Change in Pull-Out Force during Resorption of Magnesium Compression Screws for Osteosynthesis of Mandibular Condylar Fractures
Abstract
:1. Introduction
2. Materials and Methods
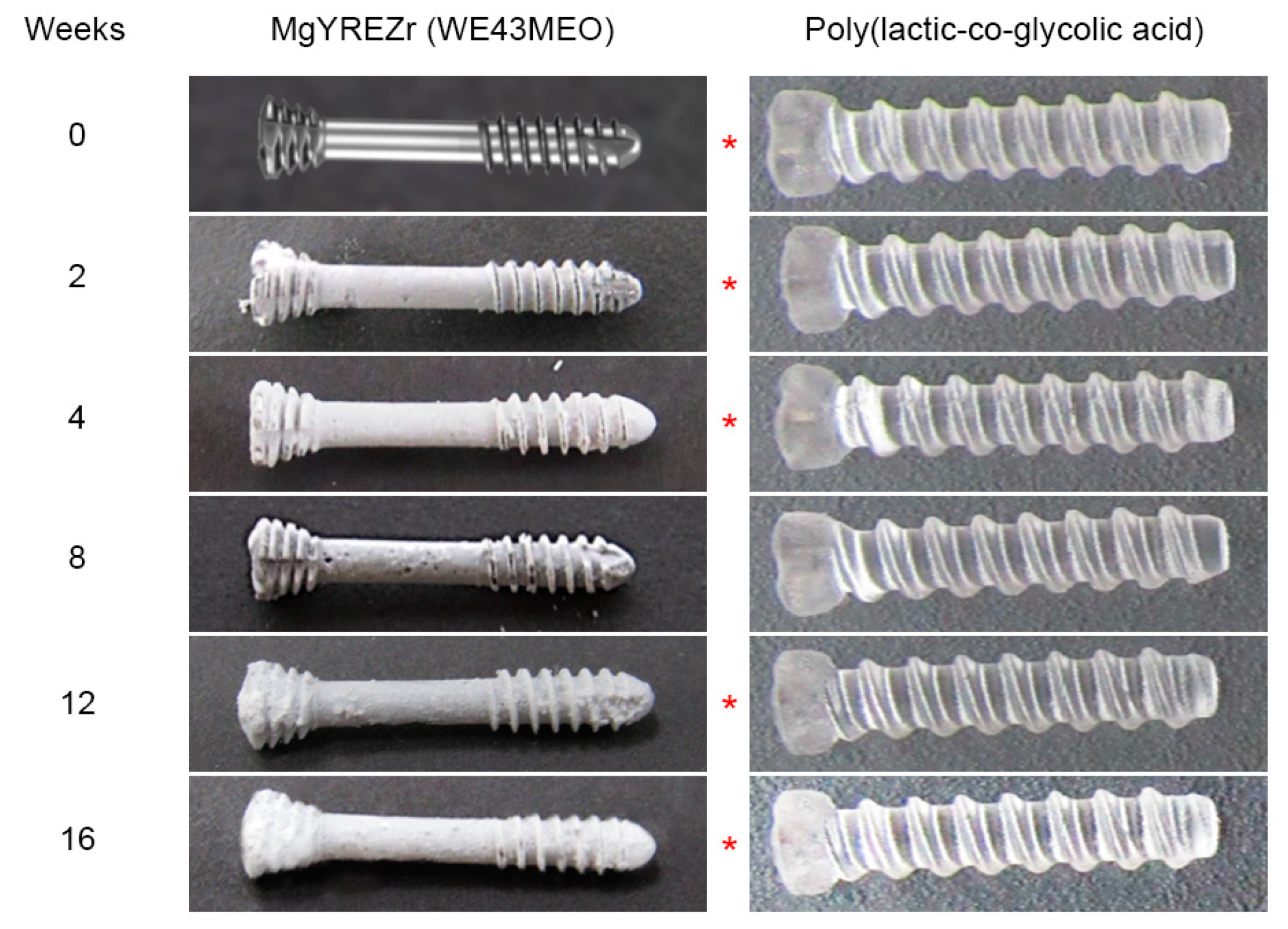
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korzon, T. Nowa metoda chirurgicznego leczenia złamań wyrostków kłykciowych żuchwy. Pol. Tyg. Lek. 1970, 25, 1391–1394. [Google Scholar]
- Eckelt, U.; Rasse, M. Controle clinique, radiographique et axiographique apres osteosynthese par vis de traction des fractures de la region condylienne de la mandibule. Rev. Stomatol. Chir. Maxillofac. 1995, 96, 158–165. [Google Scholar]
- Neff, A.; Kolk, A.; Neff, F.; Horch, H.H. Surgical vs. conservative therapy of diacapitular and high condylar fractures with dislocation. A comparison between MRI and axiography. Mund Kiefer Gesichtschir. 2002, 6, 66–73. [Google Scholar] [CrossRef]
- Meyer, C.; Zink, S.; Chatelain, B.; Wilk, A. Clinical experience with osteosynthesis of subcondylar fractures of the mandible using TCP plates. J. Cranio-Maxillofac. Surg. 2008, 36, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Galil, K.; Loukota, R. Fractures of the mandibular condyle: Evidence base and current concepts of management. Br. J. Oral Maxillofac. Surg. 2010, 48, 520–526. [Google Scholar] [CrossRef]
- Sikora, M.; Chęciński, M.; Sielski, M.; Chlubek, D. The Use of 3D Titanium Miniplates in Surgical Treatment of Patients with Condylar Fractures. J. Clin. Med. 2020, 9, 2923. [Google Scholar] [CrossRef]
- Pavlychuk, T.; Chernogorskyi, D.; Chepurnyi, Y.; Neff, A.; Kopchak, A. Biomechanical evaluation of type p condylar head osteosynthesis using conventional small-fragment screws reinforced by a patient specific two-component plate. Head Face Med. 2020, 16, 25. [Google Scholar] [CrossRef]
- Pavlychuk, T.; Shydlovsky, M.; Kopchak, A. A comparative biomechanical evaluation of different osteosynthesis techniques used for intracapsular condylar head fractures. J. Oral Biol. Craniofac. Res. 2019, 9, 123–127. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Sidun, J.; Świderska, M.; Zalewska, A. Free radical production, inflammation and apoptosis in patients treated with titanium mandibular fixations-an observational study. Front. Immunol. 2019, 10, 2662. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Kretowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V titanium alloy leads to redox abnormalities, oxidative stress, and oxidative damage in patients treated for mandible fractures. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Kretowski, A.; Sidun, J.; Domel, E.; Dąbrowski, J.; Ładny, J.R.; Morawska, K.; Zalewska, A. Glutathione metabolism, mitochondria activity, and nitrosative stress in patients treated for mandible fractures. J. Clin. Med. 2019, 8, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacifici, L.; De Angelis, F.; Orefici, A.; Cielo, A. Metals used in maxillofacial surgery. Oral Implantol. 2017, 9, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.C.; Valderrama, P.; Wilson, T.G.; Palmer, K.; Thomas, A.; Sridhar, S.; Adapalli, A.; Burbano, M.; Wadhwani, C. Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef] [Green Version]
- Noumbissi, S.; Scarano, A.; Gupta, S. A Literature Review Study on Atomic Ions Dissolution of Titanium and Its Alloys in Implant Dentistry. Materials 2019, 12, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolk, A.; Neff, A. Long-term results of ORIF of condylar head fractures of the mandible: A prospective 5-year follow-up study of small-fragment positional-screw osteosynthesis (SFPSO). J. Cranio-Maxillofac. Surg. 2015, 43, 452–461. [Google Scholar] [CrossRef]
- Skroch, L.; Fischer, I.; Meisgeier, A.; Kozolka, F.; Apitzsch, J.; Neff, A. Condylar remodeling after osteosynthesis of fractures of the condylar head or close to the temporomandibular joint. J. Cranio-Maxillofac. Surg. 2020, 48, 413–420. [Google Scholar] [CrossRef]
- Gareb, B.; Roossien, C.C.; van Bakelen, N.B.; Verkerke, G.J.; Vissink, A.; Bos, R.R.M.; van Minnen, B. Comparison of the mechanical properties of biodegradable and titanium osteosynthesis systems used in oral and maxillofacial surgery. Sci. Rep. 2020, 10, 18143. [Google Scholar] [CrossRef]
- Bell, R.B.; Kindsfater, C.S. The use of biodegradable plates and screws to stabilize facial fractures. J. Oral Maxillofac. Surg. 2006, 64, 31–39. [Google Scholar] [CrossRef]
- Böker, K.O.; Richter, K.; Jäckle, K.; Taheri, S.; Grunwald, I.; Borcherding, K.; von Byern, J.; Hartwig, A.; Wildemann, B.; Schilling, A.F.; et al. Current State of Bone Adhesives—Necessities and Hurdles. Materials 2019, 12, 3975. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016, 6, 27374. [Google Scholar] [CrossRef]
- Dobrosielska, M.; Przekop, R.E.; Sztorch, B.; Brząkalski, D.; Zgłobicka, I.; Łępicka, M.; Dobosz, R.; Kurzydłowski, K.J. Biogenic Composite Filaments Based on Polylactide and Diatomaceous Earth for 3D Printing. Materials 2020, 13, 4632. [Google Scholar] [CrossRef] [PubMed]
- McLeod, N.H.N.; Saeed, N.R. Treatment of fractures of the mandibular condylar head with ultrasound-activated resorbable pins: Early clinical experience. Br. J. Oral Maxillofac. Surg. 2016, 54, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.T.; Kim, W.H.; Park, B.; Lee, J.-H.; Kim, B.; Lee, J.-H. Biomechanical evaluation of unilateral subcondylar fracture of the mandible on the varying materials: A finite element analysis. PLoS ONE 2020, 15, e0240352. [Google Scholar] [CrossRef] [PubMed]
- Kallela, I.; Iizuka, T.; Salo, A.; Lindqvist, C. Lag-screw fixation of anterior mandibular fractures using biodegradable polylactide screws: A preliminary report. J. Oral Maxillofac. Surg. 1999, 57, 113–118. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Xue, A.S.; Koshy, J.C.; Weathers, W.M.; Wolfswinkel, E.M.; Kaufman, Y.; Sharabi, S.E.; Brown, R.H.; Hicks, M.J.; Hollier, L.H., Jr. Local foreign-body reaction to commercial biodegradable implants: An in vivo animal study. Craniomaxillofac. Trauma Reconstr. 2014, 7, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Athanasiou, K.A.; Agrawal, C.M.; Barber, F.A.; Burkhart, S.S. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Poircuitte, J.M.; Popkov, D.; Huber, H.; Polirsztok, E.; Lascombes, P.; Journeau, P. Resorbable osteosynthetic devices in pediatric traumatology: A prospective series of 24 cases. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 997–1004. [Google Scholar] [CrossRef]
- Korhonen, L.; Perhomaa, M.; Kyrö, A.; Pokka, T.; Serlo, W.; Merikanto, J.; Sinikumpu, J.-J. Intramedullary nailing of forearm shaft fractures by biodegradable compared with titanium nails: Results of a prospective randomized trial in children with at least two years of follow-up. Biomaterials 2018, 185, 383–392. [Google Scholar] [CrossRef]
- Hedelin, H.; Larnert, P.; Hebelka, H.; Brisby, H.; Lagerstrand, K.; Laine, T. Innominate Salter osteotomy using resorbable screws: A retrospective case series and presentation of a new concept for fixation. J. Child. Orthop. 2019, 13, 310–317. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.W.; Pang, K.M.; Kim, H.E.; Kim, S.M.; Lee, J.H. Biomechanical evaluation of magnesium-based resorbable metallic screw system in a bilateral sagittal split ramus osteotomy model using three-dimensional finite element analysis. J. Oral Maxillofac. Surg. 2014, 72, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable Osteofixation Materials for Maxillofacial Bone Surgery: A Review on Polymers and Magnesium-Based Materials. Biomaterials 2020, 8, 300. [Google Scholar] [CrossRef]
- Felice, P.; Lizio, G.; Marchetti, C.; Checchi, L.; Scarano, A. Magnesium-Substituted Hydroxyapatite Grafting Using the Vertical Inlay Technique. Int. J. Periodontics Restor. Dent. 2013, 33, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, J.R.; Harrington, R.M.; Lee, K.M.; Anderson, P.A.; Tencer, A.F.; Kowalski, D. Factors affecting the pullout strength of cancellous bone screws. J. Biomech. Eng. 1996, 118, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Darvish, K.; Ilyas, A.M. Biomechanical analysis of second-generation headless compression screws. Injury 2012, 43, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Baran, O.; Sagol, E.; Oflaz, H.; Sarikanat, M.; Havitcioglu, H. A biomechanical study on preloaded compression effect on headless screws. Arch. Orthop. Trauma Surg. 2009, 129, 1601–1605. [Google Scholar] [CrossRef]
- Ramaswamy, R.; Evans, S.; Kosashvili, Y. Holding power of variable pitch screws in osteoporotic, osteopenic and normal bone: Are all screws created equal? Injury 2010, 41, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, G.W.; Zhai, Z.J.; Liu, L.N.; Li, H.W.; Yang, K.; Tan, L.L.; Wan, P.; Liu, X.Q.; Ouyang, Z.X.; et al. Antibacterial properties of magnesium in vitro and in an in vivo model of implant-associated methicillin-resistant staphylococcus aureus infection. Antimicrob. Agents Chemother. 2014, 58, 7586–7591. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.; Lin, X.; Tan, L.L.; Yang, K. Effect of surface coating on antibacterial behawior of magnesium based metals. Mater. Lett. 2011, 65, 3509–3511. [Google Scholar] [CrossRef]
- Robinson, D.; Griffith, R.W.; Shechtman, D.; Evans, R.B.; Conzemius, M.G. In vitro antibacterial properties of magnesium metal against escherichia coli, pseudomonas aeruginosa and staphylococcus aureus. Acta Biomater. 2010, 6, 1869–1877. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Review Tetracycline-based histological analysis of bone remodeling. Calcif. Tissue Res. 1969, 3, 211–237. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M. Are Magnesium Screws Proper for Mandibular Condyle Head Osteosynthesis? Materials 2020, 13, 2641. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.A.; Kuiper, J.H.; Kelly, C.P. Biomechanical evaluation of a new composite bioresorbable screw. J. Hand Surg. 2006, 31, 208–212. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Herford, A.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive titanium surfaces: Interactions of eukaryotic and prokaryotic cells of nano devices applied to dental practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Z.J.; Qu, X.H.; Li, H.W.; Yang, K.; Wan, P.; Tan, L.L.; Ouyang, Z.X.; Liu, X.Q.; Tian, B.; Xiao, F.; et al. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-kappa B and NFATc1 signaling. Biomaterials 2014, 35, 6299–6310. [Google Scholar] [CrossRef]
- Mazur, A.; Maier, J.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef]
- Peng, Q.; Li, K.; Han, Z.; Wang, E.; Xu, Z.; Liu, R.; Tian, Y. Degradable magnesium-based implant materials with anti-inflammatory activity. J. Biomed. Mater. Res. Part A 2012, 101, 1898–1906. [Google Scholar] [CrossRef]
- Rokkanen, P.U.; Böstman, O.; Hirvensalo, E.; Mäkelä, E.; Partio, E.K.; Pätiälä, H.; Vainionpää, S.; Vihtonen, K.; Törmälä, P. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials 2000, 21, 2607–2613. [Google Scholar] [CrossRef]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable materials for bone repairs: A review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Li, J.; Rai, S.; Gao, Y.; Ze, R.; Tang, X.; Liu, R.; Hong, P. Biodegradable pins for lateral condylar fracture of the humerus with an early delayed presentation in children: A retrospective study of biodegradable pin vs. Kirschner wire. BMC Musculoskelet. Disord. 2020, 21, 735. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.; Pietak, A.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feyerabend, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Świniarski, J. Treatment of high fracture of the neck of the mandibular condylar process by rigid fixation performed by lag screws: Finite element analysis. Dent. Med. Probl. 2017, 54, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Leonhardt, H.; Franke, A.; McLeod, N.; Lauer, G.; Nowak, A. Fixation of fractures of the condylar head of the mandible with a new magnesium-alloy biodegradable cannulated headless bone screw. Br. J. Oral Maxillofac. Surg. 2017, 55, 623–625. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Kozakiewicz, M. Comparison of compression screws used for mandible head fracture treatment—Experimental study. Clin. Oral Investig. 2019, 23, 4059–4066. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.-C.J.; Chen, Y.-J.; Tung, Y.-Y.; Chiang, Y.-Y.; Lai, E.H.-H.; Chen, W.-P.; Lin, C.-P. Effects of thread depth, taper shape, and taper length on the mechanical properties of mini-implants. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 279–288. [Google Scholar] [CrossRef]
- Peltoniemi, H.; Ashammakhi, N.; Kontio, R.; Waris, T.; Salo, A.; Lindqvist, C.; Gratz, K.; Suuronen, R. The use of bioabsorbable osteofixation devices in craniomaxillofacial surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 5–14. [Google Scholar] [CrossRef]
- Bergsma, E.J.; Rozema, F.R.; Bos, R.R.; De Bruijn, W.C. Foreign body reactions to resorbable poly(l-lactide) bone plates and screws used for the fixation of unstable zygomatic fractures. J. Oral Maxillofac. Surg. 1993, 51, 666–670. [Google Scholar] [CrossRef]
- Abdel-Galil, K.; Loukota, R. Fixation of comminuted diacapitular fractures of the mandibular condyle with ultrasound-activated resorbable pins. Br. J. Oral Maxillofac. Surg. 2008, 46, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Xiong, M.; Guan, X.; Zhang, J.; Huang, H.; Pei, J.; Yuan, G. The in vivo degradation and bone-implant interface of Mg-Nd-Zn-Zr alloy screws: 18 months post-operation results. Corros. Sci. 2016, 113, 183–187. [Google Scholar] [CrossRef]
- Naujokat, H.; Seitz, J.-M.; Açil, Y.; Damm, T.; Möller, I.; Gülses, A.; Wiltfang, J. Osteosynthesis of a cranio-osteoplasty with a biodegradable magnesium plate system in miniature pigs. Acta Biomater. 2017, 62, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M. Small-diameter compression screws completely embedded in bone for rigid internal fixation of the condylar head of the mandible. Br. J. Oral Maxillofac. Surg. 2018, 56, 74–76. [Google Scholar] [CrossRef]
- Sukotjo, C.; Lima-Neto, T.J.; Santiago Júnior, J.F.; Faverani, L.P.; Miloro, M. Is There a Role for Absorbable Metals in Surgery? A Systematic Review and Meta-Analysis of Mg/Mg Alloy Based Implants. Materials 2020, 18, 3914. [Google Scholar] [CrossRef]
- Zieliński, R.; Kozakiewicz, M.; Świniarski, J. Comparison of Titanium and Bioresorbable Plates in “A” Shape Plate Properties—Finite Element Analysis. Materials 2019, 12, 1110. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lu, C.; Yang, S.; Sun, P.; Wang, Y.; Guan, Y.; Liu, S.; Cheng, D.; Meng, H.; Wang, Q.; et al. A fully biodegradable and self-electrified device for neuroregenerative medicine. Sci. Adv. 2020, 6, eabc6686. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.; Zhang, X.; Nune, K.C.; Zhao, Y.; Liu, N.; Misra, R.D.K.; Yang, K.; Tan, L.; Yan, J. The effect of different coatings on bone response and degradation behavior of porous magnesium-strontium devices in segmental defect regeneration. Bioact. Mater. 2020, 6, 1765–1776. [Google Scholar] [CrossRef]
- Yao, H.; Xu, J.; Wang, J.; Zhang, Y.; Zheng, N.; Yue, J.; Mi, J.; Zheng, L.; Dai, B.; Huang, W.; et al. Combination of magnesium ions and vitamin C alleviates synovitis and osteophyte formation in osteoarthritis of mice. Bioact. Mater. 2020, 6, 1341–1352. [Google Scholar] [CrossRef]
- Chu, W.; Li, T.; Jia, G.; Chang, Y.; Liu, Z.; Pei, J.; Yu, D.; Zhai, Z. Exposure to high levels of magnesium disrupts bone mineralization in vitro and in vivo. Ann. Transl. Med. 2020, 8, 1419. [Google Scholar] [CrossRef]
- Duan, H.; Cao, C.; Wang, X.; Tao, J.; Li, C.; Xin, H.; Yang, J.; Song, Y.; Ai, F. Magnesium-alloy rods reinforced bioglass bone cement composite scaffolds with cortical bone-matching mechanical properties and excellent osteoconductivity for load-bearing bone in vivo regeneration. Sci. Rep. 2020, 10, 18193. [Google Scholar] [CrossRef] [PubMed]


| Time [Weeks] | MgYREZr [N] | PLGA [N] | Note |
|---|---|---|---|
| 0 | 399 ± 7.5 | 138 ± 26.5 | p < 0.01 |
| 2 | 367 ± 28.6 | 147 ± 4.3 | p < 0.01 |
| 4 | 249 ± 34.2 | 143 ± 11.0 | p < 0.01 |
| 8 | 118 ± 71.1 | 97 ± 17.3 | NS |
| 12 | 201 ± 27.1 | 72 ± 27.2 | p < 0.01 |
| 16 | 102 ± 36.4 | 49 ± 7.0 | p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozakiewicz, M. Change in Pull-Out Force during Resorption of Magnesium Compression Screws for Osteosynthesis of Mandibular Condylar Fractures. Materials 2021, 14, 237. https://doi.org/10.3390/ma14020237
Kozakiewicz M. Change in Pull-Out Force during Resorption of Magnesium Compression Screws for Osteosynthesis of Mandibular Condylar Fractures. Materials. 2021; 14(2):237. https://doi.org/10.3390/ma14020237
Chicago/Turabian StyleKozakiewicz, Marcin. 2021. "Change in Pull-Out Force during Resorption of Magnesium Compression Screws for Osteosynthesis of Mandibular Condylar Fractures" Materials 14, no. 2: 237. https://doi.org/10.3390/ma14020237
APA StyleKozakiewicz, M. (2021). Change in Pull-Out Force during Resorption of Magnesium Compression Screws for Osteosynthesis of Mandibular Condylar Fractures. Materials, 14(2), 237. https://doi.org/10.3390/ma14020237





