Investigating the Size-Dependent Binding of Pristine nC60 to Bovine Serum Albumin by Multi-Spectroscopic Techniques
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Instruments
2.3. Preparation and Characterization of nC60 Dispersions
2.4. Fluorescence Spectra Measurements of Proteins
2.5. Determination of Absorbance of nC60–Protein Systems
2.6. Mathematical Correction
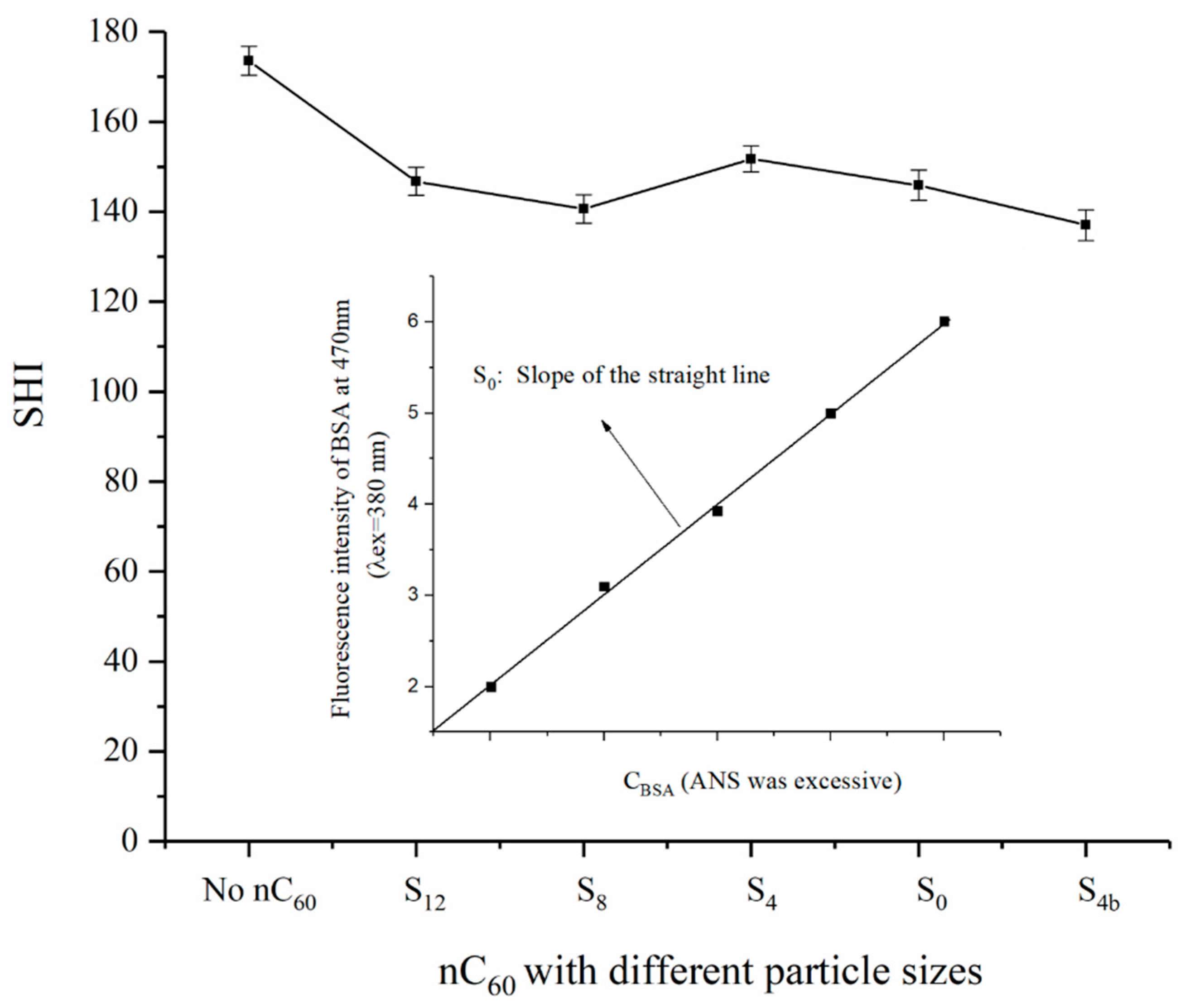
2.7. Determination of the Surface Hydrophobicity Index (SHI) of BSA
3. Results and Discussion
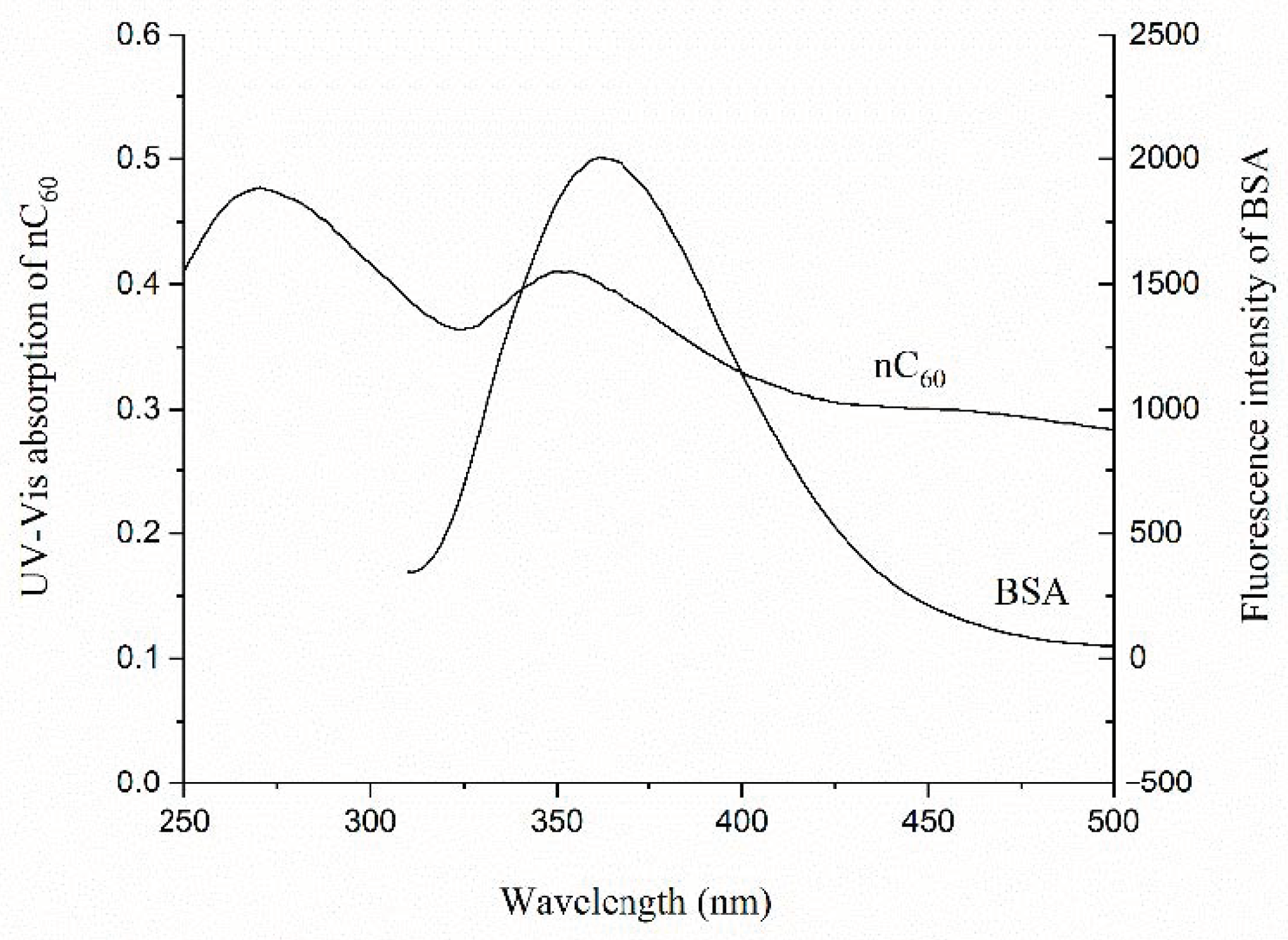
3.1. The Existence of Fluorescence Inner Filter Effect in nC60–Protein (HSA/BSA) Systems
3.2. Calculation of the Fluorescence Inner Filter Effect in the System
3.3. The Binding Constants and Binding Sites of nC60 Nanoparticles to BSA
3.4. Effect of nC60 with Different Size Distributions on the Surface Hydrophobicity of BSA and Speculation of Their Binding Sites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Allaf, A.; Balm, S. C60: Buckminsterfullerene. Chem. Rev. 1991, 91, 1213–1235. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Zakharian, T.Y.; Seryshev, A.; Sitharaman, B.; Gilbert, B.E.; Knight, V.; Wilson, L.J. A fullerene-paclitaxel chemotherapeutic: Synthesis, characterization, and study of biological activity in tissue culture. J. Am. Chem. Soc. 2005, 127, 12508–12509. [Google Scholar] [CrossRef] [PubMed]
- Pochkaeva, E.I.; Meshcheriakov, A.A.; Ageev, S.V.; Podolsky, N.E.; Petrov, A.V.; Charykov, N.A.; Vasina, L.V.; Nikolaeva, O.Y.; Gaponenko, I.N.; Sharoyko, V.V. Polythermal density and viscosity, nanoparticle size distribution, binding with human serum albumin and radical scavenging activity of the C-60-L-arginine (C-60(C6H13N4O2)(8)H-8) aqueous solutions. J. Mol. Liq. 2020, 297, 111915. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zou, Y.; Zhang, Q.W.; Chen, P.J.; Liu, Y.; Qian, Z.Y. Distinct binding dynamics, sites and interactions of fullerene and fullerenols with amyloid-peptides revealed by molecular dynamics simulations. Int. J. Mol. Sci. 2019, 20, 2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghaei, M.; Ramezanitaghartapeh, M.; Javan, M.; Hoseininezhad-Namin, M.S.; Mirzaei, H.; Rad, A.S.; Soltani, A.; Sedighi, S.; Lup, A.N.K.; Khori, V.; et al. Investigations of adsorption behavior and anti-inflammatory activity of glycine functionalized Al12N12 and Al12ON11 fullerene-like cages. Spectrochim. Acta A 2021, 246, 119023. [Google Scholar] [CrossRef]
- Bai, C.Q.; Lin, D.D.; Mo, Y.X.; Lei, J.T.; Sun, Y.X.; Xie, L.G.; Yang, X.J.; Wei, G.H. Influence of fullerenol on hIAPP aggregation: Amyloid inhibition and mechanistic aspects. Phys. Chem. Chem. Phys. 2019, 21, 4022–4031. [Google Scholar] [CrossRef] [PubMed]
- Osipov, E.M.; Hendrickson, O.D.; Tikhonova, T.V.; Zherdev, A.V.; Solopova, O.N.; Sveshnikov, P.G.; Dzantiev, B.B.; Popov, V.O. Structure of the Anti-C-60 Fullerene Antibody Fab Fragment: Structural Determinants of Fullerene Binding. Acta Nat. 2019, 11, 58–65. [Google Scholar] [CrossRef]
- Di Giosia, M.; Valle, F.; Cantelli, A.; Bottoni, A.; Zerbetto, F.; Calvaresi, M. C-60 bioconjugation with proteins: Towards a palette of carriers for all pH ranges. Materials 2018, 11, 691. [Google Scholar] [CrossRef] [Green Version]
- Junaid, M.; Almuqri, E.A.; Liu, J.J.; Zhang, H.J. Analyses of the binding between water soluble c60 derivatives and potential drug targets through a molecular docking approach. PLoS ONE 2016, 11, e0147761. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.Y.; Hua, S.Y.; Zhou, Z.Q.; Wang, G.C.; Jiang, F.L.; Liu, Y. Characterization of fullerenol-protein interactions and an extended investigation on cytotoxicity. Colloid Surf. B 2017, 157, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Gieldon, A.; Witt, M.M.; Gajewicz, A.; Puzyn, T. Rapid insight into C60 influence on biological functions of proteins. Struct. Chem. 2017, 28, 1775–1788. [Google Scholar] [CrossRef]
- Fu, X.F.; Fang, Y.L.; Zhao, H.L.; Liu, S.F. Size-dependent binding of pristine fullerene (nC60) nanoparticles to bovine/human serum albumin. J. Mol. Struct. 2018, 1166, 442–447. [Google Scholar] [CrossRef]
- Jelesarov, I.; Bosshard, H.R. Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J. Mol. Recognit. 1999, 12, 3–18. [Google Scholar] [CrossRef]
- Pollard, T.D. A guide to simple and informative binding assays. Mol. Biol. Cell 2010, 21, 4061–4067. [Google Scholar] [CrossRef] [Green Version]
- Jameson, D.M.; Ross, J.A. Fluorescence polarization/anisotropy in diagnostics and imaging. Chem. Rev. 2010, 110, 2685–2708. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.G.; Brand, L. Resonance energy transfer: Methods and applications. Anal. Biochem. 1994, 218, 1–13. [Google Scholar] [CrossRef]
- Kimball, J.; Chavez, J.; Ceresa, L.; Kitchner, E.; Nurekeyev, Z.; Doan, H.; Szabelski, M.; Borejdo, J.; Gryczynski, I.; Gryczynski, Z. On the origin and correction for inner filter effects in fluorescence Part I: Primary inner filter effect the proper approach for sample absorbance correction. Methods Appl. Fluores 2020, 8, 033002. [Google Scholar] [CrossRef]
- Gabor, G.; Walt, D.R. Sensitivity enhancement of fluorescent pH indicators by inner filter effects. Anal. Chem. 1991, 63, 793–796. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Mishra, A.K. Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. J. Photochem. Photobiol. C 2019, 41, 100318. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.L.; Wang, J.H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Steiner, R.F.; Weinryb, I. Excited States of Protein and Nucleic Acid; Plenum Press: New York, NY, USA, 1971; p. 40. [Google Scholar]
- Tabish, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potental role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.L. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; pp. 278–281. [Google Scholar]
- Raffaini, G.; Ganazzoli, F. Simulation study of the interaction of some albumin subdomains with a flat graphite surface. Langmuir 2003, 19, 3403–3412. [Google Scholar] [CrossRef]
- Haskard, C.A.; Li-Chan, E.C.Y. Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS-). J. Agric. Food Chem. 1998, 46, 2671–2677. [Google Scholar] [CrossRef]
- Alizadeh-Pasdar, N.; Li-Chan, E.C.Y. Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef]
- Liu, S.F.; Sui, Y.; Guo, K.; Yin, Z.J.; Gao, X.B. Spectroscopic study on the interaction of pristine C60 and serum albumins in solution. Nanoscale Res. Lett. 2012, 7, 433. [Google Scholar] [CrossRef] [Green Version]

| nC60 | Binding Parameters of nC60 to BSA | R2 (COD) | |||
|---|---|---|---|---|---|
| KSV (×104 L/mol) | K (×108 L/mol) | n | Kq (×1012 L/(mol·s)) | ||
| S12 | 7.25 | 0.20 | 1.41 | 7.25 | 0.998 |
| S8 | 5.13 | 1.15 | 1.53 | 5.13 | 0.998 |
| S4 | 12.1 | 7015.84 | 2.41 | 1.21 | 0.999 |
| S0 | 2.88 | 15.37 | 1.90 | 2.88 | 0.999 |
| S4b | 2.35 | 0.05 | 1.39 | 2.35 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wang, S.; Liu, Z. Investigating the Size-Dependent Binding of Pristine nC60 to Bovine Serum Albumin by Multi-Spectroscopic Techniques. Materials 2021, 14, 298. https://doi.org/10.3390/ma14020298
Liu S, Wang S, Liu Z. Investigating the Size-Dependent Binding of Pristine nC60 to Bovine Serum Albumin by Multi-Spectroscopic Techniques. Materials. 2021; 14(2):298. https://doi.org/10.3390/ma14020298
Chicago/Turabian StyleLiu, Shufang, Shu’e Wang, and Zhanzuo Liu. 2021. "Investigating the Size-Dependent Binding of Pristine nC60 to Bovine Serum Albumin by Multi-Spectroscopic Techniques" Materials 14, no. 2: 298. https://doi.org/10.3390/ma14020298




