Anti-Caking Coatings for Improving the Useful Properties of Ammonium Nitrate Fertilizers with Composition Modeling Using Box–Behnken Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Process
2.3. Analysis of Fertilizers
2.4. Analysis of Coatings
3. Results and Discussion
3.1. Analysis of Fertilizers
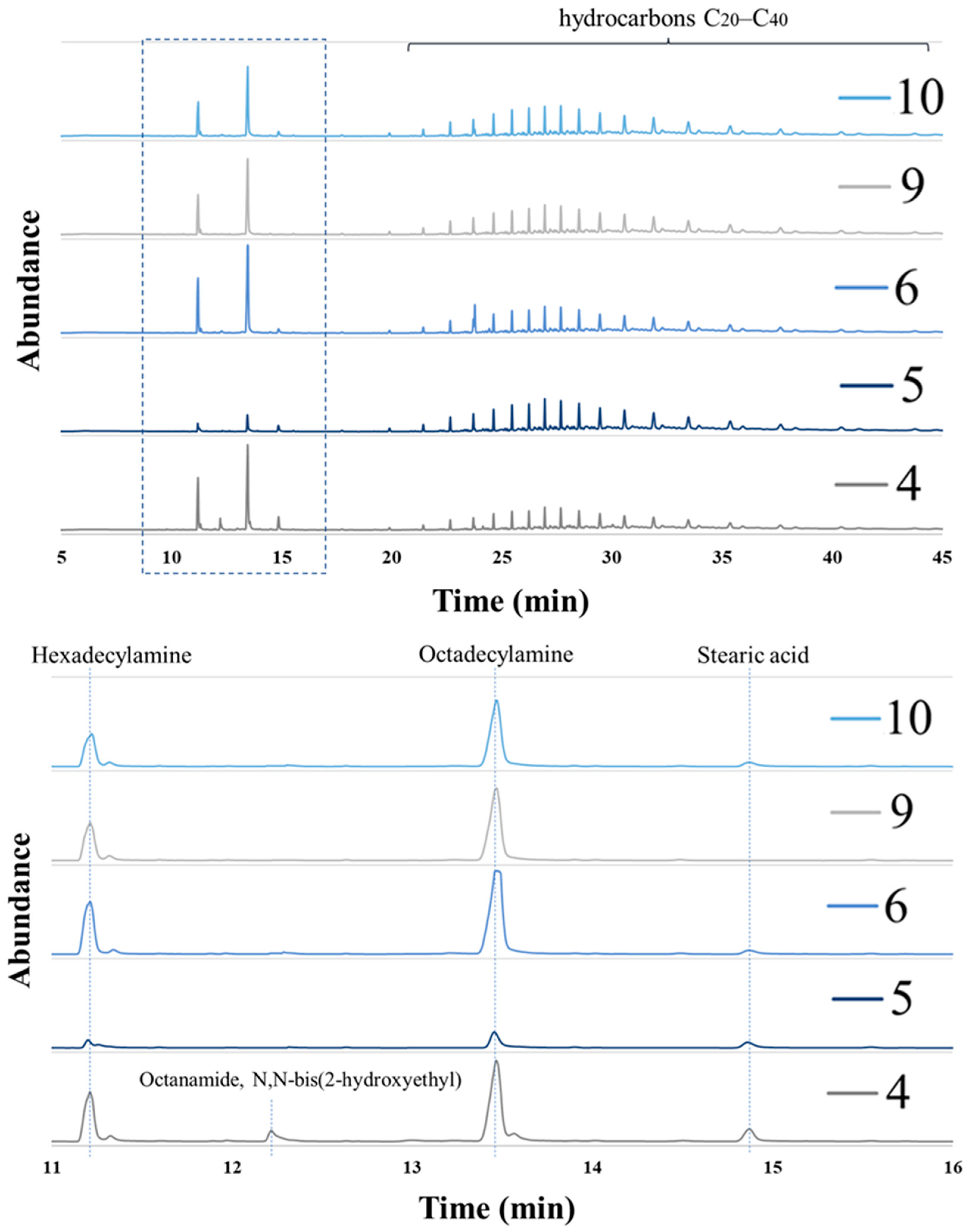
3.2. Analysis of Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wahl, M.; Bröckel, U.; Brendel, L.; Feise, H.J.; Weigl, B.; Röck, M.; Schwedes, J. Understanding powder caking: Predicting caking strength from individual particle contacts. Powder Technol. 2008, 188, 147–152. [Google Scholar] [CrossRef]
- Walker, G.M.; Holland, C.R.; Ahmad, M.N.; Fox, J.N.; Kells, A.G. Granular Fertilizer Agglomeration in Accelerated Caking Tests. Ind. Eng. Chem. Res. 1999, 38, 4100–4103. [Google Scholar] [CrossRef]
- Komunjer, L.; Affolter, C. Absorption–evaporation kinetics of water vapour on highly hygroscopic powder: Case of ammonium nitrate. Powder Technol. 2005, 157, 67–71. [Google Scholar] [CrossRef]
- Christakis, N.; Wang, J.; Patel, M.K.; Bradley, M.S.A.; Leaper, M.C.; Cross, M. Aggregation and caking processes of granular materials: Continuum model and numerical simulation with application to sugar. Adv. Powder Technol. 2006, 17, 543–565. [Google Scholar] [CrossRef]
- Videla, A.R.; Polanco, C.; Escalona, N. Phenomenological model of the effect of organic polymer addition on the control of ammonium nitrate caking. Powder Technol. 2017, 315, 114–125. [Google Scholar] [CrossRef]
- Chen, M.; Wu, S.; Xu, S.; Yu, B.; Shilbayeh, M.; Liu, Y.; Zhu, X.; Wang, J.; Gong, J. Caking of crystals: Characterization, mechanisms and prevention. Powder Technol. 2018, 337, 51–67. [Google Scholar] [CrossRef]
- Tyc, A.; Hoffmann, J.; Biskupski, A. Anti-caking agents for ammonium nitrate fertilizers. Part 1. Caking phenomenon. Przem. Chem. 2019, 98, 771–776. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, B.H. Study on Surface Modification of Ammonium Nitrate. Adv. Mater. Res. 2011, 399–401, 1989–1993. [Google Scholar] [CrossRef]
- Elzaki, B.I.; Zhang, Y.J. Coating Methods for Surface Modification of Ammonium Nitrate: A Mini-Review. Materials 2016, 9, 502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gezerman, A.O.; Corbacioglu, B.D.; Cevik, H. Improvement of Surface Features of Nitrogenous Fertilisers and Influence of Surfactant Composition on Fertiliser Surface. Int. J. Chem. 2011, 3, 201–209. [Google Scholar] [CrossRef]
- Rutland, D.W. Fertilizer caking: Mechanisms, influential factors, and methods of prevention. Nutr. Cycl. Agroecosyst. 1991, 30, 99–114. [Google Scholar] [CrossRef]
- Speight, J.G. Industrial Inorganic Chemistry. In Environmental Inorganic Chemistry for Engineers, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 111–169. [Google Scholar] [CrossRef]
- Popławski, D.; Hoffmann, J.; Hoffmann, K. Effect of carbonate minerals on the thermal stability of fertilisers containing ammonium nitrate. J. Therm. Anal. Calorim. 2016, 124, 1561–1574. [Google Scholar] [CrossRef] [Green Version]
- Shearon, H.W.; Dunwoody, W.B. Ammonium Nitrate. Ind. Eng. Chem. 1953, 45, 496–504. [Google Scholar] [CrossRef]
- Tyc, A.; Hoffmann, J.; Biskupski, A. Anti-caking agents for ammonium nitrate fertilizers. Part 2. Commercial products. Przem. Chem. 2019, 98, 948–952. [Google Scholar] [CrossRef]
- Babrauskas, V. Explosions of ammonium nitrate fertilizer in storage or transportation are preventable accidents. J. Hazard. Mater. 2016, 304, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Laboureur, D.M.; Han, Z.; Harding, B.; Pineda, A.; Pittman, W.C.; Rosas, C.; Jiang, J.; Mannan, M.S. Case study and lessons learned from the ammonium nitrate explosion at the West Fertilizer facility. J. Hazard. Mater. 2016, 308, 164–172. [Google Scholar] [CrossRef]
- El Diwani, G.; Hawash, S.; Elibiari, N.; Imam, I. Treatment of Ammonium Nitrate for Cake Prevention. Ind. Eng. Chem. Res. 1994, 33, 1620–1622. [Google Scholar] [CrossRef]
- Buczkowski, D. Explosive Properties of Mixtures of Ammonium Nitrate (V) and Materials of Plant Origin—Danger of Unintended Explosion. Cent. Eur. J. Energ. Mater. 2014, 11, 115–127. [Google Scholar]
- Liang, R.; Liu, M. Preparation and Properties of Coated Nitrogen Fertilizer with Slow Release and Water Retention. Ind. Eng. Chem. Res. 2006, 45, 8610–8616. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S.; Xie, L.; Zhang, X.; Wang, Y. Novel Slow-Release Multielement Compound Fertilizer with Hydroscopicity and Moisture Preservation. Ind. Eng. Chem. Res. 2010, 49, 4546–4552. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.; Zhao, C. Bio-based Interpenetrating Network Polymer Composites from Locust Sawdust as Coating Material for Environmentally Friendly Controlled-Release Urea Fertilizers. J. Agric. Food Chem. 2016, 64, 5692–5700. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Xu, J.; Zhao, Q. Biomimetic Superhydrophobic Biobased Polyurethane-Coated Fertilizer with Atmosphere “Outerwear”. ACS Appl. Mater. Interfaces 2017, 9, 15868–15879. [Google Scholar] [CrossRef]
- Tian, H.; Liu, Z.; Zhang, M.; Guo, Y.; Zheng, L.; Li, Y.C. Biobased Polyurethane, Epoxy Resin, and Polyolefin Wax Composite Coating for Controlled-Release Fertilizer. ACS Appl. Mater. Interfaces 2019, 11, 5380–5392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Tong, Z.; Gao, B.; Gao, N.; Shen, T.; Wan, Y.; Yu, Z.; Liu, L.; Ma, X.; et al. Self-Assembly of Hydrophobic and Self-Healing Bionanocomposite-Coated Controlled-Release Fertilizers. ACS Appl. Mater. Interfaces 2020, 12, 27598–27606. [Google Scholar] [CrossRef] [PubMed]
- Lubkowski, K.; Smorowska, A.; Grzmil, B.; Kozłowska, A. Controlled-Release Fertilizer Prepared Using a Biodegradable Aliphatic Copolyester of Poly(butylene succinate) and Dimerized Fatty Acid. J. Agric. Food Chem. 2015, 63, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, S.; Fernández-Pérez, M.; Villafranca-Sánchez, M.; González-Pradas, E.; Flores-Céspedes, F. Controlled Release of Ammonium Nitrate from Ethylcellulose Coated Formulations. Ind. Eng. Chem. Res. 2007, 46, 3304–3311. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, F.; Yang, L.; Li, X.; Wang, J.; Linghu, C.; Luo, Z. Low-Temperature Flowable Poly(lactic acid)/Polycaprolactone Blends for the Solvent-Free Preparation of Slow-Released Urea Fertilizer in a Thermal Shear Field. Ind. Eng. Chem. Res. 2020, 59, 20601–20611. [Google Scholar] [CrossRef]
- Elzaki, B.I.; Zhang, Y.J. Anti-hygroscopic surface modification of ammonium nitrate (NH4NO3) coated by surfactants. Arab. J. Chem. 2018, 13, 3460–3473. [Google Scholar] [CrossRef]
- Nagayama, S.; Katoh, K.; Higashi, E.; Hayashi, M.; Kumagae, K.; Habu, H.; Wada, Y.; Nakano, K.; Arai, M. Moisture Proofing of Spray Dried Particles Comprising Ammonium Nitrate/Potassium Nitrate/Polymer. Propellants Explos. Pyrotech. 2015, 40, 544–550. [Google Scholar] [CrossRef]
- Gezerman, A.O.; Çorbacıoğlu, B.D. Effects of sodium silicate, calcium carbonate, and silicic acid on ammonium nitrate degradation, and analytical investigations of the degradation process on an industrial scale. Chem. Ind. Chem. Eng. Q. 2015, 21, 359–367. [Google Scholar] [CrossRef]
- Gezerman, A.O. A novel industrial-scale strategy to prevent degradation and caking of ammonium nitrate. Heliyon 2020, 6, e03628. [Google Scholar] [CrossRef] [PubMed]
- Krauklis, A.E.; Kreicbergs, I.; Dreyer, I. Modified ginstling–brounshtein model for wet precipitation synthesis of hydroxy-apatite: Analytical and experimental study. Acta Bioeng. Biomech. 2018, 20, 47–57. [Google Scholar] [CrossRef]
- Pérez-Beltrán, C.H.; García-Guzmán, J.J.; Ferreira, B.; Estévez-Hernández, O.; López-Iglesias, D.; Cubillana-Aguilera, L.; Link, W.; Stănică, N.; da Costa, A.M.R.; Palacios-Santander, J.M. One-minute and green synthesis of magnetic iron oxide nanoparticles assisted by design of experiments and high energy ultrasound: Application to biosensing and immunoprecipitation. Mater. Sci. Eng. C 2021, 123, 112023. [Google Scholar] [CrossRef] [PubMed]
- Beres, D.L.; Hawkins, D.M. Plackett–Burman technique for sensitivity analysis of many-parametered models. Ecol. Model. 2001, 141, 171–183. [Google Scholar] [CrossRef]
- Miller, A.; Sitter, R. Choosing columns from the 12-run Plackett–Burman design. Stat. Probab. Lett. 2004, 67, 193–201. [Google Scholar] [CrossRef]
- Garud, S.S.; Karimi, I.A.; Kraft, M. Design of computer experiments: A review. Comput. Chem. Eng. 2017, 106, 71–95. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Hamed, E.; Sakr, A. Application of multiple response optimization technique to extended release formulations design. J. Control. Release 2001, 73, 329–338. [Google Scholar] [CrossRef]
- Robinson, T.J. Box-Behnken Designs. In Wiley StatsRef Stat. Ref. Online; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–7. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Javed, N.; Alam, S.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Nayak, A.K.; Beg, S. Purple heart plant leaves extract-mediated silver nanoparticle synthesis: Optimization by Box-Behnken design. Mater. Sci. Eng. C 2019, 99, 1105–1114. [Google Scholar] [CrossRef]
- Peng, X.; Yang, G.; Shi, Y.; Zhou, Y.; Zhang, M.; Li, S. Box-Behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Czyrski, A.; Sznura, J. The application of Box-Behnken-Design in the optimization of HPLC separation of fluoroquinolones. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyc, A.; Nieweś, D.; Penkala, S.; Grzesik, R.; Hoffmann, K.; Hoffmann, J. Influence of Anti-Caking Agents on the Highly Effective Organic Coatings for Preventing the Caking of Ammonium Nitrate Fertilizers. Coatings 2020, 10, 1093. [Google Scholar] [CrossRef]
- Tyc, A.; Penkala, S.; Biegun, M.; Nieweś, D.; Huculak-Mączka, M.; Hoffmann, K. The effectiveness of commercial anticaking agents for ammonium nitrate fertilizers. Ecol. Chem. Eng. A 2020, 26, 127–135. [Google Scholar] [CrossRef]




| Levels | Independent Parameters | ||
|---|---|---|---|
| Fatty Amine Content (X1) (Mass %) | Stearic Acid Content (X2) (Mass %) | Surfactant Content (X3) (Mass %) | |
| −1 | 5 | 0 | 0 |
| 0 | 15 | 4 | 8 |
| 1 | 25 | 8 | 16 |
| Run | Independent Variables | Responses | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | |
| 1 | 5 | 0 | 8 | 20.6 ± 7.3 | 75.3 ± 2.1 |
| 2 | 25 | 0 | 8 | 83.8 ± 5.0 | 95.9 ± 0.2 |
| 3 | 5 | 8 | 8 | 43.8 ± 3.4 | 86.2 ± 2.7 |
| 4 | 25 | 8 | 8 | 89.3 ± 2.5 | 94.3 ± 0.6 |
| 5 | 5 | 4 | 0 | 96.6 ± 0.5 | 98.3 ± 1.6 |
| 6 | 25 | 4 | 0 | 95.2 ± 1.8 | 99.2 ± 0.7 |
| 7 | 5 | 4 | 16 | 38.3 ± 2.4 | 83.1 ± 0.7 |
| 8 | 25 | 4 | 16 | 75.5 ± 5.4 | 94.1 ± 1.5 |
| 9 | 15 | 0 | 0 | 98.9 ± 1.1 | 100 ± 0 |
| 10 | 15 | 4 | 0 | 98.1 ± 1.7 | 100 ± 0 |
| 11 | 15 | 0 | 16 | 31.0 ± 3.7 | 83.0 ± 0.8 |
| 12 | 15 | 8 | 16 | 43.2 ± 1.8 | 95.7 ± 0.3 |
| 13 | 15 | 4 | 8 | 75.6 ± 1.1 | 98.6 ± 2.4 |
| 14 | 15 | 4 | 8 | 78.1 ± 1.6 | 99.3 ± 1.2 |
| 15 | 15 | 4 | 8 | 76.7 ± 1.8 | 99.0 ± 0.9 |
| Parameter | Effect | Standard Error | p-Value | Remarks | Confidence Interval | |
|---|---|---|---|---|---|---|
| −95% | +95% | |||||
| X1 | 36.12 | 9.78 | 0.0141 | significant | 10.99 | 61.26 |
| X12 | 4.41 | 7.20 | 0.5665 | not significant | −14.08 | 22.91 |
| X2 | 10.02 | 9.78 | 0.3522 | not significant | −15.11 | 35.16 |
| X22 | 13.01 | 7.20 | 0.1303 | not significant | −5.48 | 31.51 |
| X3 | −50.20 | 9.77 | 0.0037 | significant | −75.33 | −25.07 |
| X32 | −4.01 | 7.20 | 0.6011 | not significant | −22.51 | 14.48 |
| X1∙X2 | −8.85 | 13.83 | 0.5503 | not significant | −44.39 | 26.69 |
| X1∙X3 | 19.30 | 13.83 | 0.2215 | not significant | −16.24 | 54.84 |
| X2∙X3 | 6.50 | 13.83 | 0.6580 | not significant | −29.04 | 42.04 |
| Parameter | Effect | Standard Error | p-Value | Remarks | Confidence Interval | |
|---|---|---|---|---|---|---|
| −95% | +95% | |||||
| X1 | 10.15 | 1.93 | 0.0033 | significant | 2.60 | 7.55 |
| X12 | 6.02 | 1.42 | 0.0081 | significant | 1.19 | 4.83 |
| X2 | 5.50 | 1.93 | 0.0356 | significant | 0.27 | 5.23 |
| X22 | 5.02 | 1.42 | 0.0165 | significant | 0.69 | 4.33 |
| X3 | −10.40 | 1.93 | 0.0029 | significant | −7.68 | −2.72 |
| X32 | −0.73 | 1.41 | 0.6289 | not significant | −2.19 | 1.46 |
| X1∙X2 | −6.25 | 2.72 | 0.0702 | not significant | −6.63 | 0.38 |
| X1∙X3 | 5.05 | 2.72 | 0.1229 | not significant | −0.98 | 6.03 |
| X2∙X3 | 6.35 | 2.72 | 0.0671 | not significant | −0.33 | 6.68 |
| Type of Sample | Optimal Content of Components (Mass %) | Predicted Anti–Caking Prevention Efficiency (%) | ||
|---|---|---|---|---|
| X1 | X2 | X3 | ||
| Fertilizer after granulation | 18.87 | 4.43 | 0 | 100 |
| Fertilizer after 30 days storage | 22.08 | 4.41 | 0 | 100 |
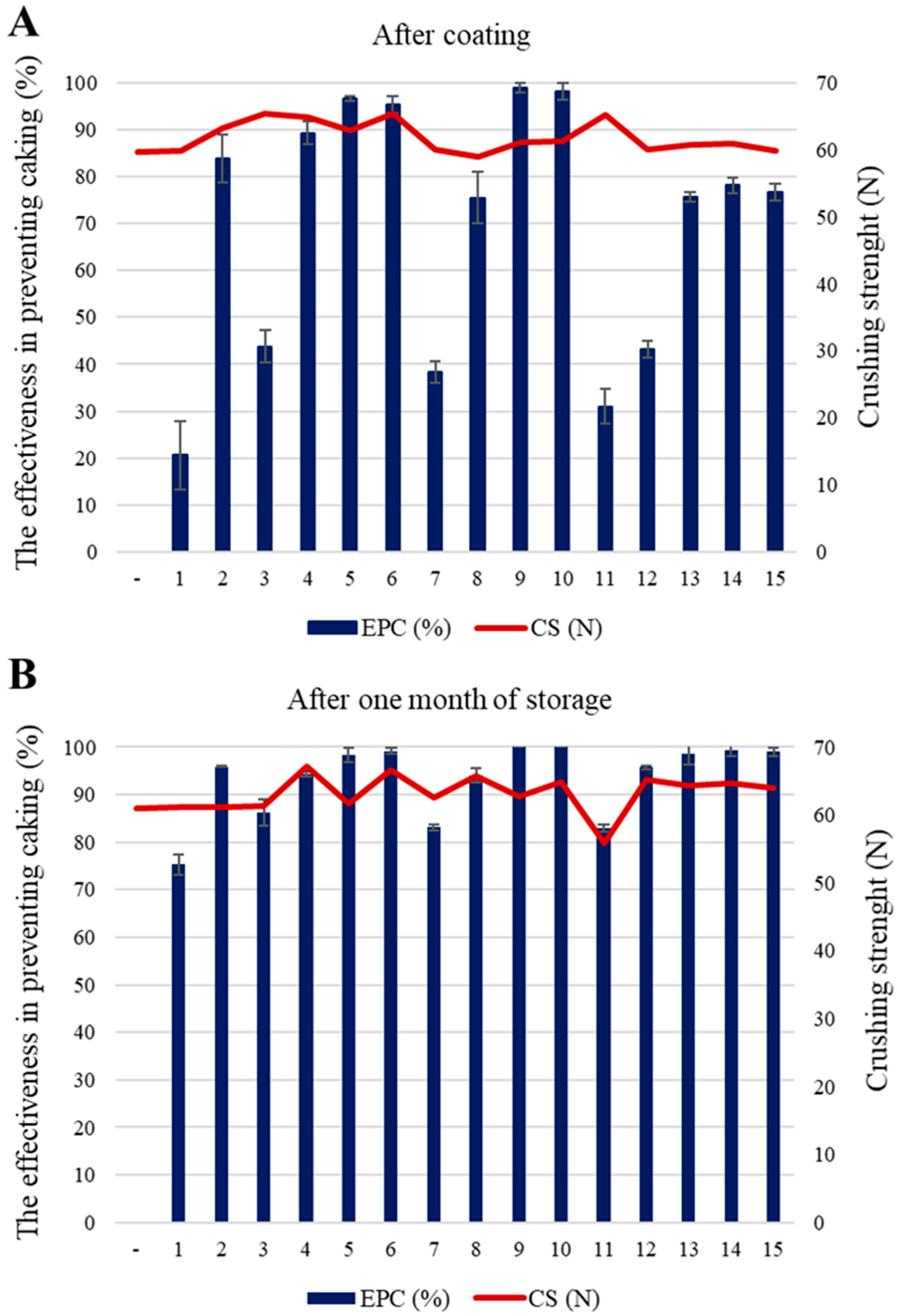
| Anti-Caking Coatings | Composition (X1:X2:X3) (Mass %) | After Coating | After One Month of Storage | ||
|---|---|---|---|---|---|
| EPC (%) | CS (N) | EPC (%) | CS (N) | ||
| - | - | - | 59.7 | - | 61.0 |
| 1 | 5:0:8 | 20.6 ± 7.3 | 59.8 | 75.3 ± 2.1 | 61.2 |
| 2 | 25:0:8 | 83.8 ± 5.0 | 63.2 | 95.9 ± 0.2 | 61.2 |
| 3 | 5:8:8 | 43.8 ± 3.4 | 65.4 | 86.2 ± 2.7 | 61.4 |
| 4 | 25:8:8 | 89.3 ± 2.5 | 64.8 | 94.3 ± 0.6 | 67.1 |
| 5 | 5:4:0 | 96.6 ± 0.5 | 62.9 | 98.3 ± 1.6 | 61.6 |
| 6 | 25:4:0 | 95.2 ± 1.8 | 65.4 | 99.2 ± 0.7 | 66,7 |
| 7 | 5:4:16 | 38.3 ± 2.4 | 60.0 | 83.1 ± 0.7 | 62.5 |
| 8 | 25:4:16 | 75.5 ± 5.4 | 59.0 | 94.1 ± 1.5 | 65.7 |
| 9 | 15:0:0 | 98.9 ± 1.1 | 61.1 | 100 ± 0 | 62.7 |
| 10 | 15:4:0 | 98.1 ± 1.7 | 61.2 | 100 ± 0 | 64.8 |
| 11 | 15:0:16 | 31.0 ± 3.7 | 65.2 | 83.0 ± 0.8 | 55.8 |
| 12 | 15:8:16 | 43.2 ± 1.8 | 60.1 | 95.7 ± 0.3 | 65.2 |
| 13 | 15:4:8 | 75.6 ± 1.1 | 60.7 | 98.6 ± 2.4 | 64.3 |
| 14 | 15:4:8 | 78.1 ± 1.6 | 60.9 | 99.3 ± 1.2 | 64.7 |
| 15 | 15:4:8 | 76.7 ± 1.8 | 59.8 | 99.0 ± 0.9 | 64.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyc, A.; Nieweś, D.; Pankalla, E.; Huculak-Mączka, M.; Hoffmann, K.; Hoffmann, J. Anti-Caking Coatings for Improving the Useful Properties of Ammonium Nitrate Fertilizers with Composition Modeling Using Box–Behnken Design. Materials 2021, 14, 5761. https://doi.org/10.3390/ma14195761
Tyc A, Nieweś D, Pankalla E, Huculak-Mączka M, Hoffmann K, Hoffmann J. Anti-Caking Coatings for Improving the Useful Properties of Ammonium Nitrate Fertilizers with Composition Modeling Using Box–Behnken Design. Materials. 2021; 14(19):5761. https://doi.org/10.3390/ma14195761
Chicago/Turabian StyleTyc, Aleksandra, Dominik Nieweś, Ewa Pankalla, Marta Huculak-Mączka, Krystyna Hoffmann, and Józef Hoffmann. 2021. "Anti-Caking Coatings for Improving the Useful Properties of Ammonium Nitrate Fertilizers with Composition Modeling Using Box–Behnken Design" Materials 14, no. 19: 5761. https://doi.org/10.3390/ma14195761
APA StyleTyc, A., Nieweś, D., Pankalla, E., Huculak-Mączka, M., Hoffmann, K., & Hoffmann, J. (2021). Anti-Caking Coatings for Improving the Useful Properties of Ammonium Nitrate Fertilizers with Composition Modeling Using Box–Behnken Design. Materials, 14(19), 5761. https://doi.org/10.3390/ma14195761






