A Non-Isokinetic Approach for Modeling Solid-State Transformations: Application to Crystallization of a Fe-B Amorphous Alloy
Abstract
:1. Introduction
2. Theoretical Background
2.1. Transformation Rate
2.2. Non-Isokinetic Analysis
3. Materials and Methods
4. Results and Discussion
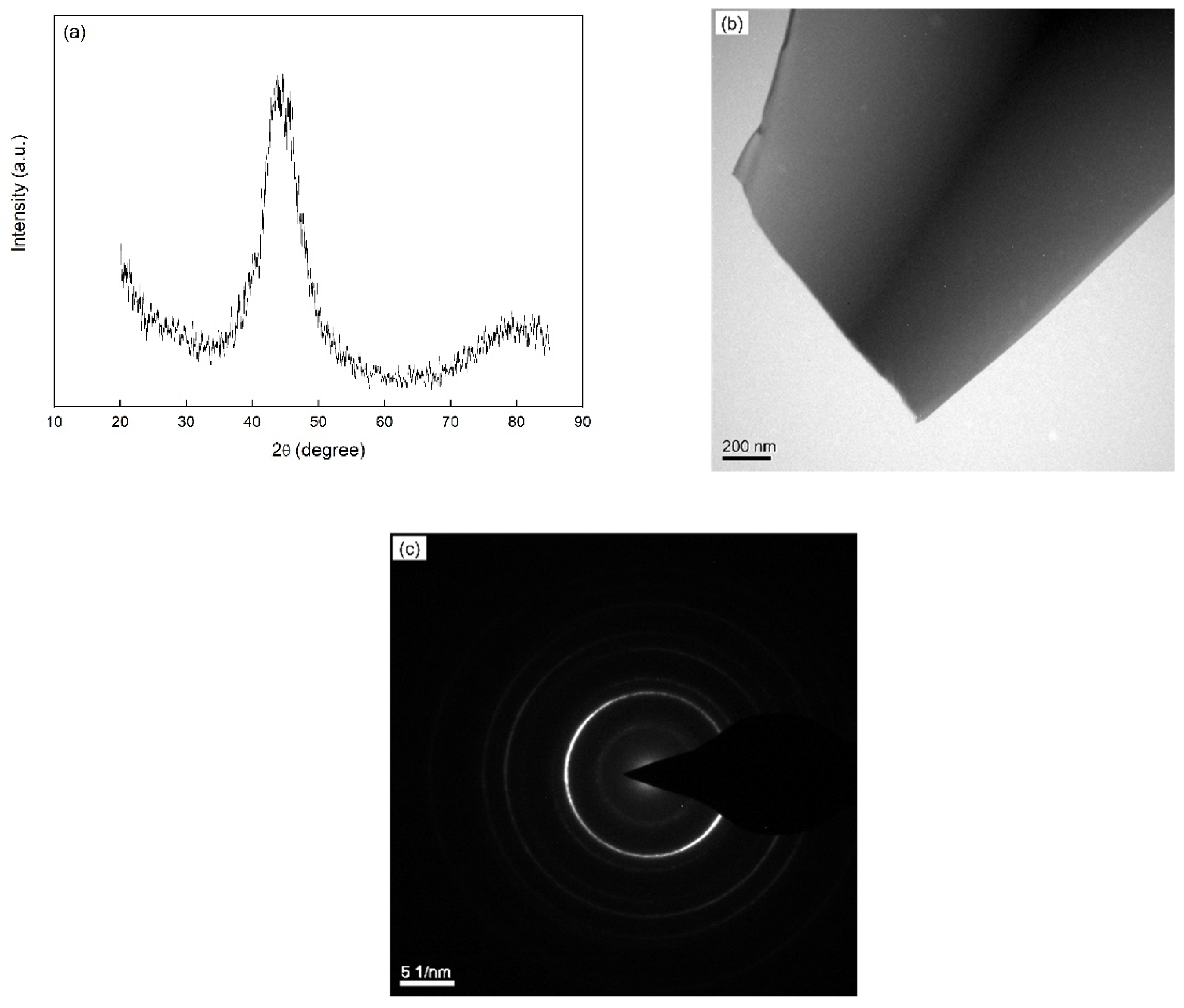
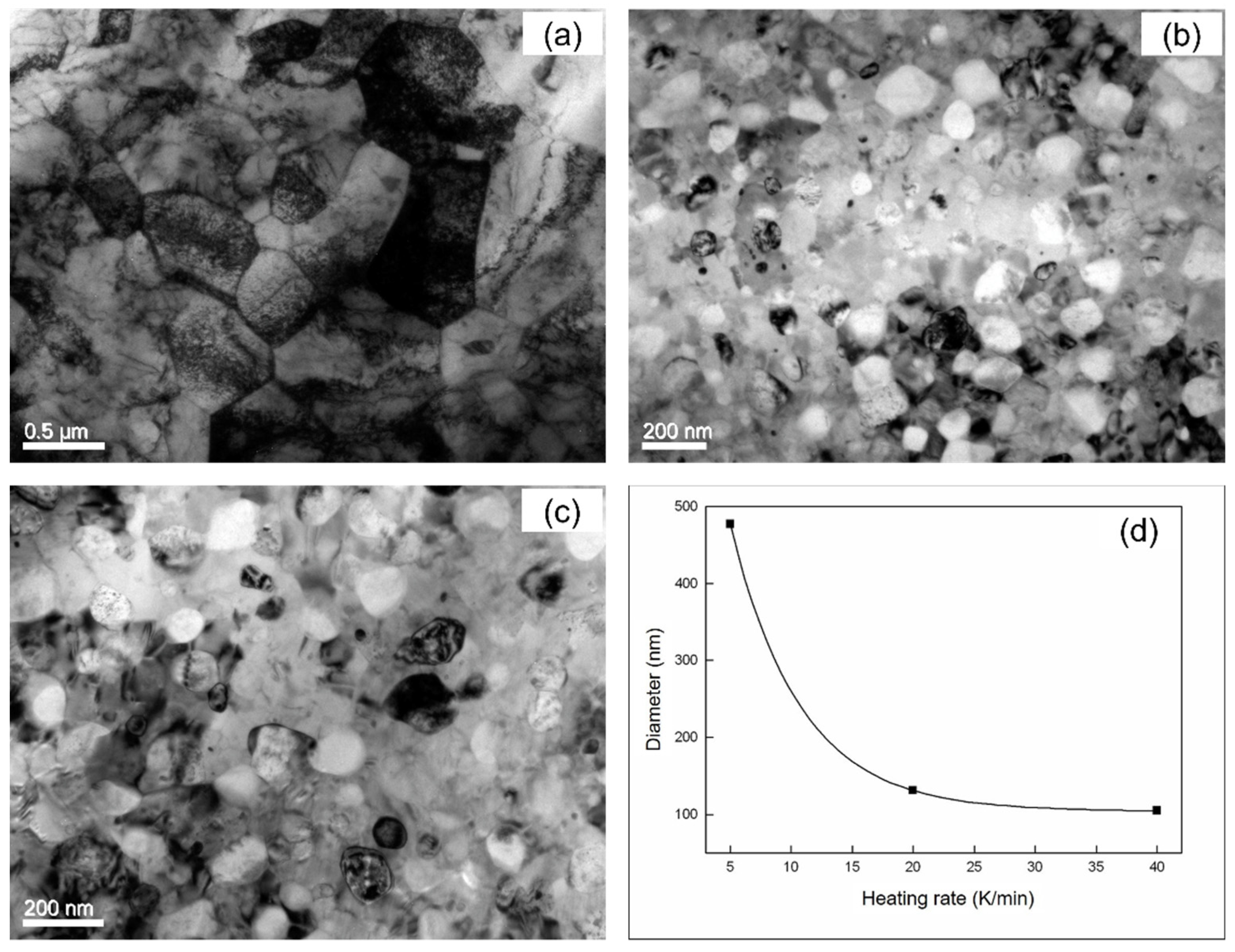
4.1. Amorphous Structure
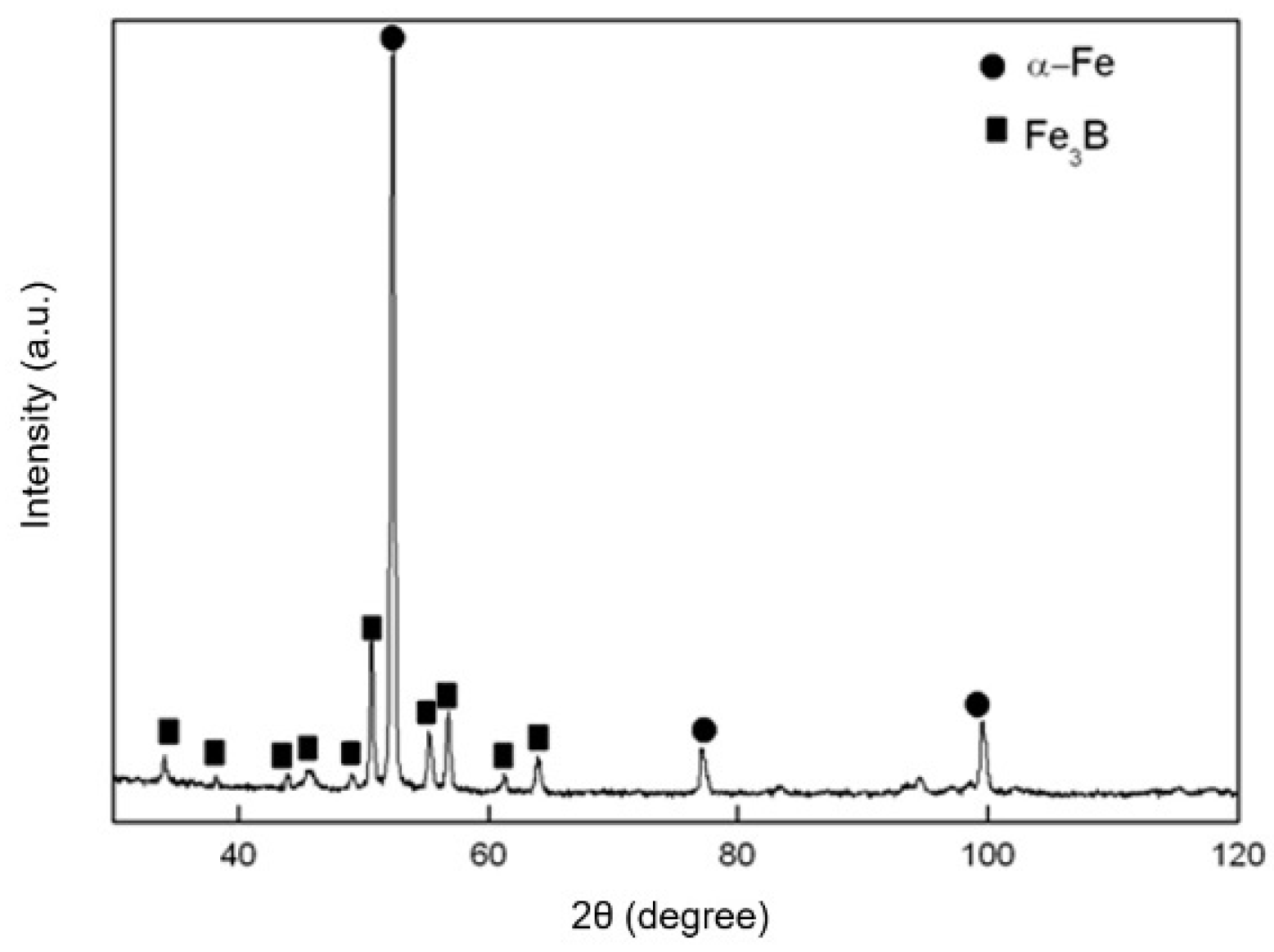
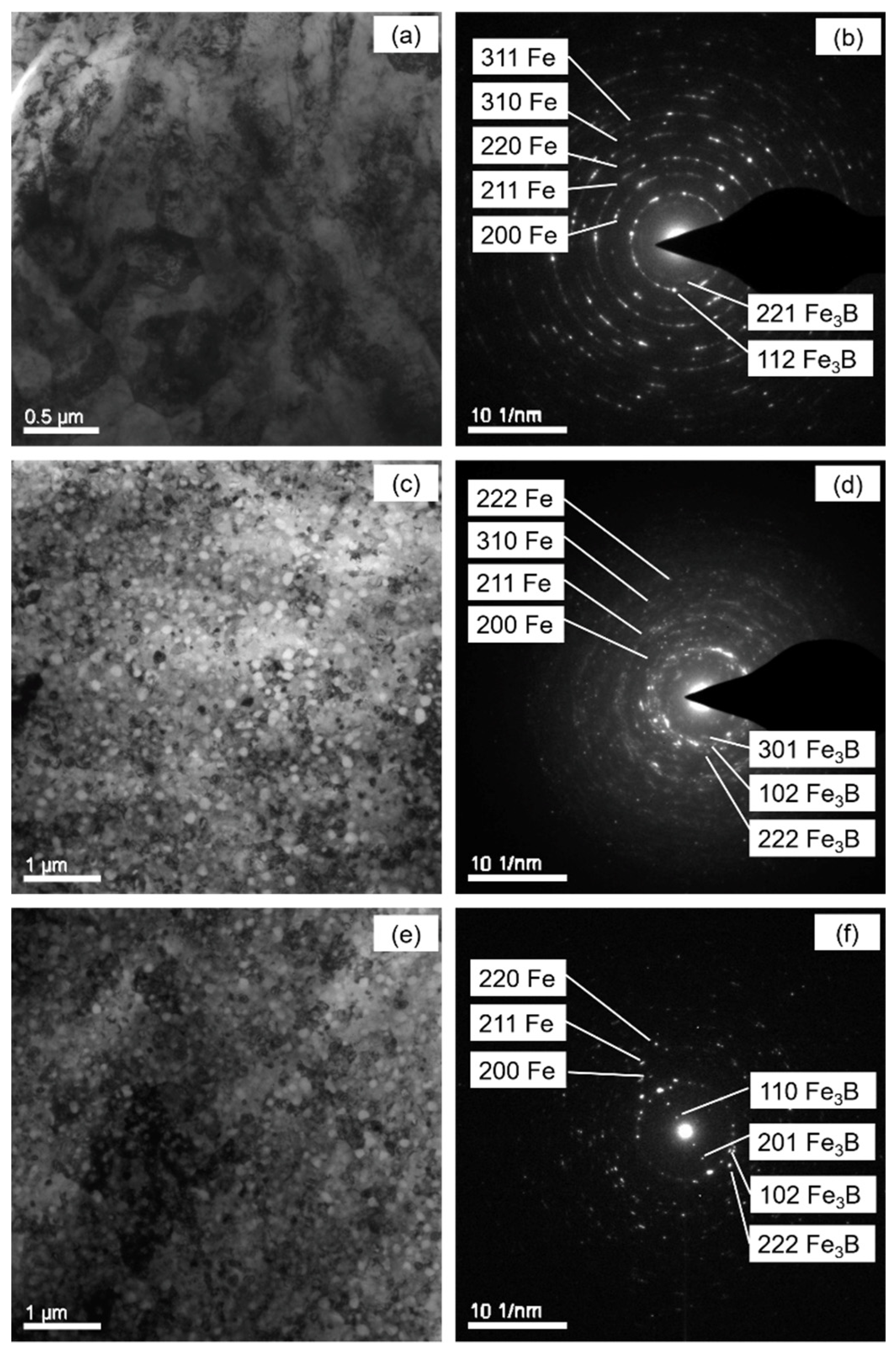
4.2. Crystallization Product
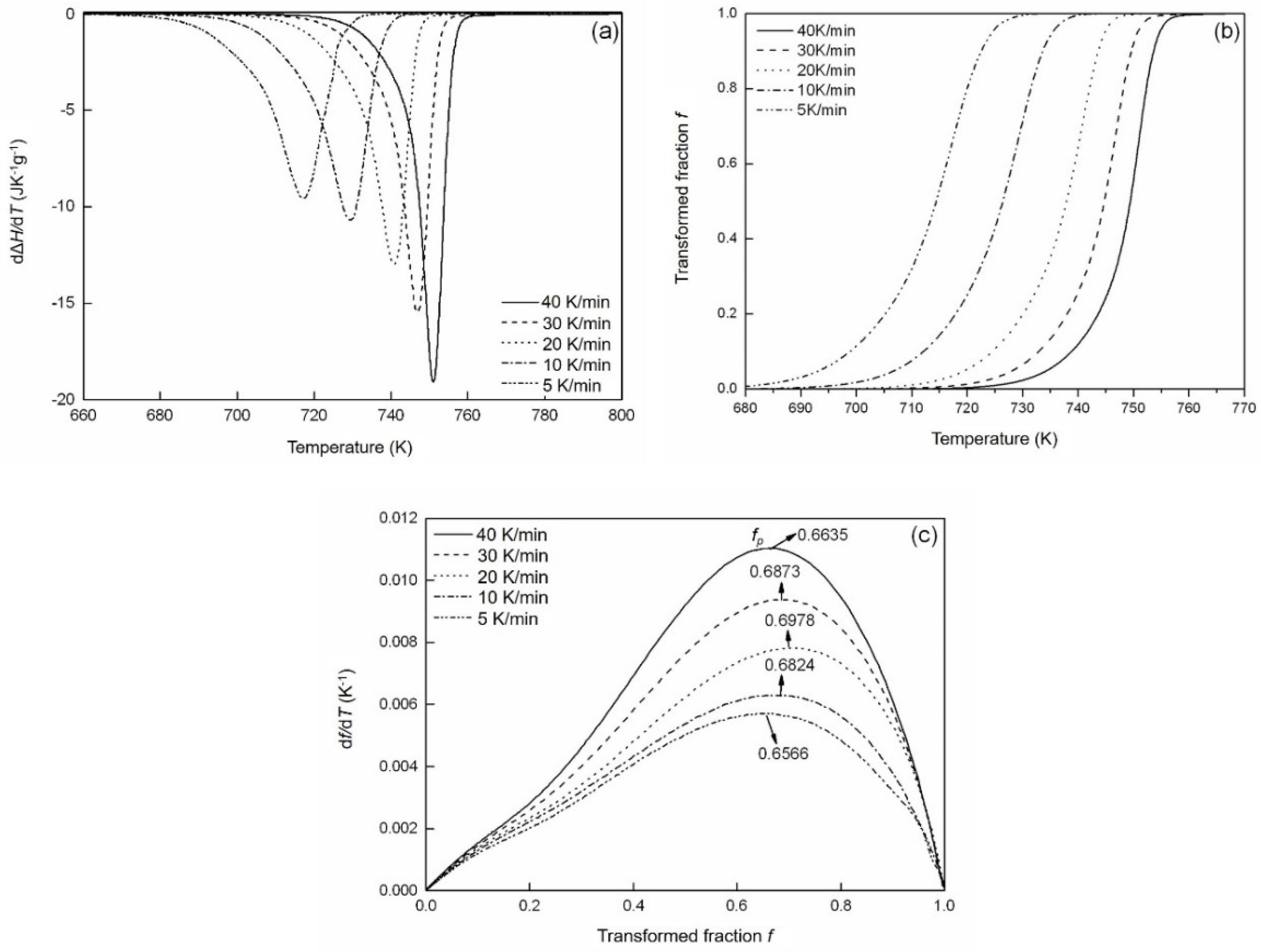
4.3. Crystallization Kinetics
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Johnson, W.A.; Mehl, R.F. Reaction kinetics in proceses of nucleation and growth. Trans. Amer. Inst. Min. (Metall.) Engrs. 1939, 135, 416–458. [Google Scholar]
- Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Kolmogorov, A.N.; Shiryayev, A.N. Selected Works of AN Kolmogorov: Volume II Probability Theory and Mathematical Statistics; Springer Science & Business Media: Berlin, Germany, 1992. [Google Scholar]
- Christian, J.W. The Theory of Transformations in Metals and Alloys; Newnes Books of Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Sha, W. Computer modelling of isothermal crystallisation kinetics of electroless and melt quenched amorphous solids using Johnson-Mehl-Avrami theory. Mater. Sci. Technol. 2005, 21, 69–75. [Google Scholar] [CrossRef]
- Peixoto, E.; Mendonça, E.; Mercena, S.; Jesus, A.; Barbosa, C.; Meneses, C.; Duque, J.; Da Silva, R.A.G. Study of the dynamic of crystallization of an amorphous Fe40Ni40P14B6 ribbon through Johnson-Mehl-Avrami model. J. Alloy Compd. 2018, 731, 1275–1279. [Google Scholar] [CrossRef]
- Tavoosi, M.; Karimzadeh, F.; Enayati, M.H.; Lee, S.; Kim, H.S. Kinetic study of non-isothermal crystallization in Al80Fe10Ti5Ni5 metallic glass. Met. Mater. Int. 2013, 19, 901–906. [Google Scholar] [CrossRef]
- Svoboda, R.; Málek, J. Non-isothermal crystallization kinetics of As2Se3 glass studied by DSC. Thermochim. Acta 2014, 579, 56–63. [Google Scholar] [CrossRef]
- Farjas, J.; Roura, P. Modification of the Kolmogorov-Johnson-Mehl-Avrami rate equation for non-isothermal experiments and its analytical solution. Acta Mater. 2006, 54, 5573–5579. [Google Scholar] [CrossRef] [Green Version]
- Adelfar, R.; Mirzadeh, H.; Ataie, A.; Malekan, M. Crystallization kinetics of mechanically alloyed amorphous Fe-Ti alloys during annealing. Adv. Powder Technol. 2020, 31, 3215–3221. [Google Scholar] [CrossRef]
- Yinnon, H.; Uhlmann, D. Applications of thermoanalytical techniques to the study of crystallization kinetics in glass-forming liquids, part I: Theory. J. Non-Crysta. Solids 1983, 54, 253–275. [Google Scholar] [CrossRef]
- Henderson, D.W. Experimental analysis of non-isothermal transformations involving nucleation and growth. J. Therm. Anal. Calorim. 1979, 15, 325–331. [Google Scholar] [CrossRef]
- De Bruijn, T.; De Jong, W.; Berg, P.V.D. Kinetic parameters in Avrami-Erofeev type reactions from isothermal and non-isothermal experiments. Thermochim. Acta 1981, 45, 315–325. [Google Scholar] [CrossRef]
- Rashidi, R.; Malekan, M.; Gholamipour, R. Crystallization kinetics of Cu47Zr47Al6 and (Cu47Zr47Al6)99Sn1 bulk metallic glasses. J. Non-Cryst. Solids 2018, 498, 272–280. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Mittemeijer, E.J. An analytical model for isothermal and isochronal transformation kinetics. J. Mater. Sci. 2004, 39, 1621–1634. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Mittemeijer, E. Parameter determination of an analytical model for phase transformation kinetics: Application to crystallization of amorphous Mg-Ni alloys. J. Mater. Res. 2004, 19, 2586–2596. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Mittemeijer, E. Determination of nucleation and growth mechanisms of the crystallization of amorphous alloys; application to calorimetric data. Acta Mater. 2004, 52, 3207–3216. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Mittemeijer, E.J. Analysis of the kinetics of phase transformations; roles of nucleation index and temperature dependent site saturation, and recipes for the extraction of kinetic parameters. J. Mater. Sci. 2006, 42, 573–587. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Bos, C.; Mittemeijer, E.J. Analysis of solid state phase transformation kinetics: Models and recipes. Int. Mater. Rev. 2007, 52, 193–212. [Google Scholar] [CrossRef]
- Liu, F.; Song, S.; Xu, J.; Wang, J. Determination of nucleation and growth modes from evaluation of transformed fraction in solid-state transformation. Acta Mater. 2008, 56, 6003–6012. [Google Scholar] [CrossRef]
- Liu, F.; Yang, G. Effects of anisotropic growth on the deviations from Johnson-Mehl-Avrami kinetics. Acta Mater. 2007, 55, 1629–1639. [Google Scholar] [CrossRef]
- Liu, F.; Yang, C.; Yang, G.; Zhou, Y. Additivity rule, isothermal and non-isothermal transformations on the basis of an analytical transformation model. Acta Mater. 2007, 55, 5255–5267. [Google Scholar] [CrossRef]
- Liu, F.; Yang, G.; Liu, J. Comparison between an analytical model and JMA kinetics for isothermally and isochronally conducted transformations. Thermochim. Acta 2005, 438, 83–89. [Google Scholar] [CrossRef]
- Liu, F.; Huang, K.; Jiang, Y.-H.; Song, S.-J.; Gu, B. Analytical description for solid-state phase transformation kinetics: Extended works from a modular model, a review. J. Mater. Sci. Technol. 2016, 32, 97–120. [Google Scholar] [CrossRef]
- Ruitenberg, G.; Petford-Long, A.K.; Doole, R.C. Determination of the isothermal nucleation and growth parameters for the crystallization of thin Ge2Sb2Te5 films. J. Appl. Phys. 2002, 92, 3116–3123. [Google Scholar] [CrossRef]
- Ranganathan, S.; Von Heimendahl, M. The three activation energies with isothermal transformations: Applications to metallic glasses. J. Mater. Sci. 1981, 16, 2401–2404. [Google Scholar] [CrossRef]
- Turnbull, D. Phase changes. Methods Exp. Phys. 1956, 3, 225–306. [Google Scholar] [CrossRef]
- Scott, M.; Ramachandrarao, P. The kinetics of crystallisation of an Fe-P-C glass. Mater. Sci. Eng. 1977, 29, 137–144. [Google Scholar] [CrossRef]
- Shen, T.; Schwarz, R. Bulk ferromagnetic glasses in the Fe-Ni-P-B system. Acta Mater. 2001, 49, 837–847. [Google Scholar] [CrossRef]
- Biswas, K.; Ram, S.; Schultz, L.; Eckert, J. Crystallization kinetics of amorphous Fe67Co9.5Nd3Dy0.5B20. J. Alloy Compd. 2005, 397, 104–109. [Google Scholar] [CrossRef]
- Blazquez, J.; Conde, C.; Conde, A. Non-isothermal approach to isokinetic crystallization processes: Application to the nanocrystallization of HITPERM alloys. Acta Mater. 2005, 53, 2305–2311. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Song, T.; Wang, G.; Zhai, Q. Non-isothermal crystallization kinetics of FeZrB amorphous alloy. Thermochim. Acta 2011, 522, 166–172. [Google Scholar] [CrossRef]
- Mittemeijer, E.J. Analysis of the kinetics of phase transformations. J. Mater. Sci. 1992, 27, 3977–3987. [Google Scholar] [CrossRef]
- Zhang, H.; Mitchell, B.S. A Method for Determining Crystallization Kinetic Parameters from one Nonisothermal Calorimetric Experiment. J. Mater. Res. 2000, 15, 1000–1007. [Google Scholar] [CrossRef]
- Liu, F.; Song, S.; Sommer, F.; Mittemeijer, E. Evaluation of the maximum transformation rate for analyzing solid-state phase transformation kinetics. Acta Mater. 2009, 57, 6176–6190. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wang, L.; Chen, H.; Cheng, X.; Zhong, X.; Feng, Y. The formation and crystallization of amorphous Al65Fe20Zr15. J. Non-Cryst. Solids 2008, 354, 5555–5558. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, K.; Li, Q. Comparative study of non-isothermal crystallization kinetics between Fe80P13C7 bulk metallic glass and melt-spun glassy ribbon. J. Alloy. Compd. 2012, 540, 6–15. [Google Scholar] [CrossRef]
- Paul, T.; Loganathan, A.; Agarwal, A.; Harimkar, S.P. Kinetics of isochronal crystallization in a Fe-based amorphous alloy. J. Alloy Compd. 2018, 753, 679–687. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Y.; Huang, J.; Pi, J. Non-isothermal crystallization kinetics and glass-forming ability of Cu-Zr-Ti-In bulk metallic glasses. Thermochim. Acta 2013, 552, 15–22. [Google Scholar] [CrossRef]
- Takahashi, M.; Koshimura, M.; Abuzuka, T. Phase Diagram of Amorphous and Crystallized Fe-B Alloy System. Jpn. J. Appl. Phys. 1981, 20, 1821–1832. [Google Scholar] [CrossRef]
- Zhang, Y.; Budnick, J.; Ford, J.; Hines, W.; Sanchez, F.; Hasegawa, R. Crystallization of Fe-B amorphous alloys: A NMR and x-ray study. J. Appl. Phys. 1987, 61, 3231–3233. [Google Scholar] [CrossRef]
- Kempen, A.; Sommer, F.; Mittemeijer, E. The isothermal and isochronal kinetics of the crystallisation of bulk amorphous Pd40Cu30P20Ni10. Acta Mater. 2002, 50, 1319–1329. [Google Scholar] [CrossRef]
- Antonione, C.; Battezzati, L.; MARINO, F.; COCCO, G. Thermomechanical and structural investigation of crystallization in Fe-B and Fe-Si-B metallic glasses. Z. Metallk. 1984, 75, 714–718. [Google Scholar]
- Palumbo, M.; Baricco, M. Modelling of primary bcc-Fe crystal growth in a FeB amorphous alloy. Acta Mater. 2005, 53, 2231–2239. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A. Numerical recipes: Does this paradigm have a future? Comput. Phys. 1997, 11, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Busby, P.E.; Warga, M.E.; Wells, C. Diffusion and Solubility of Boron in Iron and Steel. JOM 1953, 5, 1463–1468. [Google Scholar] [CrossRef]
- Busby, P.E.; Wells, C. Diffusion of Boron in Alpha Iron. JOM 1954, 6, 972. [Google Scholar] [CrossRef] [Green Version]
- Fors, D.H.R.; Wahnström, G. Nature of boron solution and diffusion in α-iron. Phys. Rev. B 2008, 77, 132102. [Google Scholar] [CrossRef]
- Al-Haj, M.; Barry, J. Nanocrystallization kinetics of Fe87.5Zr6B5.5Al1 amorphous alloy. J. Mater. Sci. Lett. 1997, 16, 1640–1642. [Google Scholar] [CrossRef]
- Köster, U.; Schünemann, U.; Blank-Bewersdorff, M.; Brauer, S.; Sutton, M.; Stephenson, G. Nanocrystalline materials by crystallization of metal-metalloid glasses. Mater. Sci. Eng. A 1991, 133, 611–615. [Google Scholar] [CrossRef]
- Rios, P.R.; Godiksen, R.; Schmidt, S.; Jensen, D.J.; VanderMeer, R. Analytical expression for the evolution of interfacial area density between transformed grains during nucleation and growth transformations. Scr. Mater. 2006, 54, 1509–1513. [Google Scholar] [CrossRef]
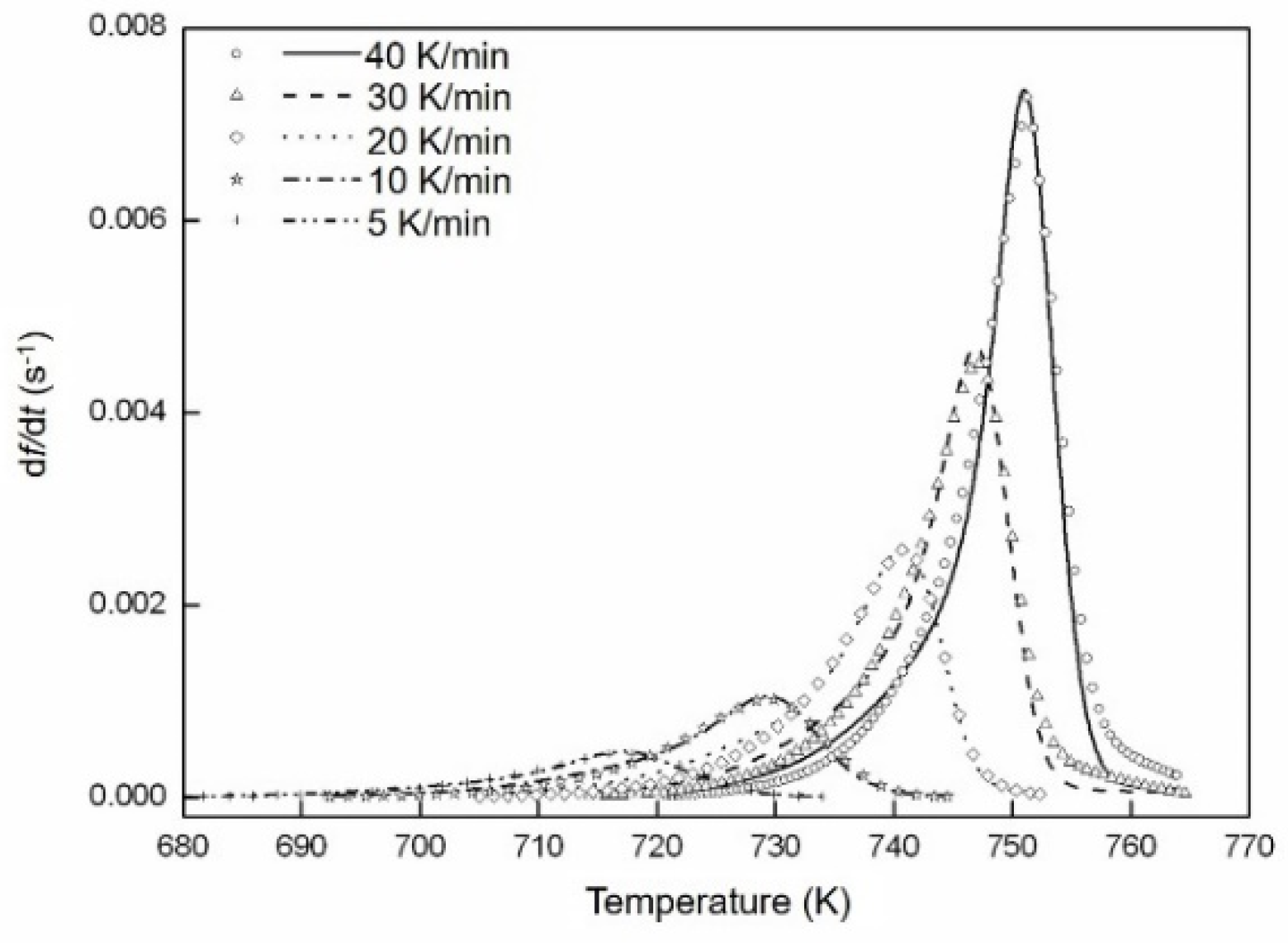
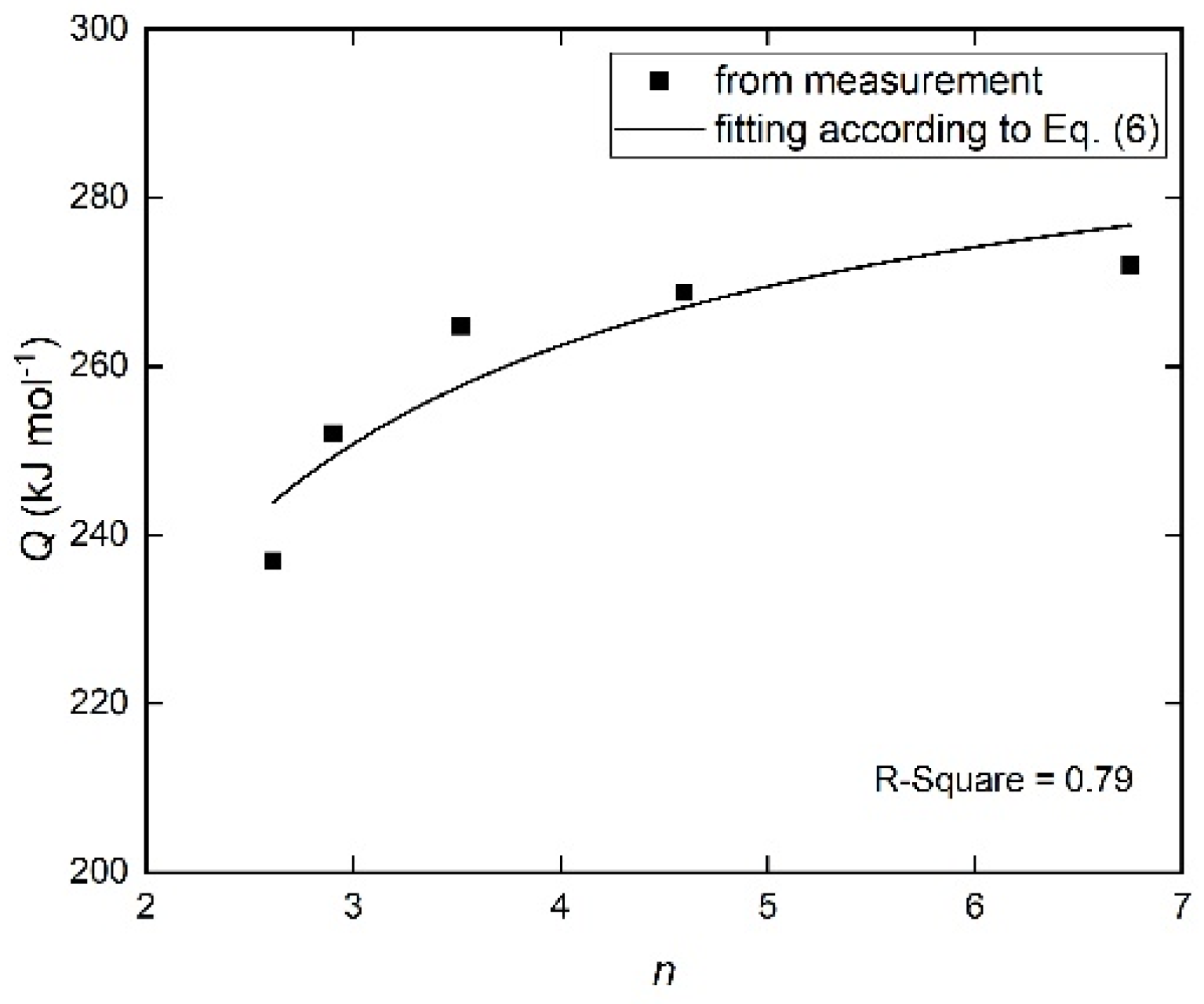
| Φ (K min−1) | n | Q (kJ mol−1) | K0 (s−1) | ε | a | Error (%) |
|---|---|---|---|---|---|---|
| 5 | 2.61 | 236.9 | 2.8 × 108 | 1.88 | 1.11 | 4.68 |
| 10 | 2.90 | 252.0 | 3.1 × 109 | 2.23 | 1.40 | 1.83 |
| 20 | 3.52 | 264.8 | 2.2 × 1010 | 3.72 | 2.02 | 6.61 |
| 30 | 4.60 | 268.8 | 4.2 × 1010 | 3.28 | 3.08 | 4.81 |
| 40 | 6.75 | 272.1 | 6.1 × 1010 | 2.75 | 5.25 | 2.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, Y.; Liu, F. A Non-Isokinetic Approach for Modeling Solid-State Transformations: Application to Crystallization of a Fe-B Amorphous Alloy. Materials 2021, 14, 292. https://doi.org/10.3390/ma14020292
Ma Y, Zhang Y, Liu F. A Non-Isokinetic Approach for Modeling Solid-State Transformations: Application to Crystallization of a Fe-B Amorphous Alloy. Materials. 2021; 14(2):292. https://doi.org/10.3390/ma14020292
Chicago/Turabian StyleMa, Yazhu, Yubing Zhang, and Feng Liu. 2021. "A Non-Isokinetic Approach for Modeling Solid-State Transformations: Application to Crystallization of a Fe-B Amorphous Alloy" Materials 14, no. 2: 292. https://doi.org/10.3390/ma14020292




