Tribological Properties of High-Entropy Alloys under Dry Conditions for a Wide Temperature Range—A Review
Abstract
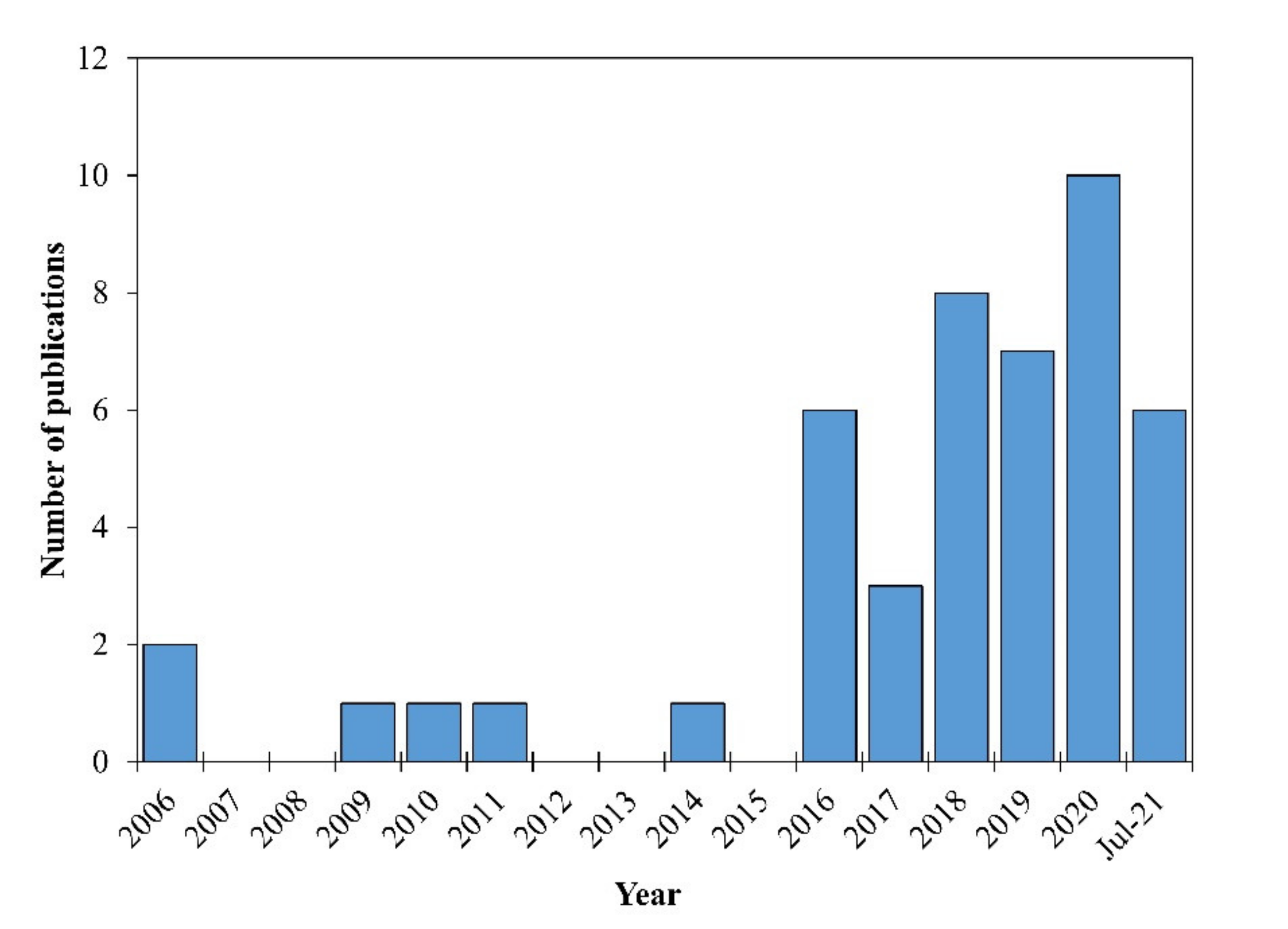
:1. Introduction
2. Design of HEAs
3. HEAs: Manufacturing Methods
3.1. Arc Melting
3.2. Induction Remelting
3.3. Spark Plasma Sintering
4. Mechanical and Tribological Properties of the HEAs
4.1. Tribological Properties: Room Temperature
4.1.1. Tribological Properties of BCC HEAs
4.1.2. Tribological Properties of FCC and FCC + BCC Phase HEAs
4.1.3. Tribological Properties of HEAs at Elevated Temperatures
5. Future Scope of the HEAs for Tribological Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-high entropy alloys with multiple pricipal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Miracle, D.B. High entropy alloys as a bold step forward in alloy development. Nat. Commun. 2019, 10, 1–3. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2015, 19, 349–362. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Sharma, A.S.; Yadav, S.; Biswas, K.; Basu, B. High-entropy alloys and metallic nanocomposites: Processing challenges, microstructure development and property enhancement. Mater. Sci. Eng. R Reports 2018, 131, 1–42. [Google Scholar] [CrossRef]
- Diao, H.Y.; Feng, R.; Dahmen, K.A.; Liaw, P.K. Fundamental deformation behavior in high-entropy alloys: An overview. Curr. Opin. Solid State Mater. Sci. 2017, 21, 252–266. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A Review on High Entropy Alloys Coatings: Fabrication Processes and Property Assessment. Adv. Eng. Mater. 2019, 1900343, 1–27. [Google Scholar] [CrossRef]
- Koch, C.C. Nanocrystalline high-entropy alloys. J. Mater. Res. 2017, 32, 3435–3444. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Alaneme, K.K.; Bodunrin, M.O.; Oke, S.R. Processing, alloy composition and phase transition effect on the mechanical and corrosion properties of high entropy alloys: A review. J. Mater. Res. Technol. 2016, 5, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wang, G.; Wu, S.; Liaw, P.K. Creep, fatigue, and fracture behavior of high-entropy alloys. J. Mater. Res. 2018, 33, 3011–3034. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, V.M.; Arulvel, S. A review on the steels, alloys/high entropy alloys, composites and coatings used in high temperature wear applications. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Torralba, J.M.; Campos, M. High entropy alloys manufactured by additive manufacturing. Metals 2020, 10, 639. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.-W.; Ranganathan, S.; Bhattacharjee, P.P. High-entropy alloys; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 0128160683. [Google Scholar]
- Gurao, N.P.; Biswas, K. High-entropy materials: Critical review and way forward. Curr. Sci. 2020, 118, 1520–1539. [Google Scholar]
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. J. Mater. Sci. 2016, 65, 1759–1771. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. 2004, A, 375–377. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, Y.; Xu, J.; Zhu, C.; Dai, P.; Xue, S. Tribological properties of nano/ultrafine-grained FeCoCrNiMnAlx high-entropy alloys over a wide range of temperatures. J. Alloys Compd. 2020, 817, 153305. [Google Scholar] [CrossRef]
- Geng, Y.; Tan, H.; Cheng, J.; Chen, J.; Sun, Q.; Zhu, S.; Yang, J. Microstructure, mechanical and vacuum high temperature tribological properties of AlCoCrFeNi high entropy alloy based solid-lubricating composites. Tribol. Int. 2020, 151, 106444. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M.; Fabijanic, D. The sliding wear behaviour of CoCrFeMnNi and AlxCoCrFeNi high entropy alloys at elevated temperatures. Wear 2019, 428–429, 32–44. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, K.; Lu, Y.; Gao, X.; Wang, T.; Li, T. Effect of vanadium addition on the microstructure and properties of AlCoCrFeNi high entropy alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Chen, M.R.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chuang, M.H. Effect of vanadium addition on the microstructure, hardness, and wear resistance of Al0.5CoCrCuFeNi high-entropy alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2006, 37, 1363–1369. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Erdoğan, A.; Gök, M.S.; Zeytin, S. Analysis of the high-temperature dry sliding behavior of CoCrFeNiTi0.5Alx high-entropy alloys. Friction 2020, 8, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Tarate, P.; Abhyankar, A.C.; Mohape, M.R.; Gowtam, D.S.; Deshmukh, V.P.; Shanmugasundaram, T. High temperature wear in CoCrFeNiCux high entropy alloys: The role of Cu. Scr. Mater. 2019, 161, 28–31. [Google Scholar] [CrossRef]
- Deng, G.; Tieu, A.K.; Lan, X.; Su, L.; Wang, L.; Zhu, Q.; Zhu, H. Effects of normal load and velocity on the dry sliding tribological behaviour of CoCrFeNiMo0.2 high entropy alloy. Tribol. Int. 2020, 144, 106116. [Google Scholar] [CrossRef]
- Xiao, J.K.; Tan, H.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and wear behavior of FeCoNiCrMn high entropy alloy coating deposited by plasma spraying. Surf. Coatings Technol. 2020, 385, 125430. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, A.; Han, J.; Meng, J. Microstructure, mechanical and tribological properties of CoCrFeNiMn high entropy alloy matrix composites with addition of Cr3C2. Tribol. Int. 2020, 151, 106436. [Google Scholar] [CrossRef]
- Yadav, S.; Sarkar, S.; Aggarwal, A.; Kumar, A.; Biswas, K. Wear and mechanical properties of novel (CuCrFeTiZn)100-xPbx high entropy alloy composite via mechanical alloying and spark plasma sintering. Wear 2018, 410–411, 93–109. [Google Scholar] [CrossRef]
- Moravcikova-Gouvea, L.; Moravcik, I.; Omasta, M.; Veselý, J.; Cizek, J.; Minárik, P.; Cupera, J.; Záděra, A.; Jan, V.; Dlouhy, I. High-strength Al0.2Co1.5CrFeNi1.5Ti high-entropy alloy produced by powder metallurgy and casting: A comparison of microstructures, mechanical and tribological properties. Mater. Charact. 2020, 159, 110046. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Z.; Pi, J. Microstructure and wear behavior of the AlCrFeCoNi high-entropy alloy fabricated by additive manufacturing. Mater. Lett. 2020, 261, 127004. [Google Scholar] [CrossRef]
- Tang, W.Y.; Chuang, M.H.; Chen, H.Y.; Yeh, J.W. Microstructure and mechanical performance of brand-new Al0.3CrFe1.5MnNi0.5 high-entropy alloys. Adv. Eng. Mater. 2009, 11, 788–794. [Google Scholar] [CrossRef]
- Zhang, A.; Han, J.; Su, B.; Meng, J. A promising new high temperature self-lubricating material: CoCrFeNiS0.5 high entropy alloy. Mater. Sci. Eng. A 2018, 731, 36–43. [Google Scholar] [CrossRef]
- Zhang, Y. Microstructures and properties of high entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- ASM Internatinal. Vacuum Induction Melting. In ASM Handbook; ASM Internatinal: Thame, UK, 2008; ISBN 978-1-62708-187-0. [Google Scholar]
- Prakasam, M.; Balima, F.; Cygan, S.; Klimczyk, P.; Jaworska, L.; Largeteau, A. Ultrahigh pressure SPS (HP-SPS) as new syntheses and exploration tool in materials science. In Spark Plasma Sintering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–218. [Google Scholar]
- Yadav, S.; Kumar, A.; Biswas, K. Wear behavior of high entropy alloys containing soft dispersoids (Pb, Bi). Mater. Chem. Phys. 2018, 210, 222–232. [Google Scholar] [CrossRef]
- Chen, M.; Shi, X.H.; Yang, H.; Liaw, P.K.; Gao, M.C.; Hawk, J.A.; Qiao, J. Wear behavior of Al0.6CoCrFeNi high-entropy alloys: Effect of environments. J. Mater. Res. 2018, 33, 3310–3320. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Annasamy, M.; Kada, S.; Hodgson, P.D.; Barnett, M.R.; Fabijanic, D.M. On the enhanced wear resistance of CoCrFeMnNi high entropy alloy at intermediate temperature. Scr. Mater. 2020, 186, 230–235. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Karantzalis, A. Evaluation of the Microstructural Aspects, Mechanical Properties and Dry Sliding Wear Response of MoTaNbVTi Refractory High Entropy Alloy. Met. Mater. Int. 2019, 25, 1529–1540. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A. Dry-Sliding Wear Response of MoTaWNbV High Entropy Alloy. Adv. Eng. Mater. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Mathiou, C.; Poulia, A.; Georgatis, E.; Karantzalis, A.E. Microstructural features and dry—Sliding wear response of MoTaNbZrTi high entropy alloy. Mater. Chem. Phys. 2018, 210, 126–135. [Google Scholar] [CrossRef]
- Lentzaris, K.; Poulia, A.; Georgatis, E.; Lekatou, A.G.; Karantzalis, A.E. Analysis of Microstructure and Sliding Wear Behavior of Co1.5CrFeNi1.5Ti0.5 High-Entropy Alloy. J. Mater. Eng. Perform. 2018, 27, 5177–5186. [Google Scholar] [CrossRef]
- Miao, J.; Liang, H.; Zhang, A.; He, J.; Meng, J.; Lu, Y. Tribological behavior of an AlCoCrFeNi2.1 eutectic high entropy alloy sliding against different counterfaces. Tribol. Int. 2021, 153, 106599. [Google Scholar] [CrossRef]
- Alvi, S.; Akhtar, F. High temperature tribology of CuMoTaWV high entropy alloy. Wear 2019, 426–427, 412–419. [Google Scholar] [CrossRef]
- Archard, J. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Yang, H.; Zhang, M.; Ma, S.; Qiao, J. Microstructure and wear properties of nitrided AlCoCrFeNi high-entropy alloy. Mater. Chem. Phys. 2018, 210, 233–239. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Wu, J.M.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chen, H.C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Löbel, M.; Lindner, T.; Pippig, R.; Lampke, T. High-temperature wear behaviour of spark plasma sintered AlCoCrFeNiTi0.5 high-entropy alloy. Entropy 2019, 21, 582. [Google Scholar] [CrossRef] [Green Version]
- Nong, Z.S.; Lei, Y.N.; Zhu, J.C. Wear and oxidation resistances of AlCrFeNiTi-based high entropy alloys. Intermetallics 2018, 101, 144–151. [Google Scholar] [CrossRef]
- Kadhim, D. Sliding friction and wear behavior of high entropy alloys at room and elevated temperatures. ProQuest Dissertations and Theses. Master’s Thesis, University of North Texas, Denton, TX, USA, 2016. [Google Scholar]
- Pole, M.; Sadeghilaridjani, M.; Shittu, J.; Ayyagari, A.; Mukherjee, S. High temperature wear behavior of refractory high entropy alloys based on 4-5-6 elemental palette. J. Alloys Compd. 2020, 843, 156004. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Lekatou, A.; Karantzalis, A.E. Microstructure and wear behavior of a refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2016, 57, 50–63. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Zhou, Q.; Zhang, F.; Han, W.C.; Du, Y.; Hua, K.; Wang, H.F. Effect of Al addition on the microstructure, mechanical and wear properties of TiZrNbHf refractory high entropy alloys. Tribol. Int. 2021, 160, 107031. [Google Scholar] [CrossRef]
- Geng, Y.; Tan, H.; Wang, L.; Tieu, A.K.; Chen, J.; Cheng, J.; Yang, J. Nano-coupled heterostructure induced excellent mechanical and tribological properties in AlCoCrFeNi high entropy alloy. Tribol. Int. 2021, 154, 106662. [Google Scholar] [CrossRef]
- Du, L.M.; Lan, L.W.; Zhu, S.; Yang, H.J.; Shi, X.H.; Liaw, P.K.; Qiao, J.W. Effects of temperature on the tribological behavior of Al0.25CoCrFeNi high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 917–925. [Google Scholar] [CrossRef]
- Yu, Y.; He, F.; Qiao, Z.; Wang, Z.; Liu, W.; Yang, J. Effects of temperature and microstructure on the triblogical properties of CoCrFeNiNbx eutectic high entropy alloys. J. Alloys Compd. 2019, 775, 1376–1385. [Google Scholar] [CrossRef]
- Xiao, J.-K.; Tan, H.; Chen, J.; Martini, A.; Zhang, C. Effect of carbon content on microstructure, hardness and wear resistance of CoCrFeMnNiCx high-entropy alloys. J. Alloys Compd. 2020, 847, 156533. [Google Scholar] [CrossRef]
- Vo, T.D.; Tran, B.; Tieu, A.K.; Wexler, D.; Deng, G.Y.; Nguyen, C. Effects of oxidation on friction and wear properties of eutectic high-entropy alloy AlCoCrFeNi2.1. Tribol. Int. 2021, 160. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.; Gao, M.C.; Zhang, C.; Zhang, T.; Yang, H.; Wang, Z.; Qiao, J. Tribological Properties of AlCrCuFeNi2 High-Entropy Alloy in Different Conditions. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 3312–3321. [Google Scholar] [CrossRef]
- Zhu, T.; Wu, H.; Zhou, R.; Zhang, N.; Yin, Y.; Liang, L.; Liu, Y.; Li, J.; Shan, Q.; Li, Q.; et al. Microstructures and Tribological Properties of TiC Reinforced FeCoNiCuAl High-Entropy Alloy at Normal and Elevated Temperature. Metals 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef] [Green Version]
- Löbel, M.; Lindner, T.; Mehner, T.; Lampke, T. Influence of titanium on microstructure, phase formation and wear behaviour of AlCoCrFeNiTix high-entropy alloy. Entropy 2018, 20, 505. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.; Tieu, A.K.; Su, L.; Wang, P.; Wang, L.; Lan, X.; Cui, S.; Zhu, H. Investigation into reciprocating dry sliding friction and wear properties of bulk CoCrFeNiMo high entropy alloys fabricated by spark plasma sintering and subsequent cold rolling processes: Role of Mo element concentration. Wear 2020, 460–461, 203440. [Google Scholar] [CrossRef]
- Molnár, D.; Vida, Á.; Huang, S.; Chinh, N.Q. The effect of cooling rate on the microstructure and mechanical properties of NiCoFeCrGa high-entropy alloy. J. Mater. Sci. 2019, 54, 5074–5082. [Google Scholar] [CrossRef]
- Cai, Z.; Cui, X.; Liu, Z.; Li, Y.; Dong, M.; Jin, G. Microstructure and wear resistance of laser cladded Ni-Cr-Co-Ti-V high-entropy alloy coating after laser remelting processing. Opt. Laser Technol. 2018, 99, 276–281. [Google Scholar] [CrossRef]
- Zhang, A.; Han, J.; Su, B.; Li, P.; Meng, J. Microstructure, mechanical properties and tribological performance of CoCrFeNi high entropy alloy matrix self-lubricating composite. Mater. Des. 2017, 114, 253–263. [Google Scholar] [CrossRef]
- Zhang, A.; Han, J.; Su, B.; Meng, J. A novel CoCrFeNi high entropy alloy matrix self-lubricating composite. J. Alloys Compd. 2017, 725, 700–710. [Google Scholar] [CrossRef]
- Geng, Y.S.; Chen, J.; Tan, H.; Cheng, J.; Zhu, S.Y.; Yang, J. Tribological performances of CoCrFeNiAl high entropy alloy matrix solid-lubricating composites over a wide temperature range. Tribol. Int. 2021, 157. [Google Scholar] [CrossRef]
- Wang, L.; Geng, Y.S.; Tieu, A.K.; Hai, G.O.J.; Tan, H.; Chen, J.; Cheng, J.; Yang, J. In-situ formed graphene providing lubricity for the FeCoCrNiAl based composite containing graphite nanoplate. Compos. Part B Eng. 2021, 221, 109032. [Google Scholar] [CrossRef]
- Cheng, J.; Li, F.; Zhu, S.; Hao, J.; Yang, J.; Li, W.; Liu, W. High temperature tribological properties of a nickel-alloy-based solid-lubricating composite: Effect of surface tribo-chemistry, counterpart and mechanical properties. Wear 2017, 386–387, 39–48. [Google Scholar] [CrossRef]
- Zhen, J.; Han, Y.; Chen, J.; Cheng, J.; Zhu, S.; Yang, J.; Kong, L. Influence of Mo and Al elements on the vacuum high temperature tribological behavior of high strength nickel alloy matrix composites. Tribol. Int. 2019, 131, 702–709. [Google Scholar] [CrossRef]
- Feng, X.; Lu, C.; Jia, J.; Xue, J.; Wang, Q.; Sun, Y.; Wang, W.; Yi, G. High temperature tribological behaviors and wear mechanisms of NiAl–NbC–Ag composites formed by in-situ decomposition of AgNbO3. Tribol. Int. 2020, 141, 105898. [Google Scholar] [CrossRef]
- John, M.; Menezes, P.L. Self-Lubricating Materials for Extreme Condition Applications. Mater. 2021, 14, 3841. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Schuh, C.A.; Olivetti, E.A. Materials selection considerations for high entropy alloys. Scr. Mater. 2017, 138, 145–150. [Google Scholar] [CrossRef]
- Canter, N. High-entropy alloys. Tribol. Lubr. Technol. 2015, 71, 14. [Google Scholar]
- Xiao, L.L.; Zheng, Z.Q.; Guo, S.W.; Huang, P.; Wang, F. Ultra-strong nanostructured CrMnFeCoNi high entropy alloys. Mater. Des. 2020, 194, 108895. [Google Scholar] [CrossRef]












| Material | Manufacturing Method | Testing Method | Counter Material | Ball/Pin Diameter | Load (N) | Distance or Time | Speed or Frequency | Temperature (°C) | Hardness (HV) | Wear Rate (mm3/Nm) | Friction | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AlCoCrFeNi | VAR | Ball-on-Block | Si3N4 | 5 mm | 3 | 1200 s | 0.2 m/s | Room Temp. | 522 | 0.00018000 | 0.42 | [49] |
| AlCoCrFeNi | VAR | Pin-on-Disk | Al2O3 | 6 mm | 15 N | 1000 m | 100 mm/s | Room Temp. | 630 | 0.00008240 | 0.73 | [22] |
| 300 | 0.00005319 | 0.56 | ||||||||||
| 600 | 0.00000924 | 0.55 | ||||||||||
| 800 | 0.00000184 | 0.42 | ||||||||||
| 900 | 0.00000103 | 0.33 | ||||||||||
| AlCoCrFe0.6Mo0.5Ni | Arc smelting | Pin-on-Disk | SKH51 | 8 mm | 29.4 | 24 h | 0.5 m/s | Room Temp. | 751 | - | 0.74 | [50] |
| AlCoCrFe1Mo0.5Ni | 726 | - | 0.74 | |||||||||
| AlCoCrFe1.5Mo0.5Ni | 631 | - | 0.74 | |||||||||
| AlCoCrFe2Mo0.5Ni | 632 | - | 0.74 | |||||||||
| Al2CoCrCuFeNi | VAR | Pin-on-Disk | SKH51 | 8 mm | 29.4 | 64,800 m | 0.5 m/s | Room Temp. | 546 | 0.00010000 | 0.32 | [51] |
| AlCoCrFeNiTi0.5 | SPS | Reciprocating Wear | Al2O3 | 10 mm | 26 | 900 s | 40 Hz | Room Temp. | 750 | 1.22 | [52] | |
| 500 | 1.12 | |||||||||||
| 650 | 1.13 | |||||||||||
| 800 | 1.06 | |||||||||||
| 900 | 0.85 | |||||||||||
| AlCrFeNiTi | VAR | Pin-on-Disk | GCrl5 Steel | 9 mm | 20 | 1000 m | 0.8 m/s | Room Temp. | 616 | 0.00000260 | 0.55 | [53] |
| AlCrFeNiTiMn0.5 | 539 | 0.00000510 | 0.55 | |||||||||
| CoCrFeNiTi0.5Al0.5 | Induction Melting | Ball-on-Disk | WC | 6 mm | 10 | 216 m | Room Temp. | 968 | 0.35 | [26] | ||
| 250 | 0.45 | |||||||||||
| 500 | 0.55 | |||||||||||
| CoCrFeNiTi0.5Al | Room Temp. | 1264 | ||||||||||
| 250 | 0.37 | |||||||||||
| 500 | 0.47 | |||||||||||
| Co0.5CrCu0.5FeNi1.5AlTi0.4 | Arc Melting | Pin-on-Disk | Si3N4 | 3.175 mm | 0.25 | 200 m | 8.5 mm/s | Room Temp. | 626 | 0.00000003 | 0.55 | [54] |
| 100 | 0.6 | |||||||||||
| 300 | 0.25 | |||||||||||
| TiNbCrCoAl | Room Temp. | 365 | 0.00000011 | 0.6 | ||||||||
| 100 | 0.9 | |||||||||||
| 300 | 0.6 | |||||||||||
| CuMoTaWV | SPS | Ball-on-Disk | Alloy Steel | 9.33 mm | 5 | 200 m | 0.1 m/s | Room Temp. | 600 | 0.00000050 | 0.5 | [47] |
| Si3N4 | Room Temp. | 600 | 0.00400000 | 0.45 | ||||||||
| 200 | 0.00230000 | 0.6 | ||||||||||
| 400 | 0.00500000 | 0.67 | ||||||||||
| 600 | 0.04500000 | 0.54 | ||||||||||
| FeCoCrNiMnAl1 | Hot Press Sintering | Ball-on-Disk | Si3N4 | 5 mm | 10 | 15 min | 450 RPM | Room Temp. | 684 | 0.00010000 | 0.31 | [20] |
| 200 | 650 | 0.00012500 | 0.4 | |||||||||
| 500 | 580 | 0.00012000 | 0.275 | |||||||||
| 800 | 150 | 0.00250000 | 0.19 | |||||||||
| HfTaTiVZr | VAR | Ball-on-Disk | Si3N4 | 3 mm | 50 | 190 m | 5 Hz | Room Temp. | 693 | 0.00031600 | 0.25 | [55] |
| 150 | 635 | 0.00072100 | 0.28 | |||||||||
| 300 | 642 | 0.00024800 | 0.27 | |||||||||
| 450 | 622 | 0.00023800 | 0.25 | |||||||||
| TaTiVWZr | Room Temp. | 825 | 0.00027900 | 0.32 | ||||||||
| 150 | 785 | 0.00079000 | 0.35 | |||||||||
| 300 | 764 | 0.00011100 | 0.32 | |||||||||
| 450 | 683 | 0.00011100 | 0.3 | |||||||||
| MoTaNbZrTi | VAR | Ball-on-Disk | 100Cr6 Steel | 6 mm | 5 | 400 m | 10 cm/s | Room Temp. | 688 | 0.00013500 | 0.5 | [44] |
| 1000 m | 0.19000000 | 0.5 | ||||||||||
| Al2O3 | 400 m | 0.00014100 | 0.75 | |||||||||
| 1000 m | 1.35000000 | 0.75 | ||||||||||
| MoTaNbVTi | VAR | Ball-on-Disk | 100Cr6 Steel | 6 mm | 5 | 400 m | 10 cm/s | Room Temp. | 604 | 0.00002542 | [42] | |
| 1000 m | 0.00001957 | |||||||||||
| 2000 m | 0.00002520 | |||||||||||
| Al2O3 | 400 m | 0.00013200 | ||||||||||
| 1000 m | 0.00012960 | |||||||||||
| 2000 m | 0.00012100 | |||||||||||
| MoTaWNbV | VAR | Ball-on-Disk | Al2O3 | 6 mm | 5 | 1000 m | 10 cm/s | Room Temp. | 722 | 1.57000000 | [56] | |
| 100Cr6 Steel | 2.32000000 | |||||||||||
| MoTaWNbV | VAR | Ball-on-Disk | 100Cr6 Steel | 6 mm | 5 | 400 m | 10 cm/s | Room Temp. | 772 | 0.00002980 | 0.75 | [43] |
| 1000 m | 0.00002320 | 0.8 | ||||||||||
| 2000 m | 0.00001670 | 0.8 | ||||||||||
| Al2O3 | 400 m | 0.00004840 | 0.65 | |||||||||
| 1000 m | 0.00001570 | 0.8 | ||||||||||
| 2000 m | 0.00001050 | 0.8 | ||||||||||
| TiZrNbHf | VAR | Pin-on-Disk | GCr15 Steel Ball | 6 mm | 5 | 5400 mm | 3 Hz | RT | 225 | 0.002407 | 0.61 | [57] |
| Al0.25TiZrNbHf | 280 | 0.002074 | 0.58 | |||||||||
| Al0.5TiZrNbHf | 325 | 0.002055 | 0.55 | |||||||||
| Al0.75TiZrNbHf | 375 | 0.001740 | 0.60 | |||||||||
| Al1TiZrNbHf | 419 | 0.001629 | 0.58 |
| Material | Manufacturing Method | Testing Method | Counter Material | Ball/Pin Diameter | Load | Distance or Time | Speed or Frequency | Temperature (°C) | Hardness (HV) | Wear Rate (mm3/Nm) | Friction | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al1Co2Cr3Fe3Ni1 (FCC + σ + A2/B2) | SPS | Ball-on-Disk | Si3N4 | 6.43 mm | 5 | 340 m | 0.19 m/s | Room Temp. | 509 | 0.00015522 | 0.75 | [58] |
| 200 | 458 | 0.00020199 | 0.67 | |||||||||
| 400 | 428 | 0.00037326 | 0.65 | |||||||||
| 600 | 397 | 0.00013511 | 0.70 | |||||||||
| 800 | 336 | 0.00003800 | 0.57 | |||||||||
| Al0.2Co1.5CrFeNi1.5Ti | SPS | Ball-on-Plate | AISI 52100 | 9.51 mm | 1.2 | 30 min | 2 Hz | Room Temp. | 712 | 0.00000008 | 0.74 | [32] |
| 5 | 0.00000110 | 0.60 | ||||||||||
| VAR | 1.2 | 682 | 0.00000002 | 0.67 | ||||||||
| 5 | 0.00000003 | 0.66 | ||||||||||
| Al0.3CoCrFeNi | Arc Melting | Pin-on-Disk | Si3N4 | 3.175 mm | 0.25 N | 200 m | 8.5 mm/s | Room Temp. | 135 | 0.00000009 | 0.80 | [54] |
| 100 | 0.70 | |||||||||||
| 300 | 0.60 | |||||||||||
| Al0.3CuCrFeNi2 | Room Temp. | 140 | 0.00000007 | 0.80 | ||||||||
| 100 | 0.80 | |||||||||||
| 300 | 0.60 | |||||||||||
| Al0.5CoCrCuFeNi | VAR | Pin-on-Disk | SKH51 | 8 mm | 29.4 N | 64,800 m | 0.5 m/s | Room Temp. | 222 | 0.00070000 | 0.50 | [51] |
| Al0.25CoCrFeNi | Arc Melting | Ball-on-Disk | Si3N4 | 5.5 mm | 10 N | 30 min | 0.084 m/s | Room Temp. | 0.00014000 | 0.72 | [59] | |
| 100 | 0.00023000 | 0.65 | ||||||||||
| 200 | 0.00027700 | 0.61 | ||||||||||
| 300 | 0.00034000 | 0.57 | ||||||||||
| 400 | 0.00036000 | 0.57 | ||||||||||
| 500 | 0.00034000 | 0.52 | ||||||||||
| 600 | 0.00035000 | 0.51 | ||||||||||
| CoCrFeMnNi (FCC + σ phase) | Arc Melting | Pin-on-Disk | Al2O3 | 6mm | 5 N | 1000 m | 100 mm/s | Room Temp. | 0.00044148 | [41] | ||
| 600 | 0.00001580 | |||||||||||
| 800 | 0.00004732 | |||||||||||
| 15 N | Room Temp. | 0.00028983 | ||||||||||
| 600 | 0.00001768 | |||||||||||
| 800 | 0.00003429 | |||||||||||
| CoCrFeNiMn | SPS | Ball-on-Disk | Si3N4 | 6 mm | 5 N | 30 min | 0.28 m/s | Room Temp. | 1.81 | 0.00055000 | 0.62 | [30] |
| 200 | 0.00035000 | 0.59 | ||||||||||
| 400 | 0.00007541 | 0.44 | ||||||||||
| 600 | 0.00006494 | 0.42 | ||||||||||
| 800 | 0.00000463 | 0.37 | ||||||||||
| CoCrFeNiMn–Cr3C2 (10 wt.%) | Room Temp. | 323 | 0.00000878 | 0.46 | ||||||||
| 200 | 0.00004283 | 0.63 | ||||||||||
| 400 | 0.00002282 | 0.50 | ||||||||||
| 600 | 0.00000578 | 0.48 | ||||||||||
| 800 | 0.00000546 | 0.35 | ||||||||||
| CoCrFeNiMn- Cr3C2 (20 wt.%) | Room Temp. | 417 | 0.00000454 | 0.44 | ||||||||
| 200 | 0.00001053 | 0.56 | ||||||||||
| 400 | 0.00003708 | 0.53 | ||||||||||
| 600 | 0.00003228 | 0.62 | ||||||||||
| 800 | 0.00003704 | 0.35 | ||||||||||
| CoCrFeNiMn–Cr3C2 (40 wt.%) | Room Temp. | 681 | 0.00000447 | 0.44 | ||||||||
| 200 | 0.00000748 | 0.33 | ||||||||||
| 400 | 0.00004329 | 0.66 | ||||||||||
| 600 | 0.00001014 | 0.57 | ||||||||||
| 800 | 0.00000743 | 0.40 | ||||||||||
| CoCrFeNi | SPS | Ball-on-Disk | GCr15 Steel | 6.35 mm | 5 N | 3.6 m | 6 mm/s | Room Temp. | 415 | 0.00059000 | 0.76 | [28] |
| 50 N | 0.00041800 | 0.63 | ||||||||||
| CoCrFeNiMo0.1 | 5 N | 443 | 0.00052800 | 0.75 | ||||||||
| 50 N | 0.00036400 | 0.61 | ||||||||||
| CoCrFeNiMo0.3 | 5 N | 465 | 0.00050700 | 0.71 | ||||||||
| 50 N | 0.00033400 | 0.59 | ||||||||||
| CoCrFeNiTi0.5 (FCC + σ) | Induction Melting | Ball-on-Disk | WC | 6 mm | 10 N | 216 m | Room Temp. | [26] | ||||
| 250 | ||||||||||||
| 500 | ||||||||||||
| CoCrFeNiNb0.5 (FCC + Laves phase) | Arc Melting | Pin-on-Disk | Si3N4 | 6.55 mm | 5 N | 30 min | 0.188 m/s | Room Temp. | 546 | 0.00004095 | 0.82 | [60] |
| 200 | 494 | 0.00005959 | 0.58 | |||||||||
| 400 | 458 | 0.00007319 | 0.38 | |||||||||
| 600 | 432 | 0.00003007 | 0.54 | |||||||||
| 800 | 397 | 0.00001971 | 0.37 | |||||||||
| 1000 | 334 | |||||||||||
| CoCrFeNiNb0.65 (FCC + Laves phase) | Room Temp. | 597 | 0.00001833 | 0.77 | ||||||||
| 200 | 562 | 0.00004988 | 0.49 | |||||||||
| 400 | 526 | 0.00007504 | 0.38 | |||||||||
| 600 | 501 | 0.00004984 | 0.52 | |||||||||
| 800 | 436 | 0.00000069 | 0.60 | |||||||||
| 1000 | 391 | |||||||||||
| CoCrFeNiNb0.8 (FCC + Laves phase) | Room Temp. | 649 | 0.00001603 | 0.70 | ||||||||
| 200 | 604 | 0.00004759 | 0.45 | |||||||||
| 400 | 578 | 0.00008533 | 0.46 | |||||||||
| 600 | 552 | 0.00005825 | 0.60 | |||||||||
| 800 | 497 | 0.00000064 | 0.70 | |||||||||
| 1000 | 429 | |||||||||||
| CoCrFeMnNiC | Mechanical alloying + SPS | Ball-on-Disk | Si3N4 | 5 mm | 20 N | 100 m | 4 Hz | Room Temp. | 327 | 0.00006500 | 0.76 | [61] |
| CoCrFeMnNiC0.3 | 460 | 0.00003600 | 0.78 | |||||||||
| CoCrFeMnNiC0.6 | 566 | 0.00000470 | 0.67 | |||||||||
| CoCrFeMnNiC0.9 | 558 | 0.00000860 | 0.69 | |||||||||
| CoCrFeMnNiC1.2 | 525 | 0.00003000 | 0.77 | |||||||||
| CoCrFeNiCu | Arc Melting | Pin-on-Disk | Not Specified | 8 mm | 100 N | Room Temp. | 136 | 0.00002293 | [27] | |||
| 600 | 0.00002333 | |||||||||||
| CoCrFeNiCu0.2 | Room Temp. | 143 | 0.00001893 | |||||||||
| 600 | 0.00002013 | |||||||||||
| CoCrFeNiCu0.4 | Room Temp. | 153 | 0.00001893 | |||||||||
| 600 | 0.00001700 | |||||||||||
| CoCrFeNiCu0.6 | Room Temp. | 158 | 0.00002520 | |||||||||
| 600 | 0.00001467 | |||||||||||
| CoCrFeNiCu0.8 | Room Temp. | 160 | 0.00001353 | |||||||||
| 600 | 0.00001413 | |||||||||||
| CoCrFeNiCu1 | Room Temp. | 169 | 0.00001507 | |||||||||
| 600 | 0.00001320 | |||||||||||
| Co1.5CrFeNi1.5Ti0.5 | VAR | Ball-on-Disk | Al2O3 | 6 mm | 5 N | 1000 m | 0.1 m/s | Room Temp. | 368 | 0.00113000 | [45] | |
| 100Cr6 Steel | 0.00052000 | |||||||||||
| CoCrFeMnNi | VAR | Pin-on-Disk | Al2O3 | 6 mm | 15 N | 1000 m | 100 mm/s | Room Temp. | 255 | 0.00026630 | 0.61 | [22] |
| 300 | 0.00007836 | 0.57 | ||||||||||
| 600 | 0.00001573 | 0.55 | ||||||||||
| 800 | 0.00001837 | 0.43 | ||||||||||
| 900 | 0.00002193 | 0.35 | ||||||||||
| Al0.3CoCrFeNi | Room Temp. | 176 | 0.00022324 | 0.64 | ||||||||
| 300 | 0.00008199 | 0.55 | ||||||||||
| 600 | 0.00001760 | 0.51 | ||||||||||
| 800 | 0.00001702 | 0.44 | ||||||||||
| 900 | 0.00001556 | 0.34 | ||||||||||
| FeCoCrNiMn | Hot Press Sintering | Ball-on-Disk | Si3N4 | 5 mm | 10 N | 15 min | 450 RPM | Room Temp. | 415 | 0.00027500 | 0.25 | [20] |
| 200 | 400 | 0.00037500 | 0.25 | |||||||||
| 500 | 375 | 0.00030700 | 0.25 | |||||||||
| 800 | 90 | 0.00005000 | 0.25 | |||||||||
| AlCoCrFeNi2.1 | VAR | Reciprocating | Si3N4 | 6.35 | 30 | 6300 mm | 1 Hz | 500 | - | 100 | 0.36 | [62] |
| 600 | - | 650 | 0.47 | |||||||||
| 700 | - | 1100 | 0.64 | |||||||||
| 800 | - | 1800 | 0.37 | |||||||||
| 900 | - | 1600 | 0.40 |
| Material | Manufacturing Method | Testing Method | Counter Material | Ball/Pin Diameter | Load | Distance or Time | Speed or Frequency | Temperature | Hardness (HV) | Wear Rate (mm3/Nm) | Friction | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al0.3CrFe1.5MnNi0.5 | VAR | Pin-on-Disk | SKH51 | 29.4 N | 64800 m | 0.5 m/s | Room Temp. | 462 | 0.00002510 | 0.56 | [34] | |
| Air melting | 444 | 0.00002930 | 0.51 | |||||||||
| Al1.3CoCuFeNi2 | VAR | Ball-on-Block | Si3N4 | 5 mm | 3 N | 1200 s | 0.2 m/s | Room Temp. | 340 | 0.00120000 | 0.63 | [61] |
| Al1CoCrCuFeNi | VAR | Pin-on-Disk | SKH-51 Steel | 8 mm | 29.4 N | 5400 m | 0.5 m/s | Room Temp. | 349 | 0.00050000 | 0.48 | [51] |
| Al0.6CoCrFeNi | VAR | Pin-on-Disk | Al2O3 | 6 mm | 15 N | 1000 m | 100 mm/s | Room Temp. | 251 | 0.00021328 | 0.57 | [22] |
| 300 | 0.00007927 | 0.56 | ||||||||||
| 600 | 0.00001627 | 0.51 | ||||||||||
| 800 | 0.00002369 | 0.39 | ||||||||||
| 900 | 0.00003022 | 0.32 | ||||||||||
| AlCrCuFeNi2 | VAR | Ball-on-Block | Si3N4 | 5 mm | 5 N | 30 min | 0.2 m/s | Room Temp. | 0.00216300 | 0.35 | [63] | |
| 10 N | 0.00047800 | 0.45 | ||||||||||
| 15 N | 0.00030500 | 0.25 | ||||||||||
| AlCoCrFeNi2.1 | VAR | Ball-on-Disk | Al2O3 | 6 mm | 4.9 N | 30 min | 0.28 m/s | room temp | 319 | 0.00042000 | 0.59 | [46] |
| Si3N4 | 0.00037000 | 0.54 | ||||||||||
| GCr15 Steel | 0.00034000 | 0.57 | ||||||||||
| SiC | 0.00009700 | 0.5 | ||||||||||
| 200 | 306 | 0.00021200 | 0.68 | |||||||||
| 400 | 282 | 0.00041400 | 0.69 | |||||||||
| 600 | 270 | 0.00061800 | 0.73 | |||||||||
| 800 | 258 | 0.00081900 | 0.34 | |||||||||
| 900 | 197 | 0.00092200 | 0.29 | |||||||||
| AlCoCrFeNi (FCC + A2/B2 + σ) | SPS | Ball-on-Disk | Si3N4 | 6.43 mm | 5 N | 340 m | 0.19 m/s | Room Temp. | 551 | 0.00010540 | 0.79 | [58] |
| 200 | 489 | 0.00016656 | 0.7 | |||||||||
| 400 | 469 | 0.00031334 | 0.63 | |||||||||
| 600 | 428 | 0.00010688 | 0.66 | |||||||||
| 800 | 377 | 0.00001911 | 0.43 | |||||||||
| Al1.3Co1.15Cr1Fe1Ni1.3 (FCC + A2/B2 + σ) | Room Temp. | 581 | 0.00010160 | 0.69 | ||||||||
| 200 | 510 | 0.00013830 | 0.63 | |||||||||
| 400 | 489 | 0.00018005 | 0.615 | |||||||||
| 600 | 459 | 0.00004840 | 0.632 | |||||||||
| 800 | 387 | 0.00001675 | 0.384 | |||||||||
| Al0.6CoCrFeNi | VAR | Ball-on-Block | GCr15 Steel | 5 mm | 5 N | 1800 s | 2 Hz | Room Temp. | 280 | 0.00065200 | 0.542 | [40] |
| 3 Hz | 0.00063600 | 0.536 | ||||||||||
| 4 Hz | 0.00015900 | 0.365 | ||||||||||
| 5 Hz | 0.00019000 | 0.402 | ||||||||||
| CoCrFeNiAl0.25Ti0.75 | Arc Melting | Pin-on-Disk | Si3N4 | 3.175 mm | 0.25 N | 200 m | 8.5 mm/s | Room Temp. | 866 | 0.00000003 | 0.6 | [54] |
| 100 | 0.8 | |||||||||||
| 300 | 0.55 | |||||||||||
| FeCoCrNiMnAl0.5 | Hot Press Sintering | Ball-on-Disk | Si3N4 | 5 mm | 10 N | 15 min | 450 RPM | Room Temp. | 553 | 0.00015000 | 0.32 | [20] |
| 200 | 510 | 0.00020000 | 0.32 | |||||||||
| 500 | 410 | 0.00017500 | 0.275 | |||||||||
| 800 | 125 | 0.00003500 | 0.19 | |||||||||
| FeCoNiCuAl | SPS | Ball-on-Disk | Si3N4 | 6 mm | 10 N | 30 min | 0.5 m/s | Room Temp. | 475 | 0.00006500 | 0.53 | [64] |
| 600 | 0.00076000 | 0.55 | ||||||||||
| FeCoNiCuAl–TiC(10wt.%) | Room Temp. | 525 | 0.00004890 | 0.67 | ||||||||
| 600 | 0.00005440 | 0.46 | ||||||||||
| FeCoNiCuAl–TiC(20wt.%) | Room Temp. | 575 | 0.00002920 | 0.67 | ||||||||
| 600 | 0.00001730 | 0.39 | ||||||||||
| FeCoNiCuAl–TiC(30 wt.%) | Room Temp. | 750 | 0.00001979 | 0.6 | ||||||||
| 600 | 0.00001680 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasar, A.K.; Scalaro, K.; Menezes, P.L. Tribological Properties of High-Entropy Alloys under Dry Conditions for a Wide Temperature Range—A Review. Materials 2021, 14, 5814. https://doi.org/10.3390/ma14195814
Kasar AK, Scalaro K, Menezes PL. Tribological Properties of High-Entropy Alloys under Dry Conditions for a Wide Temperature Range—A Review. Materials. 2021; 14(19):5814. https://doi.org/10.3390/ma14195814
Chicago/Turabian StyleKasar, Ashish K., Kelsey Scalaro, and Pradeep L. Menezes. 2021. "Tribological Properties of High-Entropy Alloys under Dry Conditions for a Wide Temperature Range—A Review" Materials 14, no. 19: 5814. https://doi.org/10.3390/ma14195814
APA StyleKasar, A. K., Scalaro, K., & Menezes, P. L. (2021). Tribological Properties of High-Entropy Alloys under Dry Conditions for a Wide Temperature Range—A Review. Materials, 14(19), 5814. https://doi.org/10.3390/ma14195814







