Effect of Iron Phase on Calcination and Properties of Barium Calcium Sulfoaluminate Cement
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Experimental Methods
3. Results and Analysis
3.1. Effect of C4AF Content on Barium Calcium Sulfoaluminate Cement
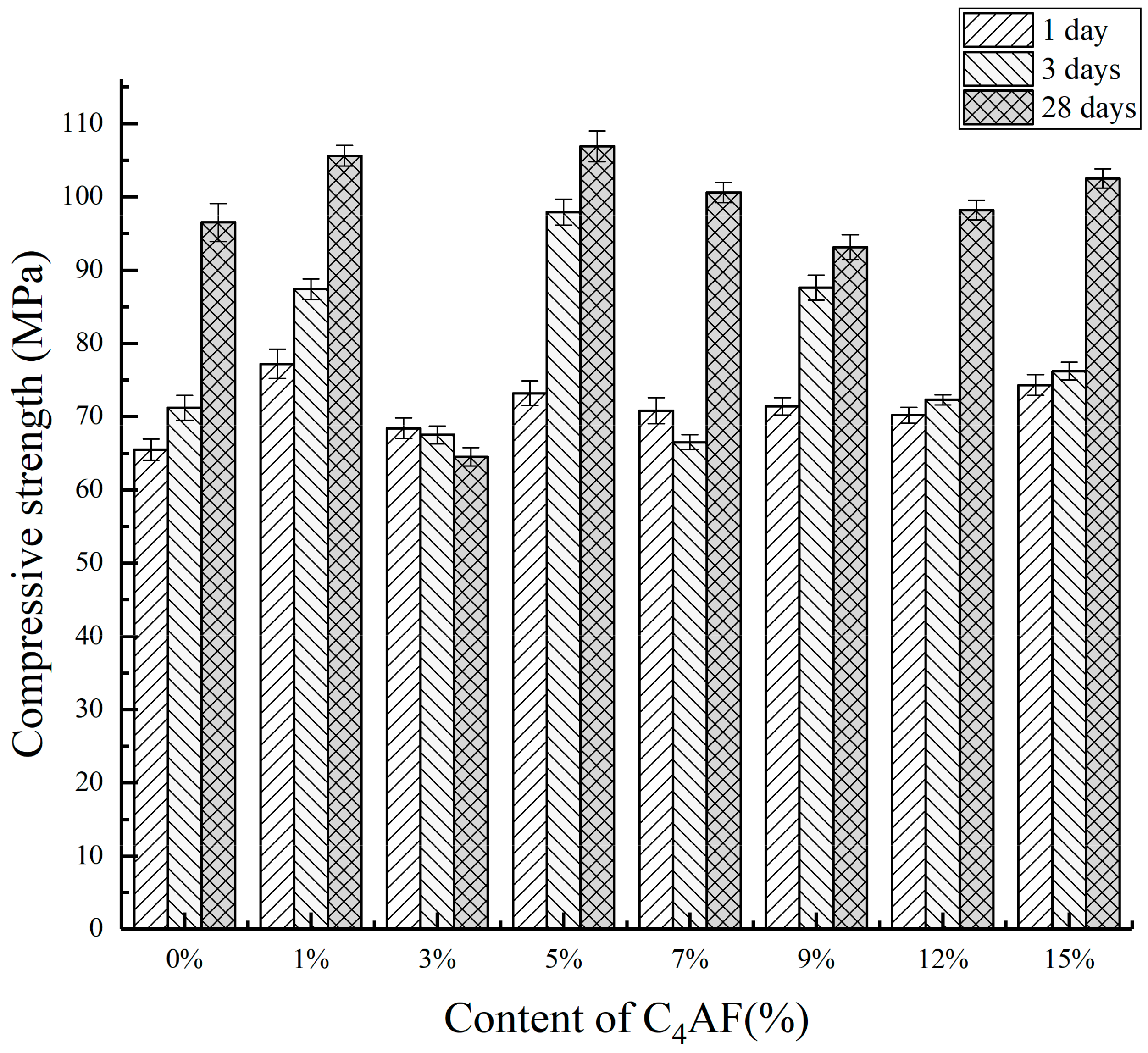
3.1.1. Mechanical Properties
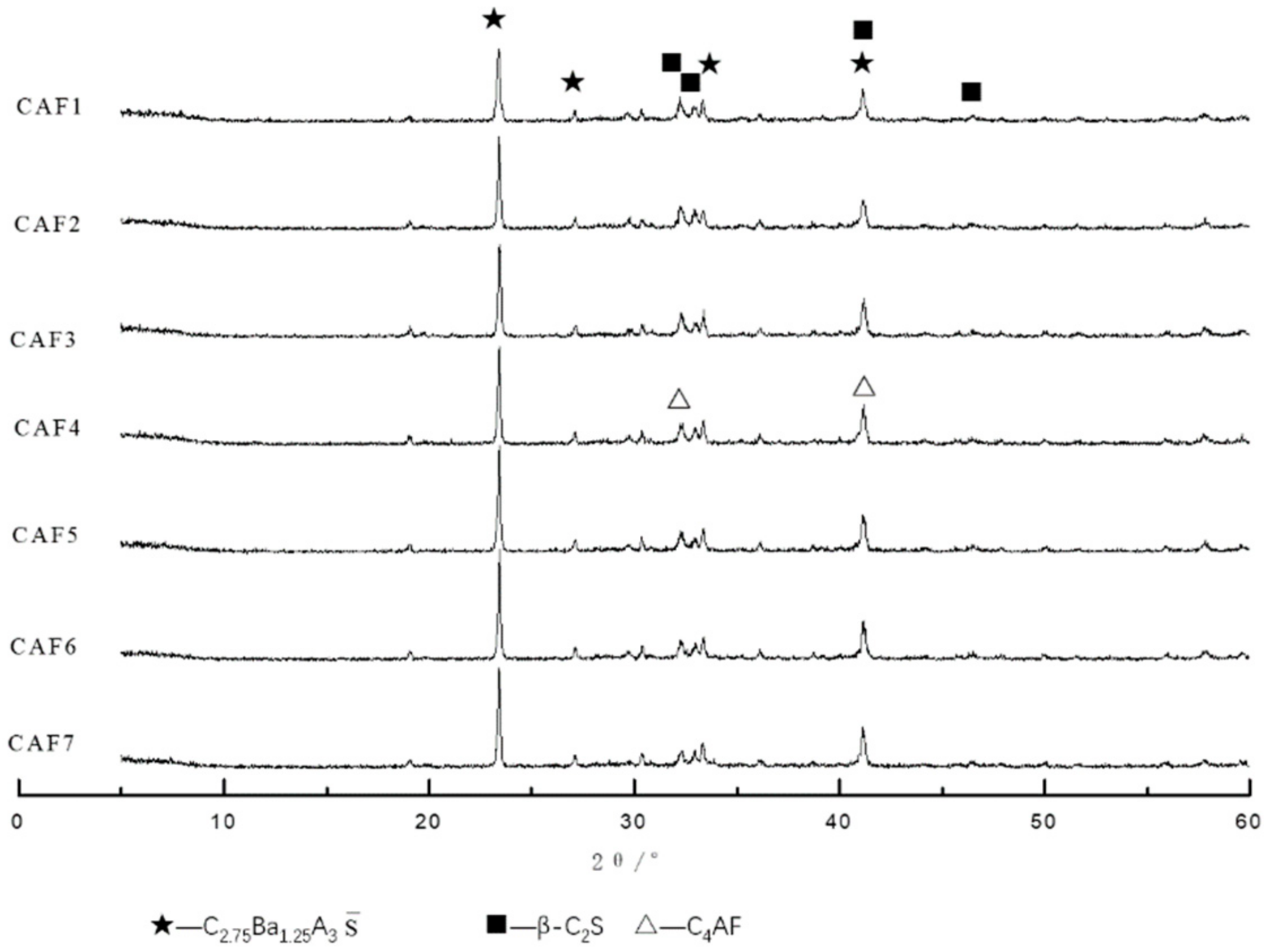
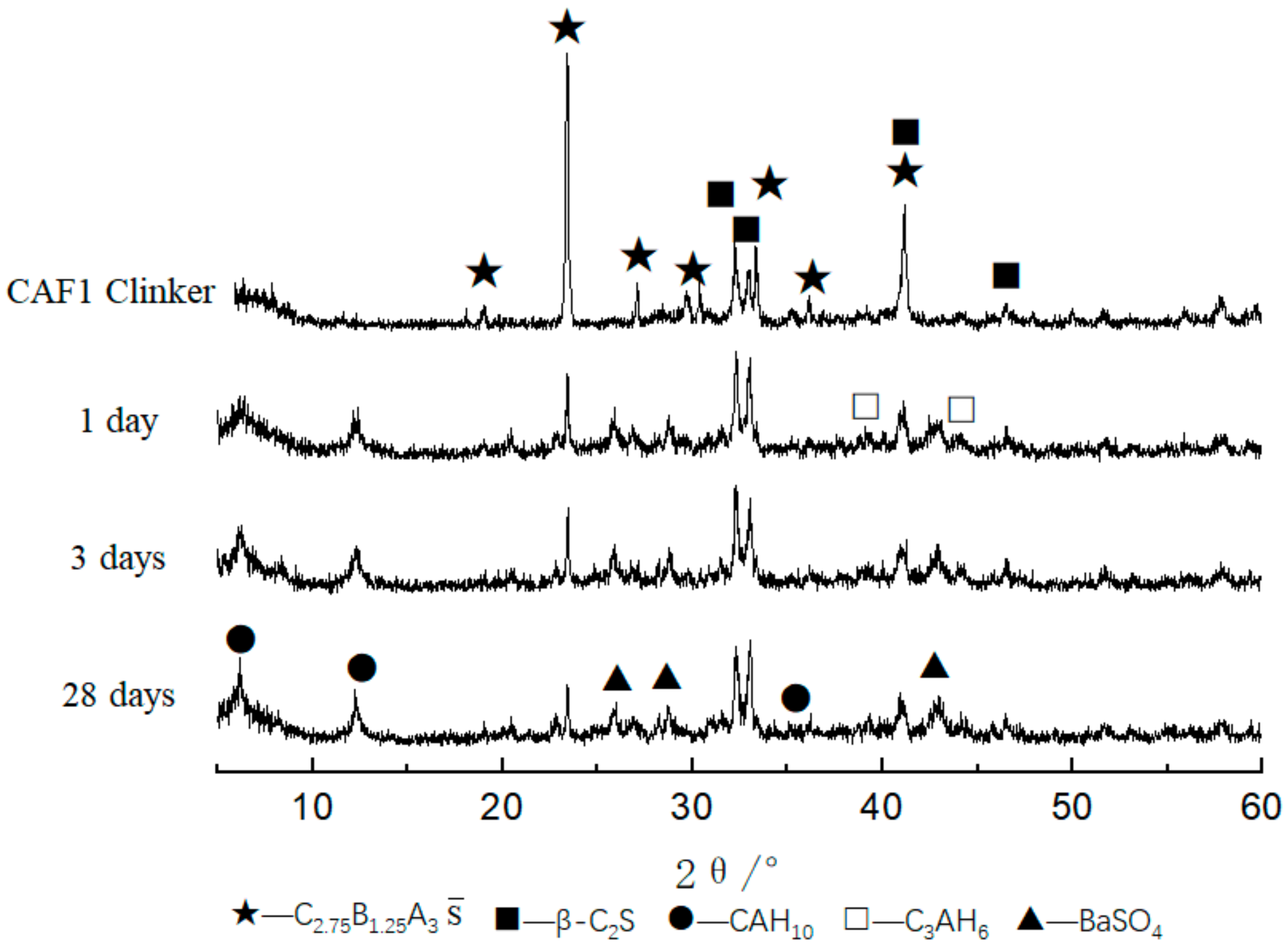
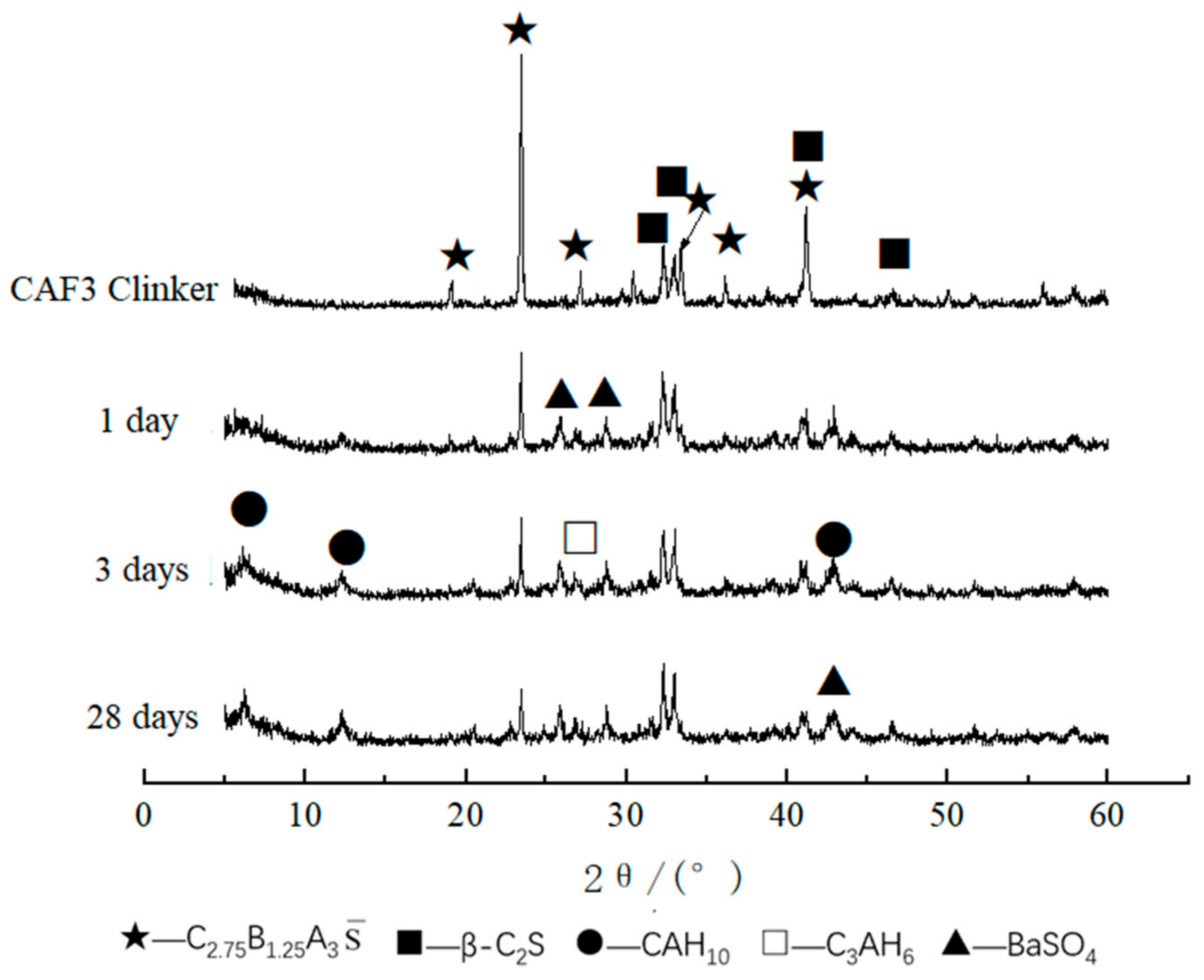
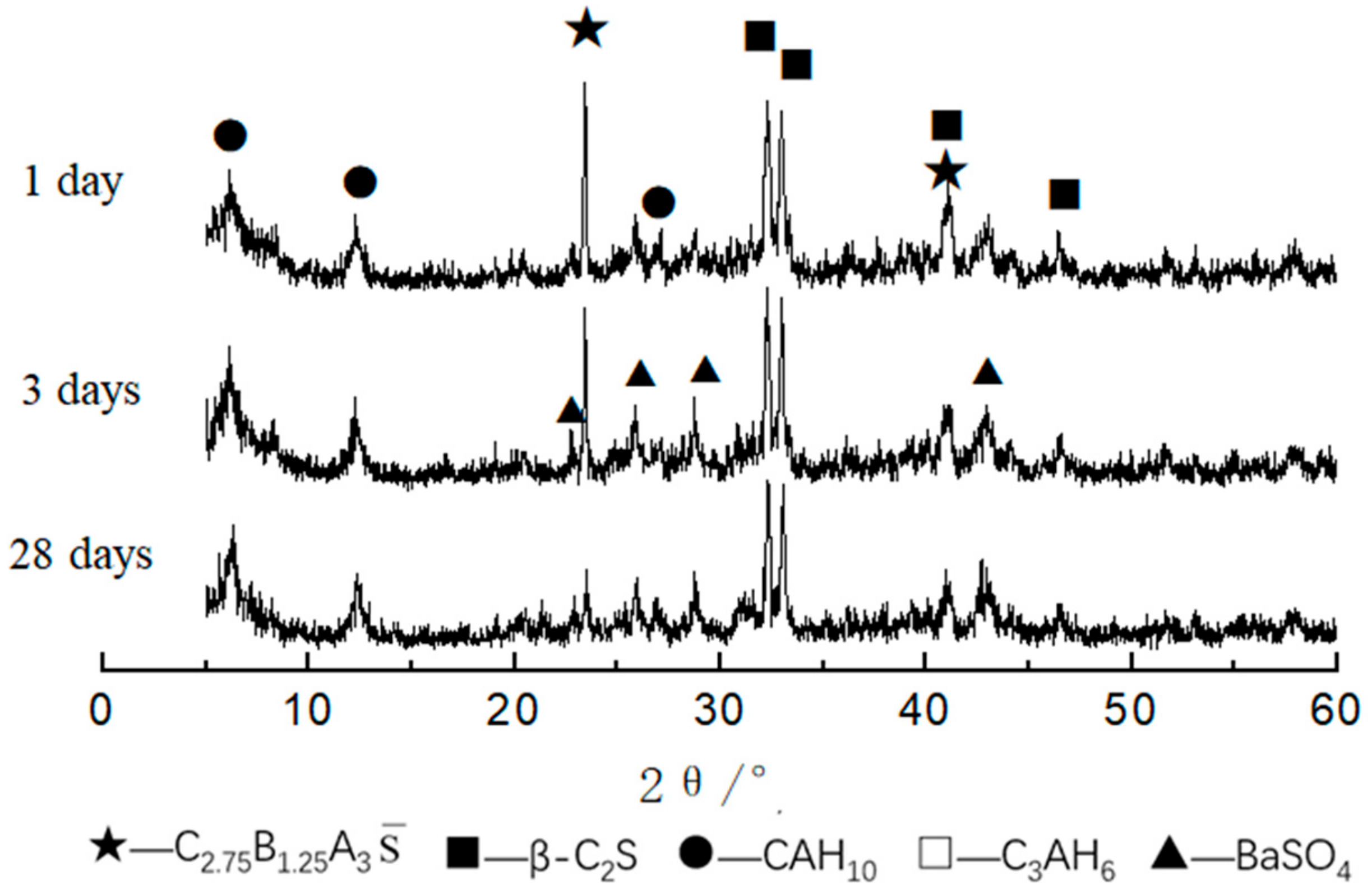
3.1.2. XRD Analysis
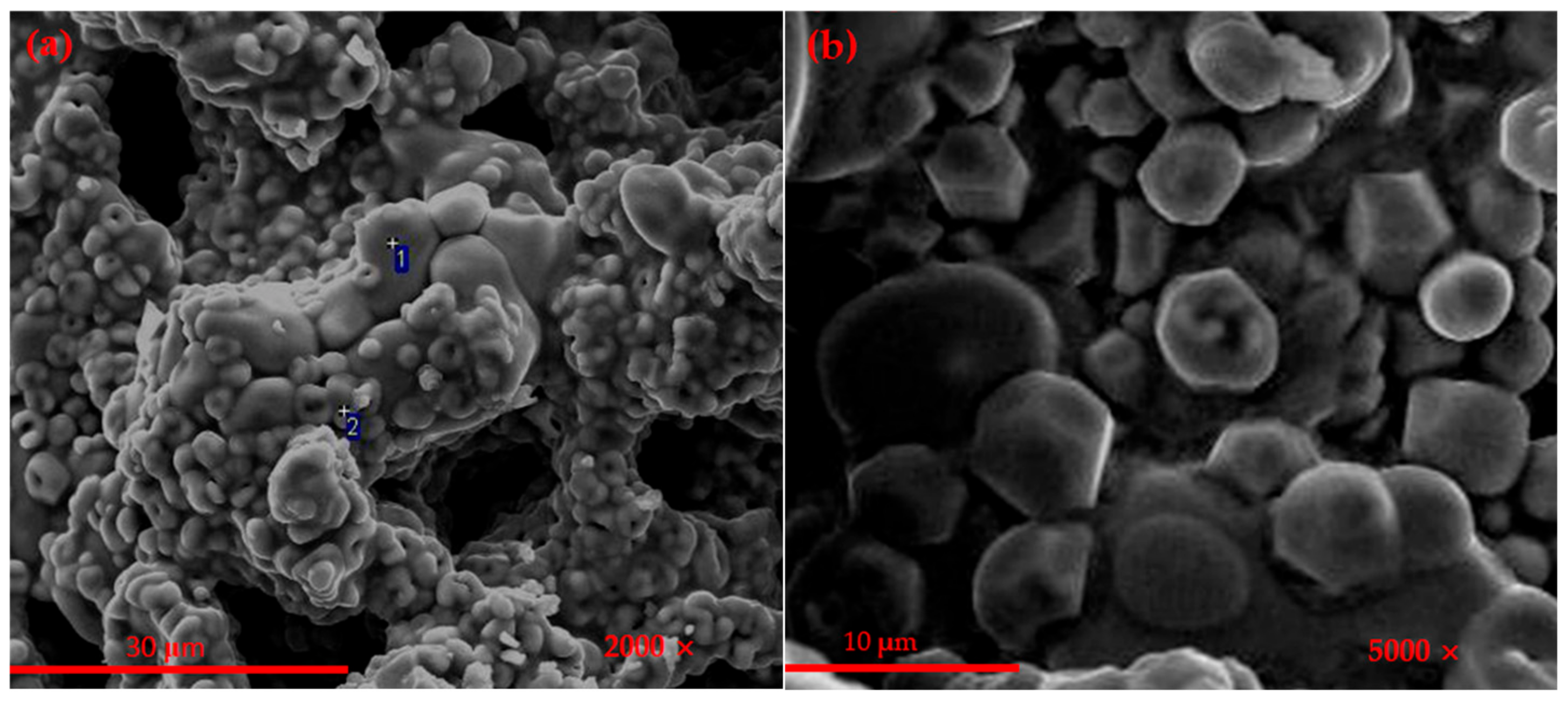
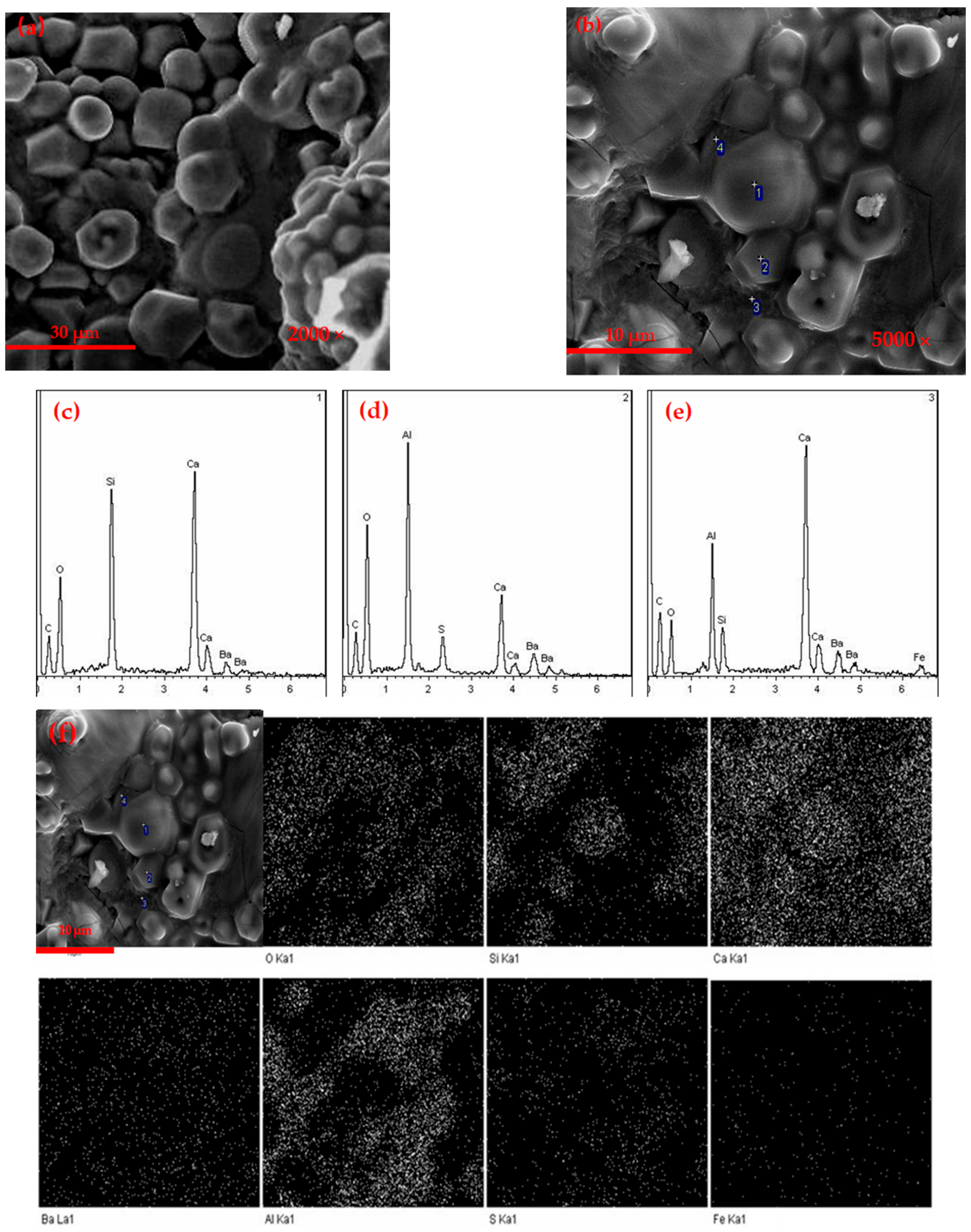
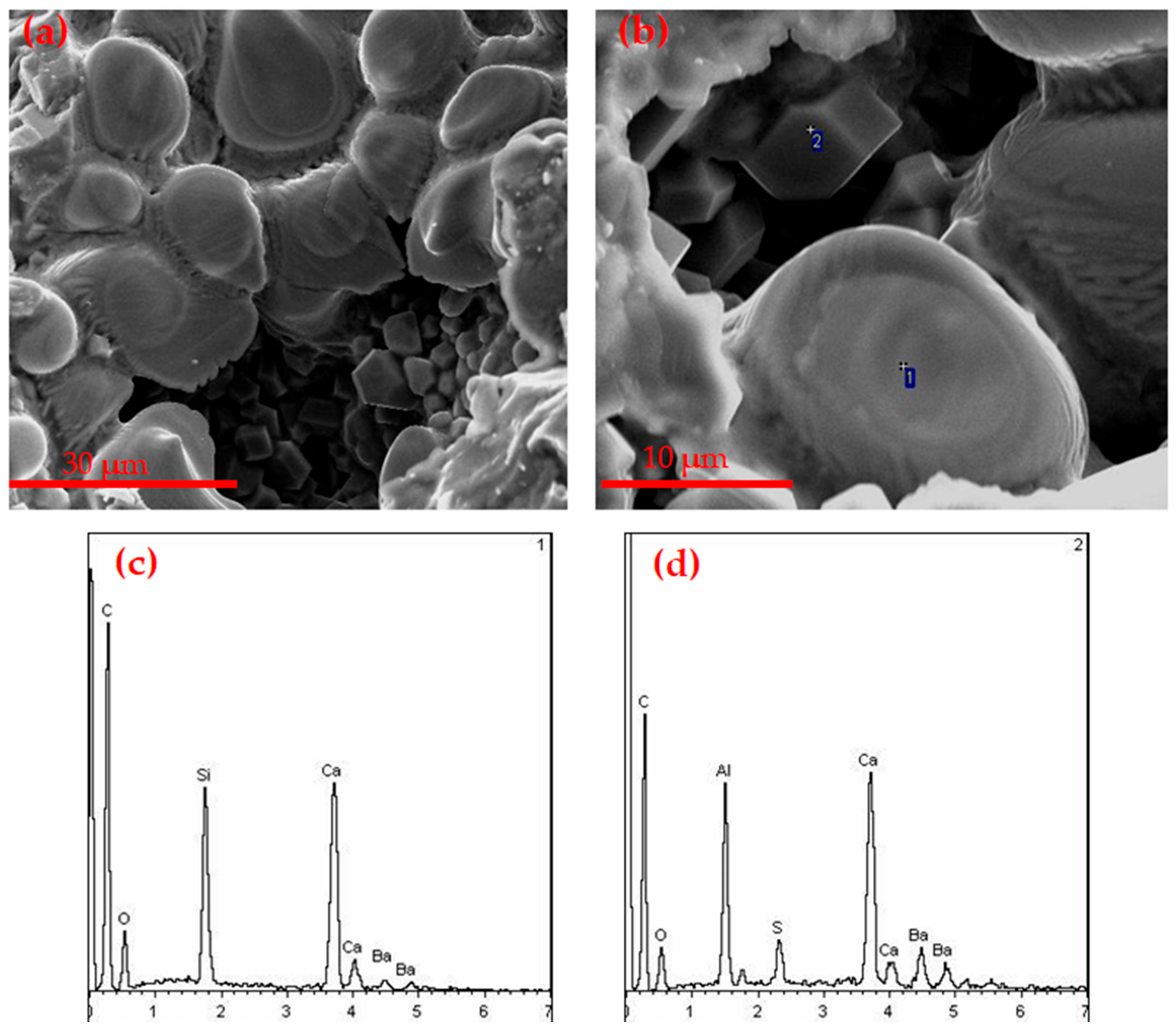
3.1.3. SEM Observation
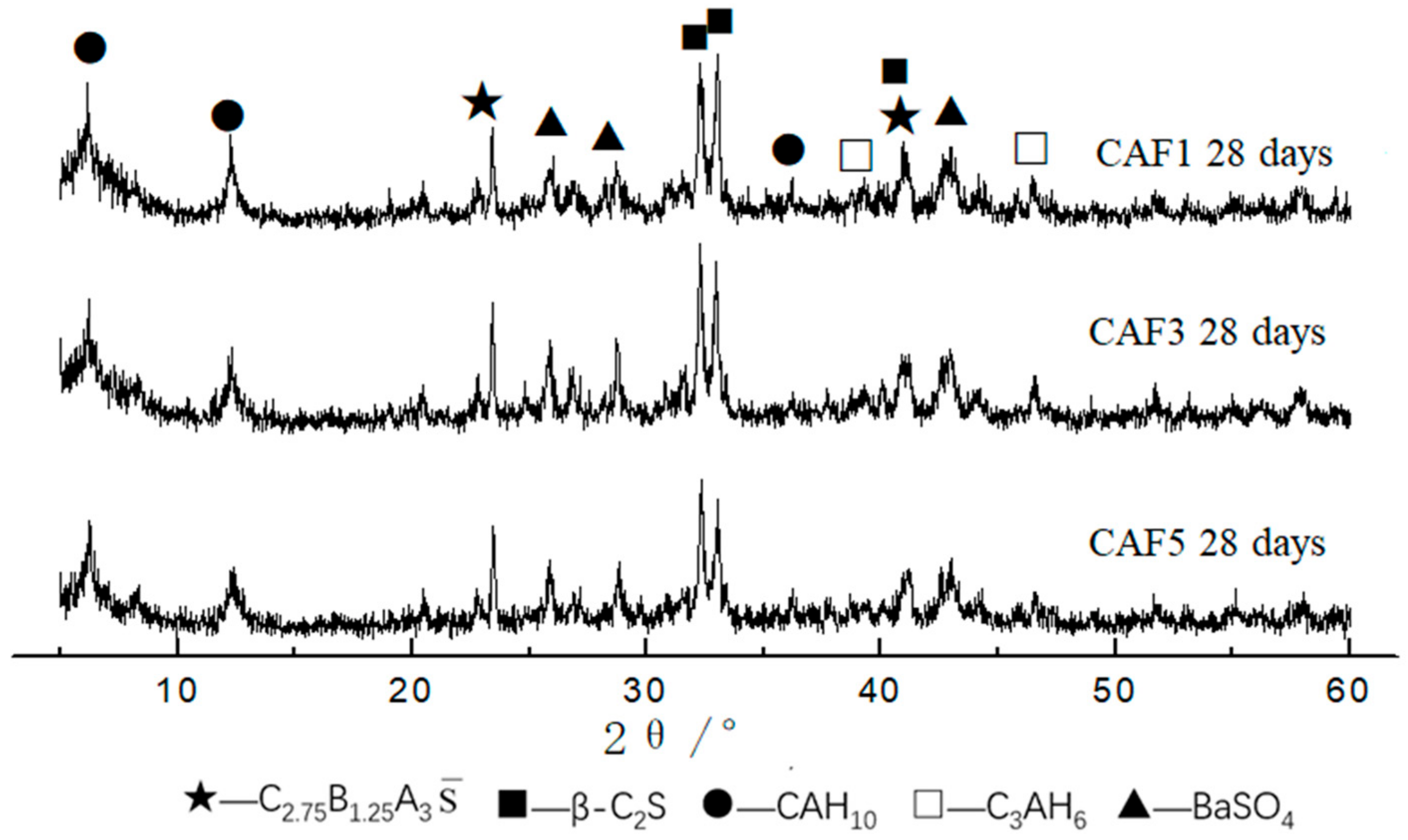
3.2. XRD and SEM Analysis of Hydration Samples
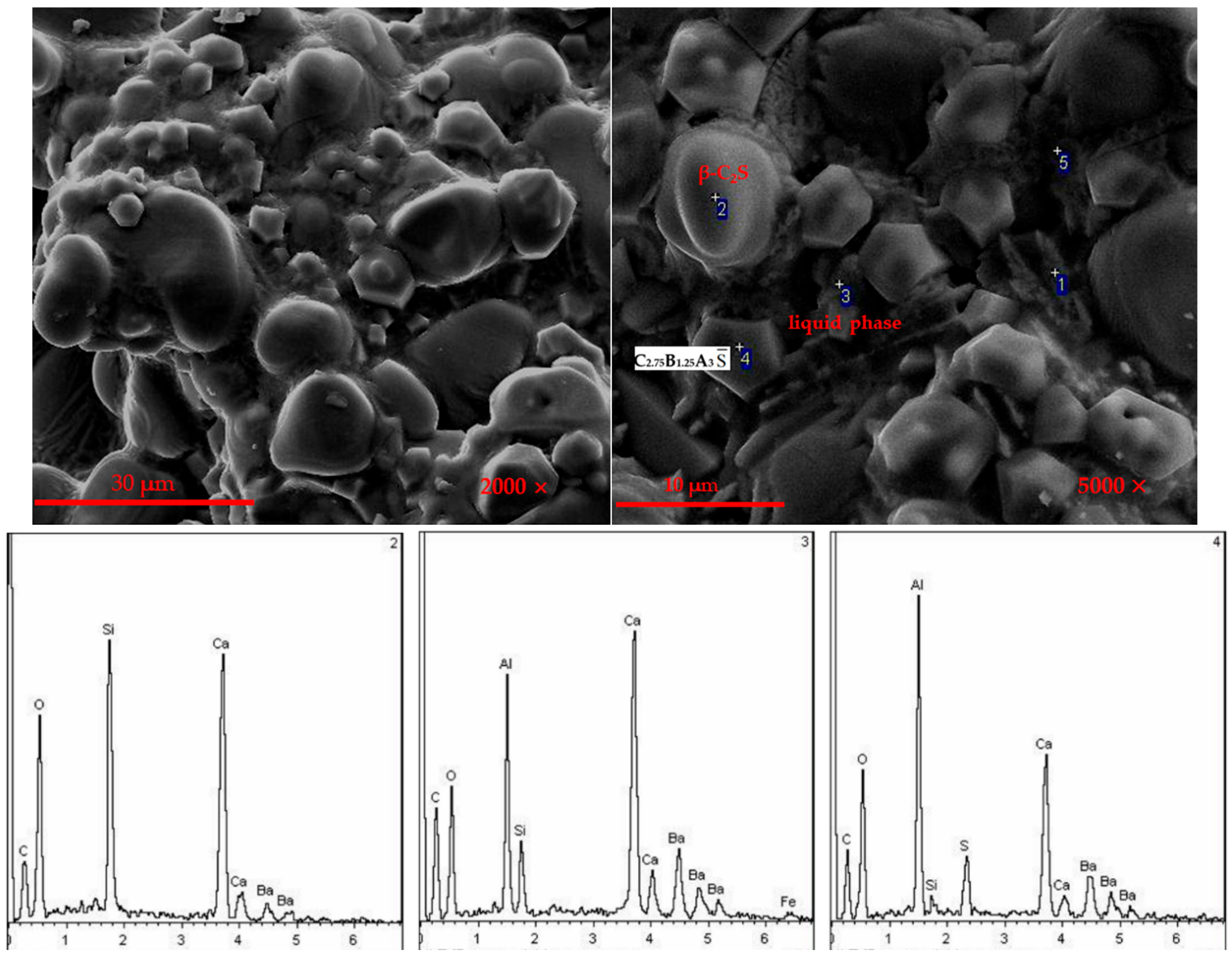
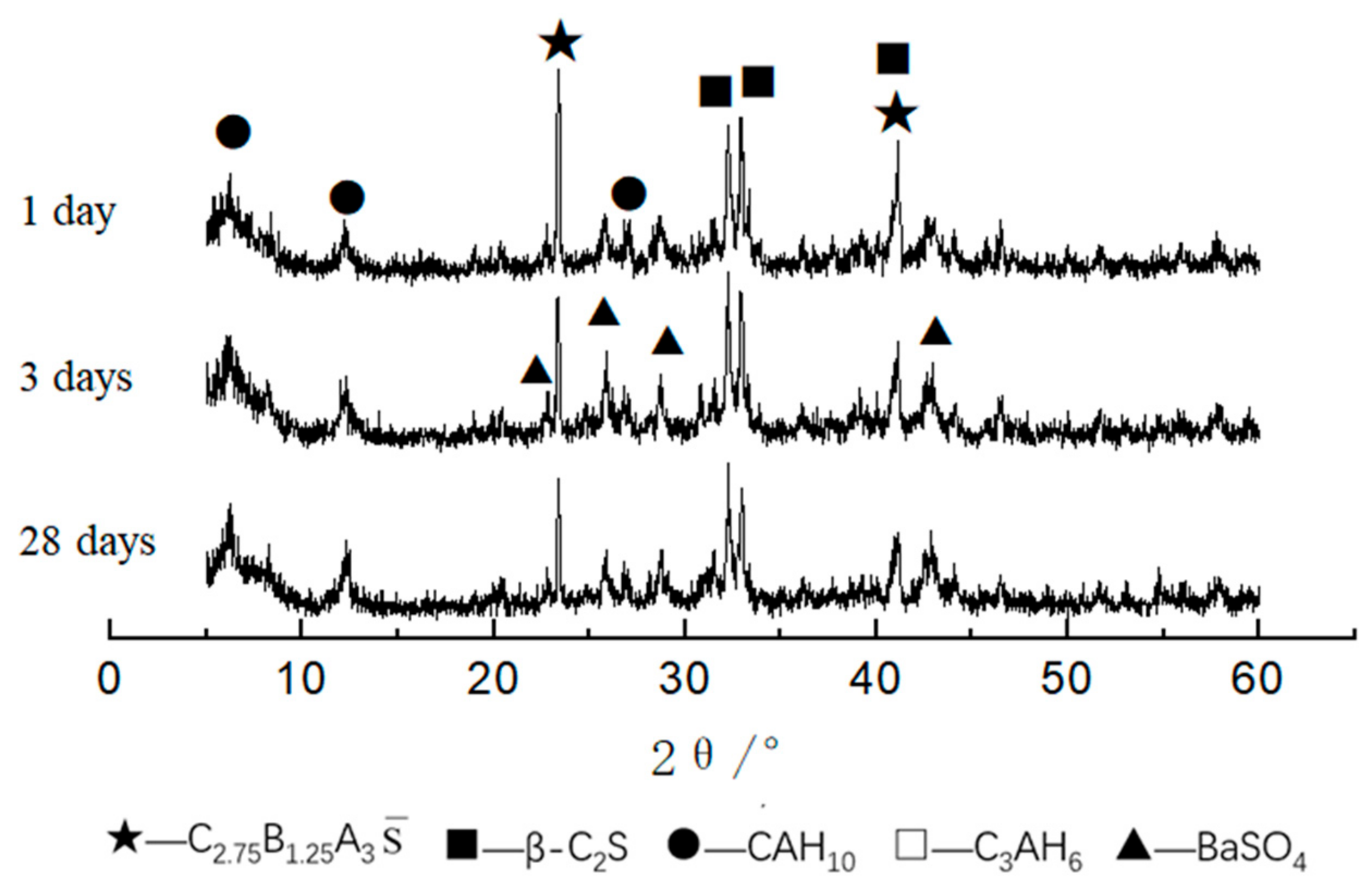
3.2.1. XRD Analysis of Hydrated Samples
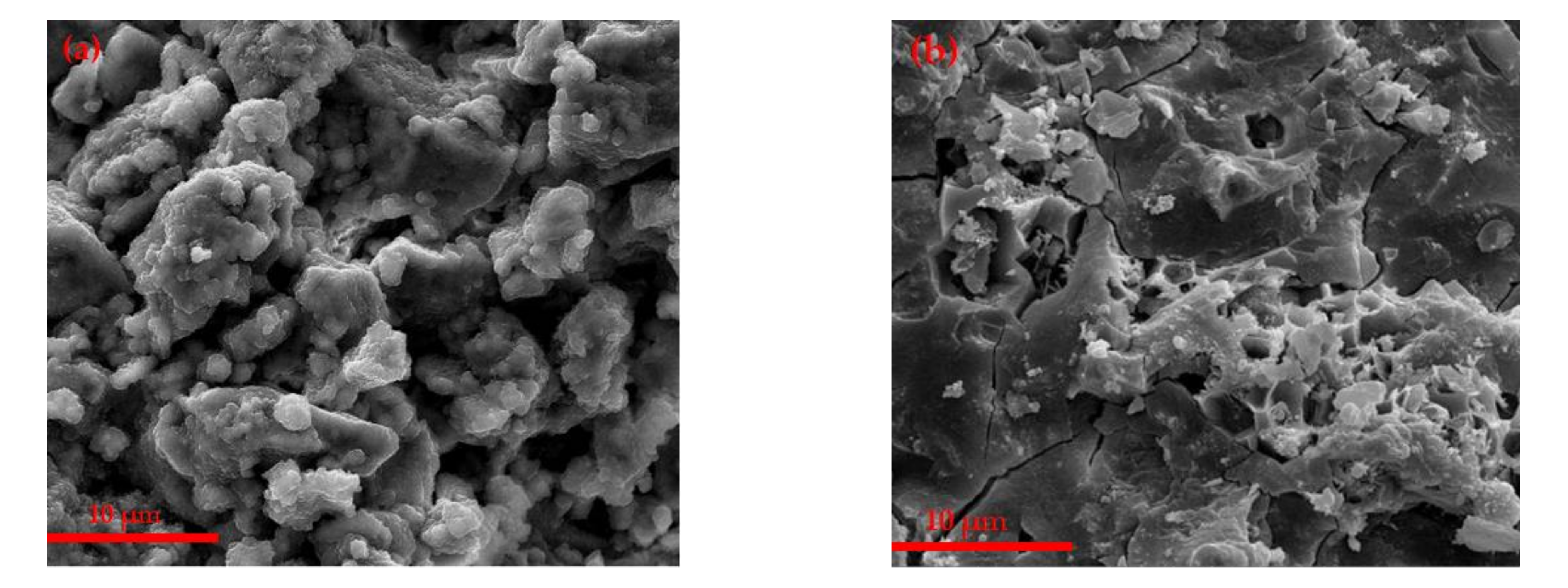
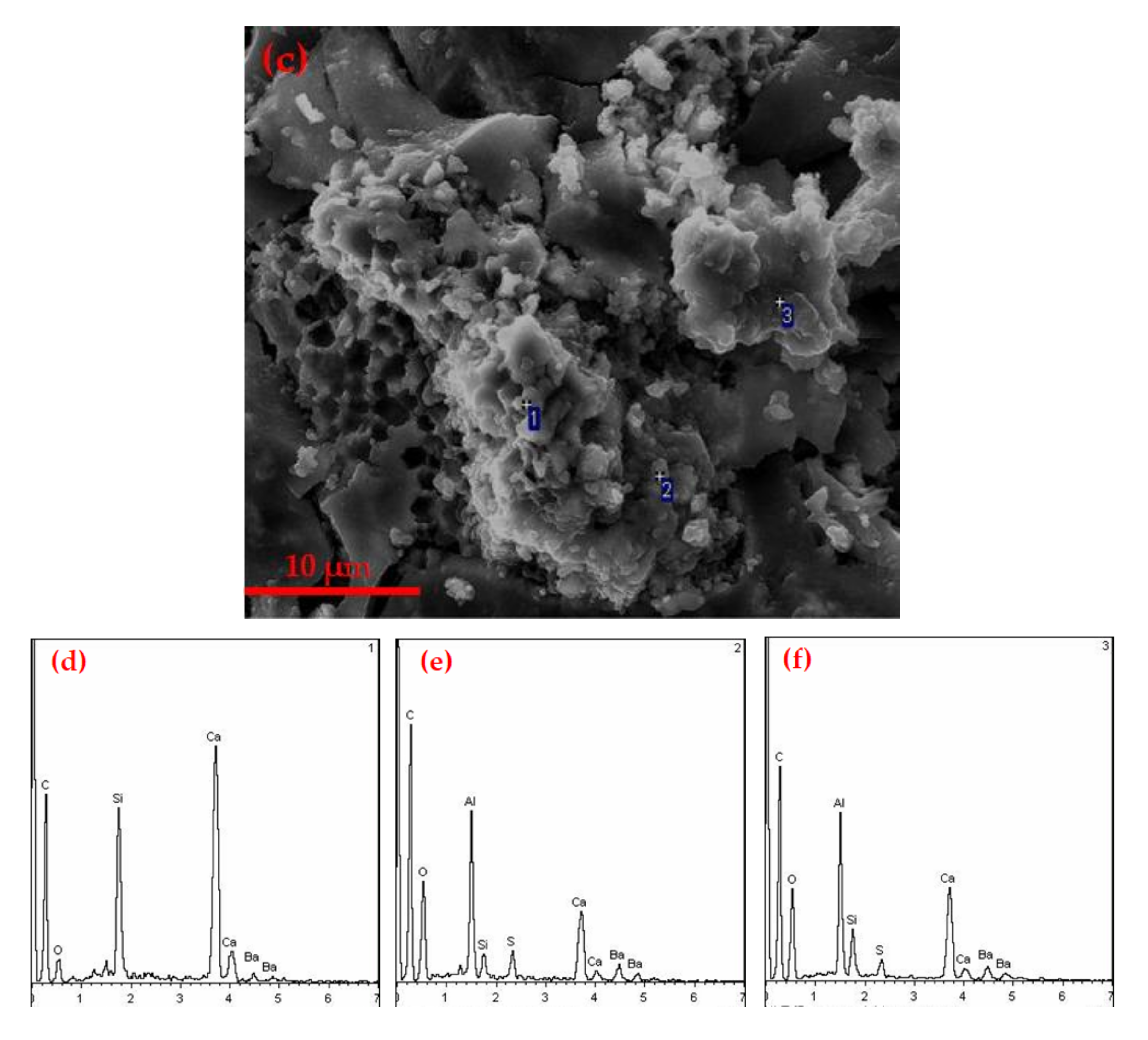
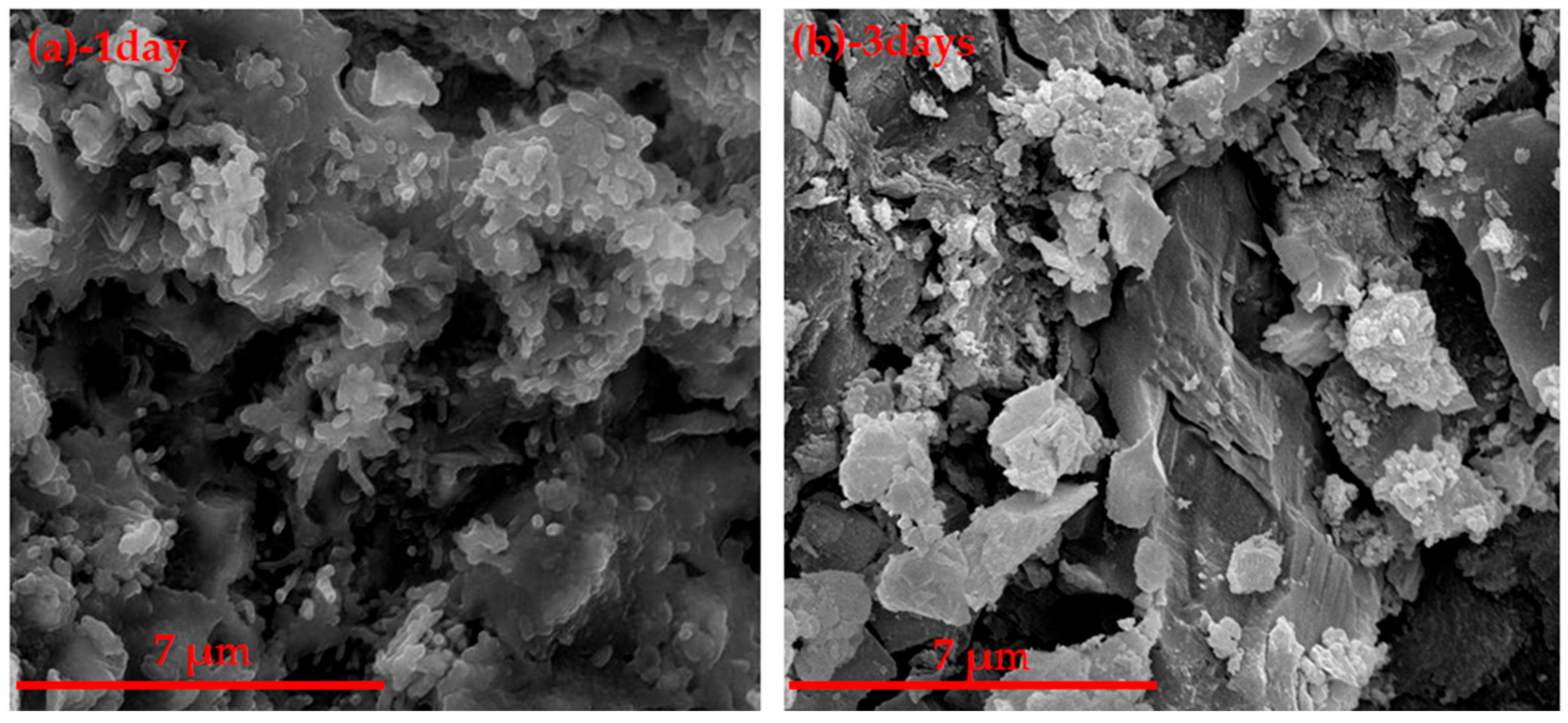
3.2.2. SEM Analysis of Hydration Samples
3.3. Effect of C2F Content on Barium Calcium Sulfoaluminate Cement
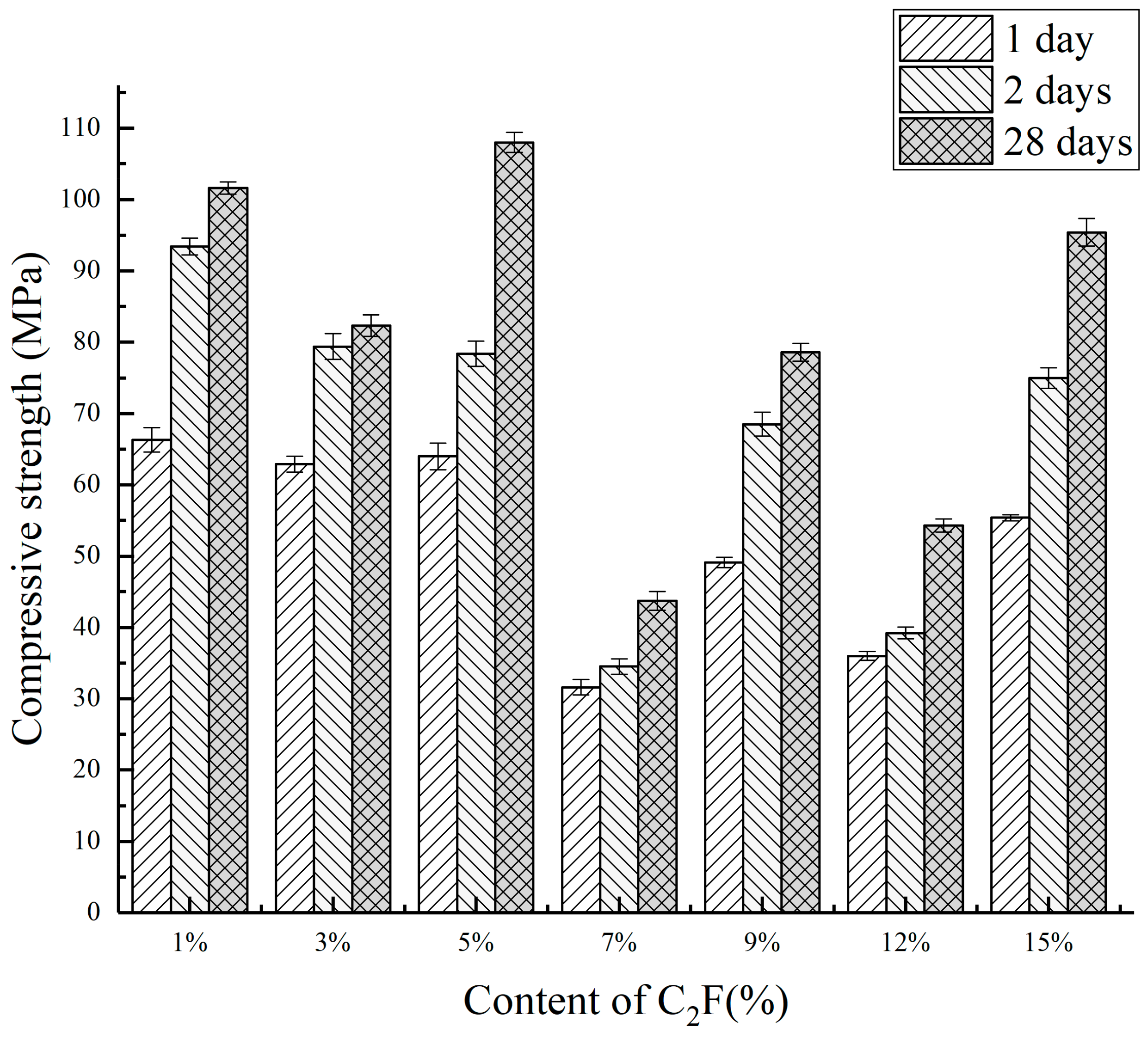
3.3.1. Mechanical Properties
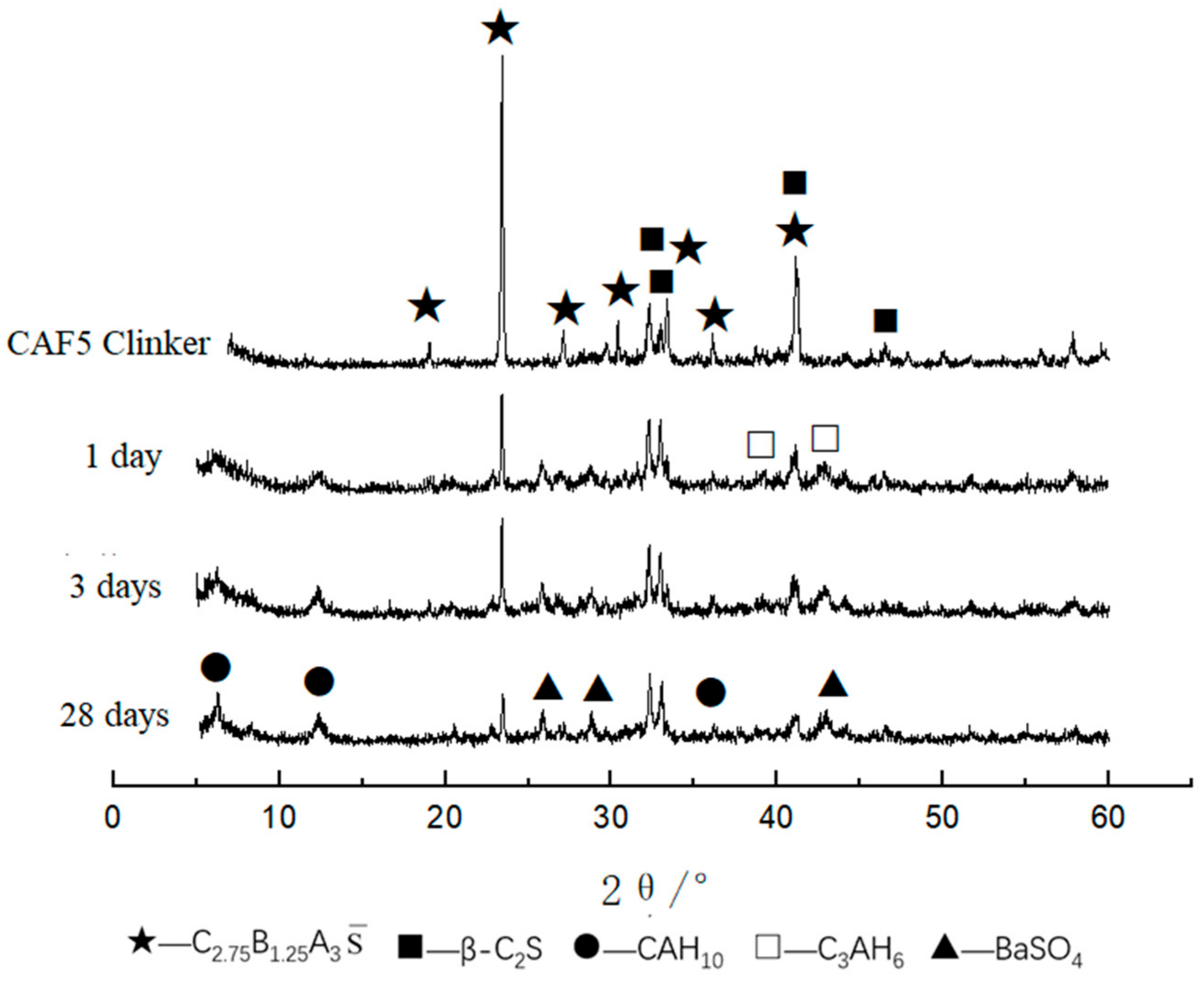
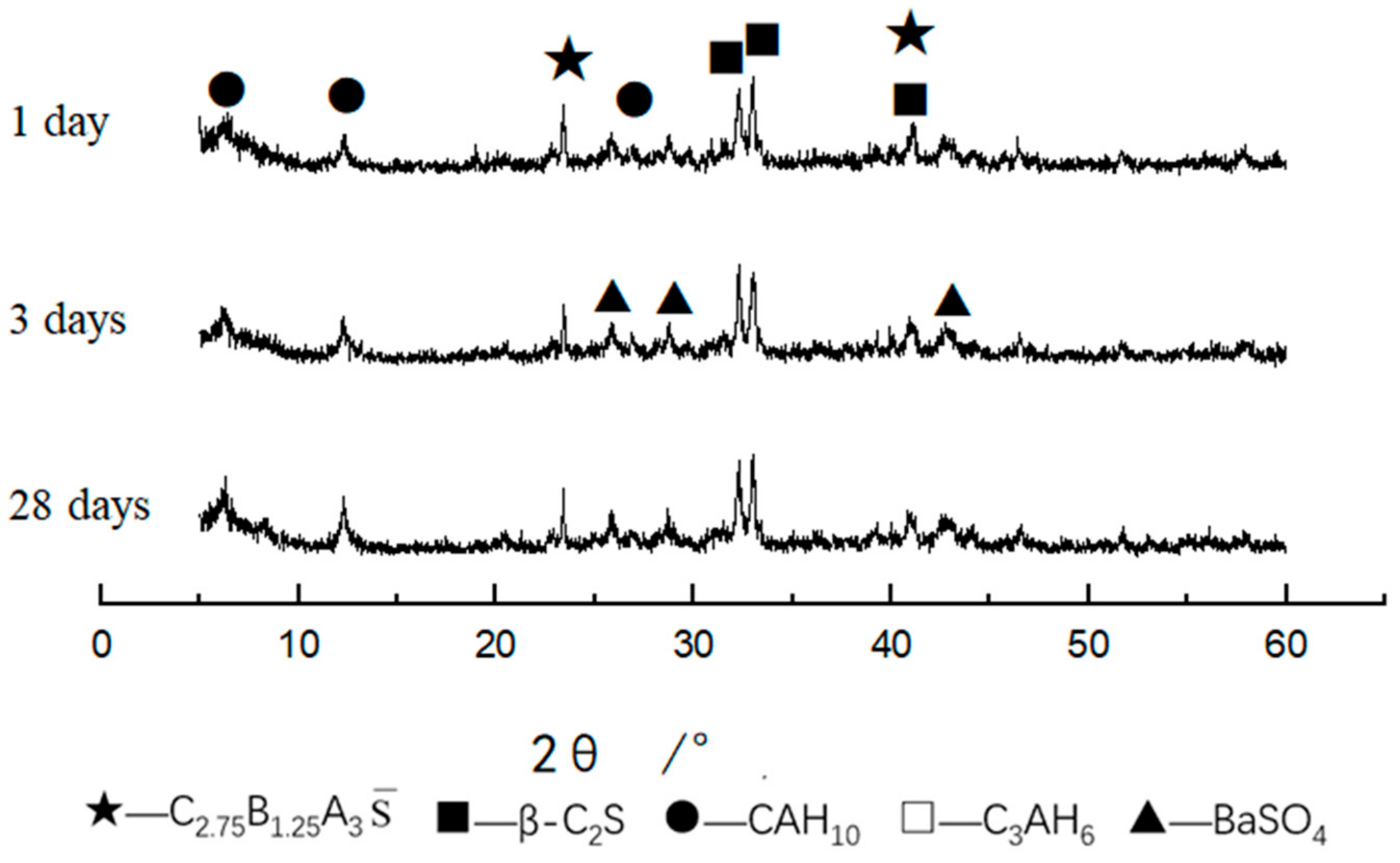
3.3.2. XRD and SEM Analysis of Clinker
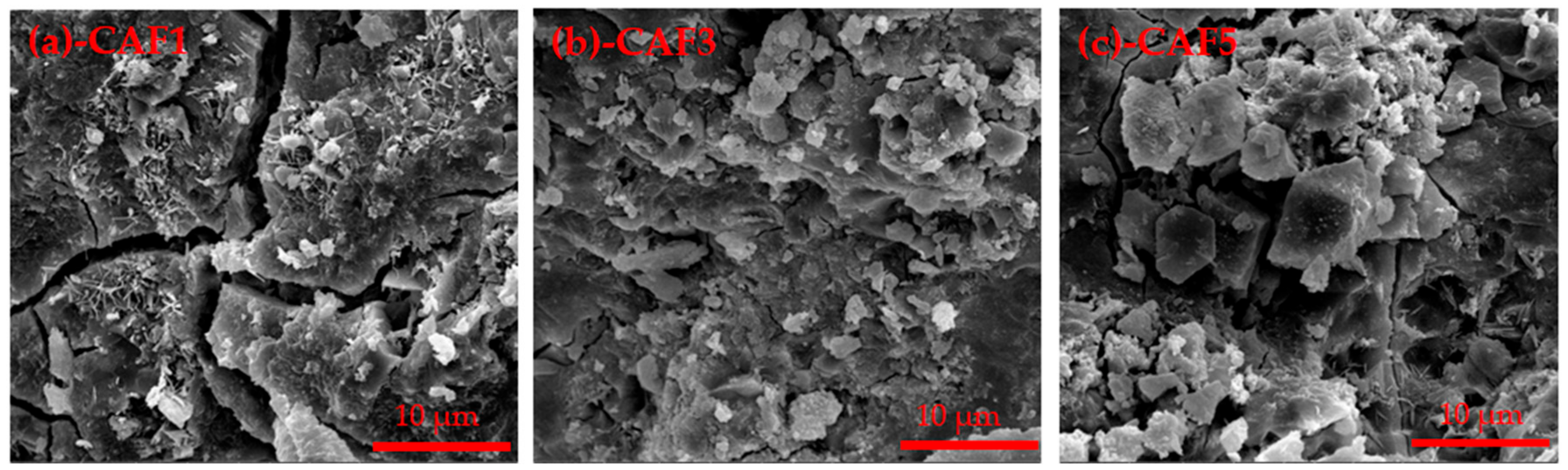
3.3.3. XRD and SEM Analysis of Hydrated Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Ye, Z.M.; Chang, J.; Lu, L.C. Repairing bridges in coastal area with Ba bearing sulfoaluminate cement. Key Eng. Mater. 2009, 787, 501–506. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Hou, P.; Chen, Y.; Gong, C.; Lu, L. Mechanisms and kinetics of the decomposition of calcium barium sulfoaluminate. J. Therm. Anal. Calorim. 2014, 119, 1731–1737. [Google Scholar] [CrossRef]
- Yu, J.C.; Qian, J.S.; Tang, J.Y.; Fan, Y.R. Effect of ettringite seed crystals on the properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 207, 249–257. [Google Scholar] [CrossRef]
- Chenchen, G.; Jibao, X.; Shoude, W.; Lingchao, L. Hydrating characteristics of modified Portland with Ba-bearing sulfoaluminate minerals. Ceram. Silik. 2016, 60, 12–18. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Chen, Y.; Gong, C.; Lu, L. Effect of waste gypsum on the setting and early mechanical properties of belite-C2.75B1.25A3$ cement. J. Therm. Anal. Calorim. 2016, 125, 75–83. [Google Scholar] [CrossRef]
- Chang, J.; Xin, C. Influence of fluorite on the Ba-bearing sulfoaluminate cement. J. Shandong Inst. Build. Mater. 2001, 31, 213–216. [Google Scholar] [CrossRef]
- Wang, Q. Inorganic non-metallic materials technology; China Building Materials Industry Press: Beijing, China, 2005; pp. 50–51. [Google Scholar]
- Zhu, M. Experimental Research on Iron Phase Formation and Hydration. Cem. Eng. 1996, 6, 14–15. [Google Scholar]
- Wang, Y.M. Sulfoaluminate cement; Beijing University of Technology Press: Beijing, China, 1996; pp. 86–102. [Google Scholar]
- Guo, Y. Study on the formation mechanism of iron phases in ferro-aluminate cement. J. Chin. Ceram. Soc. 1988, 16, 481–488. [Google Scholar]
- Huang, Y. Study on isothermal formation dynamics of calcium barium sulphaoluminate mineral. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1172–1176. [Google Scholar] [CrossRef]
- Li, Y.J. Study on the effect of Fe2O3 on the formation of alite sulfoaluminate cement clinker mineral. Cement 2000, 4, 5–7. [Google Scholar]
- Cheng, X.; Chang, J. Study of Ba-bearing calcium sulfoaluminate minerals and cement. Cem. Concr. Res. 2000, 30, 77–81. [Google Scholar] [CrossRef]
- Cheng, X.; Chang, J. Study on the hydration of Ba-bearing calcium sulfoaluminate in the presence of gypsum. Cem. Concr. Res. 2004, 34, 2009–2013. [Google Scholar]
- Wang, S.; Huang, Y.; Gong, C.; Fu, X.; Lu, L. Formation and early hydration characteristics of C2.75B1.25A3$ in binary system of C2.75B1.25A3$-C2S. Constr. Mater. 2016, 66. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Huang, Y.; Gong, C.; Lu, L.; Cheng, X. Formation mechanism of barium calcium sulfoaluminate mineral. Adv. Cem. Res. 2014, 26, 169–176. [Google Scholar] [CrossRef]
- Zhang, G. Effect of sulfoaluminate cement on the strength and water stability of magnesium potassium phosphate cement. Constr. Build. Mater. 2017, 132, 335–342. [Google Scholar] [CrossRef]
- García-Maté, M.; Londono-Zuluaga, D.; de la Torre, A.G.; Losilla, E.R.; Cabeza, A.; Aranda, M.A.G.; Santacruz, I. Tailored setting times with high compressive strengths in bassanite calcium sulfoaluminate eco-cements. Cem. Concr. Compos. 2016, 72, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Hargis, C.W. Early age hydration of calcium sulfoaluminate in the presence of gypsum and varying amounts of calcium hydroxide. Cem. Concr. Compos. 2013, 48, 105–115. [Google Scholar] [CrossRef]
- Garcia-Mate, M. Rheological and hydration characterization of calcium sullfoaluminate cement pastes. Cem. Concr. Compos. 2012, 34, 684–691. [Google Scholar] [CrossRef]
- Hargis, C.W. Calcium sulfoaluminate hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Compos. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Zhao, J.; Chang, J. Kinetic Analysis for Formation Process of Sr-Bearing Ye’elimite. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1861–1869. [Google Scholar] [CrossRef]
- Chang, J.; Li, J.; Han, J.; Zhang, T. Traces of CH in a C4A3$-C2S hydration system. Constr. Build. Mater. 2019, 197, 641–651. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Shen, X.; Wang, Q.; Pan, Z. Kinetics of calcium sulfoaluminate formation from tricalcium aluminate, calcium sulfate and calcium oxide. Cem. Concr. Res. 2014, 55, 79–87. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, Y.; Shang, X.; Zhao, J.; Yu, X. Effects of amorphous AH3 phase on mechanical properties and hydration process of C4A3S¯-CS¯H2-CH-H2O system. Constr. Build. Mater. 2017, 133, 314–322. [Google Scholar] [CrossRef]
- Cuesta, A.; de la Torre, A.G.; Losilla, E.R.; Peterson, V.K.; Rejmak, P.; Ayuela, A.; Frontera, C.; Aranda, M.A. ChemInform Abstract: Structure, Atomistic Simulations, and Phase Transition of Stoichiometric Yeelimite. ChemInform 2013, 44, 1680–1687. [Google Scholar] [CrossRef]
- Kurokawa, D.; Takeda, S.; Colas, M.; Asaka, T.; Thomas, P.; Fukuda, K. Phase transformation of Ca4[Al6O12]SO4 and its disordered crystal structure at 1073K. J. Solid State Chem. 2014, 215, 265–270. [Google Scholar] [CrossRef]
- Feng, X. The structure and quantum chemistry studies of 3CaO·3Al2O3· SrSO4. Cem. Concr. Res. 1996, 26, 955–962. [Google Scholar] [CrossRef]
- Chang, J.; Shang, X.; Zhao, J. Study on Sintering System of Calcium Barium Sulfoaluminate by XRD Quantitative Analysis. Appl. Sci. 2015, 5, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Pinazo, G.; Cuesta, A.; García-Maté, M.; Santacruz, I.; Losilla, E.R.; de la Torre, A.G.; León-Reina, L.; Aranda, M.A.G. Rietveld quantitative phase analysis of Yeelimite-containing cements. Cem. Concr. Res. 2012, 42, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Cuesta, A.; Álvarez-Pinazo, G.; Sanfélix, S.G.; Peral, I.; Aranda, M.A.G.; de la Torre, A.G. Hydration mechanisms of two polymorphs of synthetic ye’elimite. Cem. Concr. Res. 2014, 63, 127–136. [Google Scholar] [CrossRef] [Green Version]



| Number | The Content of Mineral Composition/% | ||
|---|---|---|---|
| C2.75Ba1.25A3 | C2S | C4AF | |
| CAF0 | 60 | 40 | 0 |
| CAF1 | 60 | 39 | 1 |
| CAF2 | 60 | 37 | 3 |
| CAF3 | 60 | 35 | 5 |
| CAF4 | 60 | 33 | 7 |
| CAF5 | 60 | 31 | 9 |
| CAF6 | 60 | 28 | 12 |
| CAF7 | 60 | 25 | 15 |
| Number | 1 Day | 3 Days | 28 Days |
|---|---|---|---|
| CAF0 | 65.5 | 71.2 | 96.5 |
| CAF1 | 77.2 | 87.4 | 105.6 |
| CAF2 | 68.4 | 67.5 | 64.5 |
| CAF3 | 73.2 | 97.9 | 106.9 |
| CAF4 | 70.8 | 66.5 | 100.6 |
| CAF5 | 71.4 | 87.6 | 93.1 |
| CAF6 | 70.2 | 72.3 | 98.2 |
| CAF7 | 74.3 | 76.2 | 102.5 |
| Number | The Content of Mineral Composition/% | Compressive Strength/Mpa | ||||
|---|---|---|---|---|---|---|
| C2.75Ba1.25A3 | C2S | C2F | 1 Day | 3 Days | 28 Days | |
| CF1 | 60 | 39 | 1 | 66.3 | 93.4 | 101.6 |
| CF2 | 60 | 37 | 3 | 62.9 | 79.4 | 82.3 |
| CF3 | 60 | 35 | 5 | 64.0 | 78.4 | 108.0 |
| CF4 | 60 | 33 | 7 | 31.6 | 34.5 | 43.7 |
| CF5 | 60 | 31 | 9 | 49.1 | 68.5 | 78.6 |
| CF6 | 60 | 28 | 12 | 36.0 | 39.2 | 54.3 |
| CF7 | 60 | 25 | 15 | 55.4 | 75.0 | 95.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.; Zhang, J.; Yuan, Y.; Cui, K. Effect of Iron Phase on Calcination and Properties of Barium Calcium Sulfoaluminate Cement. Materials 2021, 14, 5813. https://doi.org/10.3390/ma14195813
Chang J, Zhang J, Yuan Y, Cui K. Effect of Iron Phase on Calcination and Properties of Barium Calcium Sulfoaluminate Cement. Materials. 2021; 14(19):5813. https://doi.org/10.3390/ma14195813
Chicago/Turabian StyleChang, Jun, Jixin Zhang, Yanchen Yuan, and Kai Cui. 2021. "Effect of Iron Phase on Calcination and Properties of Barium Calcium Sulfoaluminate Cement" Materials 14, no. 19: 5813. https://doi.org/10.3390/ma14195813





