767, 122–130. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.-M.; Liu, Y.-C.; Huang, Y.; Ma, Z.-Q.; Li, H.-J. Effects of aluminum and titanium on the microstructure of ODS steels fabricated by hot pressing. Int. J. Miner. Metall. Mater. 2018, 25, 1156–1165. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takasugi, T. Mapping of 475 °C embrittlement in ferritic Fe–Cr–Al alloys. Scr. Mater. 2010, 63, 1104–1107. [Google Scholar] [CrossRef]
- Li, W.; Lu, S.; Hu, Q.-M.; Mao, H.; Johansson, B.; Vitos, L. The effect of Al on the 475 °C embrittlement of Fe–Cr alloys. Comput. Mater. Sci. 2013, 74, 101–106. [Google Scholar] [CrossRef]
- Száraz, Z.; Hähner, P.; Stráská, J.; Ripplinger, S. Effect of phase separation on tensile and Charpy impact properties of MA956 ODS steel. Mater. Sci. Eng. A 2017, 700, 425–437. [Google Scholar] [CrossRef]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high-Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar] [CrossRef]
- Xia, Y.P.; Wang, X.P.; Zhuang, Z.; Sun, Q.X.; Zhang, T.; Fang, Q.F.; Hao, T.; Liu, C.S. Microstructure and oxidation properties of 16Cr–5Al–ODS steel prepared by sol–gel and spark plasma sintering methods. J. Nucl. Mater. 2013, 432, 198–204. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, T.; Wang, X.P.; Fang, Q.F.; Liu, C.S. Effect of zirconium addition on the microstructure and mechanical properties of ODS ferritic steels containing aluminum. J. Nucl. Mater. 2014, 444, 462–468. [Google Scholar] [CrossRef]
- Rahmanifard, R.; Farhangi, H.; Novinrooz, A.J. Optimization of mechanical alloying parameters in 12YWT ferritic steel nanocomposite. Mater. Sci. Eng. A 2010, 527, 6853–6857. [Google Scholar] [CrossRef]
- Williams, C.A.; Unifantowicz, P.; Baluc, N.; Smith, G.D.W.; Marquis, E.A. The formation and evolution of oxide particles in oxide-dispersion-strengthened ferritic steels during processing. Acta Mater. 2013, 61, 2219–2235. [Google Scholar] [CrossRef]
- Waghmode, S.P.; Dabade, U.A. Optimization of process parameters during turning of Inconel 625. Mater. Today Proc. 2019, 19, 823–826. [Google Scholar] [CrossRef]
- Alinger, M.J.; Odette, G.R.; Hoelzer, D.T. On the role of alloy composition and processing parameters in nanocluster formation and dispersion strengthening in nanostuctured ferritic alloys. Acta Mater. 2009, 57, 392–406. [Google Scholar] [CrossRef]
- Gil, E.; Ordás, N.; García-Rosales, C.; Iturriza, I. ODS ferritic steels produced by an alternative route (STARS): Microstructural characterisation after atomisation, HIPping and heat treatments. Powder Metall. 2016, 59, 359–369. [Google Scholar] [CrossRef]
- Ordás, N.; Gil, E.; Cintins, A.; de Castro, V.; Leguey, T.; Iturriza, I.; Purans, J.; Anspoks, A.; Kuzmin, A.; Kalinko, A. The role of yttrium and titanium during the development of ODS ferritic steels obtained through the STARS route: TEM and XAS study. J. Nucl. Mater. 2018, 504, 8–22. [Google Scholar] [CrossRef]
- Pazos, D.; Cintins, A.; de Castro, V.; Fernández, P.; Hoffmann, J.; Vargas, W.G.; Leguey, T.; Purans, J.; Anspoks, A.; Kuzmin, A.; et al. ODS ferritic steels obtained from gas atomized powders through the STARS processing route: Reactive synthesis as an alternative to mechanical alloying. Nucl. Mater. Energy 2018, 17, 1–8. [Google Scholar] [CrossRef]
- Rieken, J.R.; Anderson, I.E.; Kramer, M.J.; Odette, G.R.; Stergar, E.; Haney, E. Reactive gas atomization processing for Fe-based ODS alloys. J. Nucl. Mater. 2012, 428, 65–75. [Google Scholar] [CrossRef]
- Gil, E.; Cortés, J.; Iturriza, I.; Ordás, N. XPS and SEM analysis of the surface of gas atomized powder precursor of ODS ferritic steels obtained through the STARS route. Appl. Surf. Sci. 2018, 427, 182–191. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, S.; Zou, L.; Schneider, Y.; Schmauder, S.; Wang, M. Enhanced strength and high temperature resistance of 25Cr20Ni ODS austenitic alloy through thermo-mechanical treatment and addition of Mo. Fusion Eng. Des. 2019, 138, 175–182. [Google Scholar] [CrossRef]
- Song, M.; Sun, C.; Fan, Z.; Chen, Y.; Zhu, R.; Yu, K.Y.; Hartwig, K.T.; Wang, H.; Zhang, X. A roadmap for tailoring the strength and ductility of ferritic/martensitic T91 steel via thermo-mechanical treatment. Acta Mater. 2016, 112, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Wang, H.; Qiu, S.; Zhang, Y.; He, K.; Wu, B. Cold-rolling & annealing process for nuclear grade wrought FeCrAl cladding alloy to enhance the strength and ductility. J. Mater. Process. Technol. 2020, 277, 116434. [Google Scholar]
- Karlsson, H.; Nyborg, L.; Berg, S. Surface chemical analysis of prealloyed water atomised steel powder. Powder Metall. 2005, 48, 51–58. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, I.S.; Kim, J.H. Design of alumina forming FeCrAl steels for lead or lead–bismuth cooled fast reactors. J. Nucl. Mater. 2013, 441, 650–660. [Google Scholar] [CrossRef]
- Wu, Y.; Haney, E.M.; Cunningham, N.J.; Odette, G.R. Transmission electron microscopy characterization of the nanofeatures in nanostructured ferritic alloy MA957. Acta Mater. 2012, 60, 3456–3468. [Google Scholar] [CrossRef]
- Hirata, A.; Fujita, T.; Liu, C.T.; Chen, M.W. Characterization of oxide nanoprecipitates in an oxide dispersion strengthened 14YWT steel using aberration-corrected STEM. Acta Mater. 2012, 60, 5686–5696. [Google Scholar] [CrossRef]
- Bai, Q.; Lin, J.; Tian, G.; Zou, J.; Dean, T.A. Review and Analysis of Powder Prior Boundary (PPB) Formation in Powder Metallurgy Processes for Nickel-based Super Alloys. J. Powder Metall. Min. 2015, 4, 2. [Google Scholar]
- Hou, J.; Dong, J.X.; Yao, Z.H.; Jiang, H.; Zhang, M.C. Influences of PPB, PPB affect zone, grain boundary and phase boundary on crack propagation path for a P/M superalloy FGH4096. Mater. Sci. Eng. A 2018, 724, 17–28. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Z.; Li, M.; Wang, M.; Zhang, G. Microstructure characterization and tensile properties of 18Cr–4Al-oxide dispersion strengthened ferritic steel. J. Alloys Compd. 2015, 648, 39–45. [Google Scholar] [CrossRef]
- Kasada, R.; Toda, N.; Yutani, K.; Cho, H.S.; Kishimoto, H.; Kimura, A. Pre- and post-deformation microstructures of oxide dispersion strengthened ferritic steels. J. Nucl. Mater. 2007, 367–370, 222–228. [Google Scholar] [CrossRef]
- Sun, Z.; Edmondson, P.D.; Yamamoto, Y. Effects of Laves phase particles on recovery and recrystallization behaviors of Nb-containing FeCrAl alloys. Acta Mater. 2018, 144, 716–727. [Google Scholar] [CrossRef]
- Martínez-de-Guerenu, A.; Jorge-Badiola, D.; Gutiérrez, I. Assessing the recovery and recrystallization kinetics of cold rolled microalloyed steel through coercive field measurements. Mater. Sci. Eng. A 2017, 691, 42–50. [Google Scholar] [CrossRef]
- Guan, D.; Rainforth, W.M.; Gao, J.; Sharp, J.; Wynne, B.; Ma, L. Individual effect of recrystallisation nucleation sites on texture weakening in a magnesium alloy: Part 1- double twins. Acta Mater. 2017, 135, 14–24. [Google Scholar] [CrossRef]
- Dadé, M.; Malaplate, J.; Garnier, J.; De Geuser, F.; Barcelo, F.; Wident, P.; Deschamps, A. Influence of microstructural parameters on the mechanical properties of oxide dispersion strengthened Fe-14Cr steels. Acta Mater. 2017, 127, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Boegelein, T.; Louvis, E.; Dawson, K.; Tatlock, G.J.; Jones, A.R. Characterisation of a complex thin walled structure fabricated by selective laser melting using a ferritic oxide dispersion strengthened steel. Mater. Charact. 2016, 112, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Morris, D.G.; Muñoz-Morris, M.A. Nanoprecipitation of oxide particles and related high strength in oxide-dispersion-strengthened iron–aluminium–chromium intermetallics. Acta Mater. 2013, 61, 4636–4647. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.Z.; Zou, T.T.; Zhang, S.; Sheng, L.Z. Identification of Oxide Phases in Oxide Dispersion Strengthened PM2000 Steel. ISIJ Int. 2013, 53, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Li, Y.; Zhu, L.; Liu, Y.; Lei, X.; Wang, G.; Wu, Y.; Mi, Z.; Liu, J.; Wang, H.; et al. Evading the strength-ductility trade-off dilemma in steel through gradient hierarchical nanotwins. Nat. Commun. 2014, 5, 3580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, G.J.; Jiang, F.; Ding, X.D.; Sun, Y.J.; Sun, J.; Ma, E. Nanostructured high-strength molybdenum alloys with unprecedented tensile ductility. Nat. Mater. 2013, 12, 344–350. [Google Scholar] [CrossRef]
- Zerbst, U.; Madia, M.; Klinger, C.; Bettge, D.; Murakami, Y. Defects as a root cause of fatigue failure of metallic components. II: Non-metallic inclusions. Eng. Fail. Anal. 2019, 98, 228–239. [Google Scholar] [CrossRef]
- Yamakov, V.; Wolf, D.; Phillpot, S.R.; Mukherjee, A.K.; Gleiter, H. Deformation-mechanism map for nanocrystalline metals by molecular-dynamics simulation. Nat. Mater. 2004, 3, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Y.; Yu, L.; Ma, Z.; Guo, Q.; Huang, Y.; Li, H. Microstructure characteristic and mechanical property of transformable 9Cr-ODS steel fabricated by spark plasma sintering. Mater. Des. 2017, 132, 158–169. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liao, X.Z.; Cheng, S.; Ma, E.; Zhu, Y.T.J.A.M. Simultaneously Increasing the Ductility and Strength of Nanostructured Alloys. Adv. Mater. 2006, 18, 2280–2283. [Google Scholar] [CrossRef]
- Sha, G.; Wang, Y.B.; Liao, X.Z.; Duan, Z.C.; Ringer, S.P.; Langdon, T.G. Influence of equal-channel angular pressing on precipitation in an Al–Zn–Mg–Cu alloy. Acta Mater. 2009, 57, 3123–3132. [Google Scholar] [CrossRef]
- Rao, P.N.; Singh, D.; Brokmeier, H.-G.; Jayaganthan, R. Effect of ageing on tensile behavior of ultrafine grained Al 6061 alloy. Mater. Sci. Eng. A 2015, 641, 391–401. [Google Scholar] [CrossRef]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liu, P.Z.; Li, J.J.; Liaw, P.K.; Spieckermann, F.; Kiener, D.; Qiao, J.W.; Eckert, J. Universally scaling Hall-Petch-like relationship in metallic glass matrix composites. Int. J. Plast. 2018, 105, 225–238. [Google Scholar] [CrossRef]
- Armstrong, R.; Codd, I.; Douthwaite, R.M.; Petch, N.J. The plastic deformation of polycrystalline aggregates. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1962, 7, 45–58. [Google Scholar] [CrossRef]
- Yu, H.; Xin, Y.; Wang, M.; Liu, Q. Hall-Petch relationship in Mg alloys: A review. J. Mater. Sci. Technol. 2018, 34, 248–256. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, T.S.; Hoelzer, D.T.; Kim, S.-W.; Lee, B.H. Temperature dependence of strengthening mechanisms in the nanostructured ferritic alloy 14YWT: Part I—Mechanical and microstructural observations. Mater. Sci. Eng. A 2013, 559, 101–110. [Google Scholar] [CrossRef]
- Chauhan, A.; Bergner, F.; Etienne, A.; Aktaa, J.; de Carlan, Y.; Heintze, C.; Litvinov, D.; Hernandez-Mayoral, M.; Oñorbe, E.; Radiguet, B.; et al. Microstructure characterization and strengthening mechanisms of oxide dispersion strengthened (ODS) Fe-9%Cr and Fe-14%Cr extruded bars. J. Nucl. Mater. 2017, 495, 6–19. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: II Characteristics of the L ders Deformation. Proc. Phys. Soc. Sect. B 1951, 64, 742–747. [Google Scholar] [CrossRef]
- Hansen, N. Hall–Petch relation and boundary strengthening. Scr. Mater. 2004, 51, 801–806. [Google Scholar] [CrossRef]
- Ukai, S.; Yano, Y.; Inoue, T.; Sowa, T. Solid-solution strengthening by Al and Cr in FeCrAl oxide-dispersion-strengthened alloys. Mater. Sci. Eng. A 2021, 812, 141076. [Google Scholar] [CrossRef]



| Materials | Cr | Al | Y | Si | Ti | Fe |
|---|---|---|---|---|---|---|
| FeCrAl alloy | 22.0 | 5.0 | 0.15 | 0.2 | 0.1 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, H.; Zhai, Y.; Yan, Y.; Chen, Y.; Qin, W.; Wang, T.; Cao, R. Effect of Thermo-Mechanical Treatment on the Microstructure and Tensile Properties of the Fe-22Cr-5Al-0.1Y Alloy. Materials 2021, 14, 5696. https://doi.org/10.3390/ma14195696
Che H, Zhai Y, Yan Y, Chen Y, Qin W, Wang T, Cao R. Effect of Thermo-Mechanical Treatment on the Microstructure and Tensile Properties of the Fe-22Cr-5Al-0.1Y Alloy. Materials. 2021; 14(19):5696. https://doi.org/10.3390/ma14195696
Chicago/Turabian StyleChe, Hongyan, Yazhong Zhai, Yingjie Yan, Yongqing Chen, Wei Qin, Tiejun Wang, and Rui Cao. 2021. "Effect of Thermo-Mechanical Treatment on the Microstructure and Tensile Properties of the Fe-22Cr-5Al-0.1Y Alloy" Materials 14, no. 19: 5696. https://doi.org/10.3390/ma14195696






