The Effect of Electrophoretic Deposition Parameters on the Microstructure and Adhesion of Zein Coatings to Titanium Substrates
Abstract
:1. Introduction
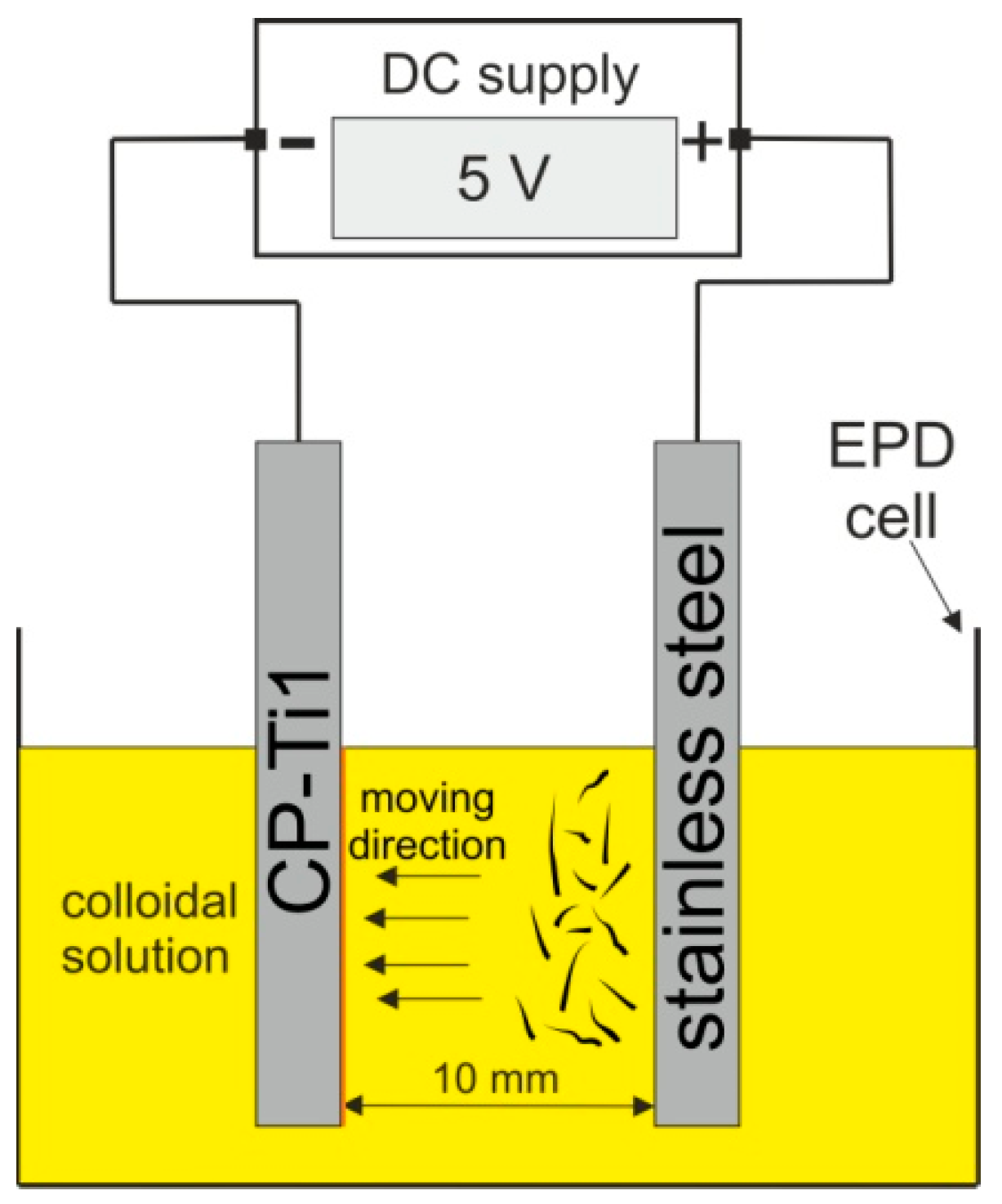
2. Material and Methods
3. Results and Discussion
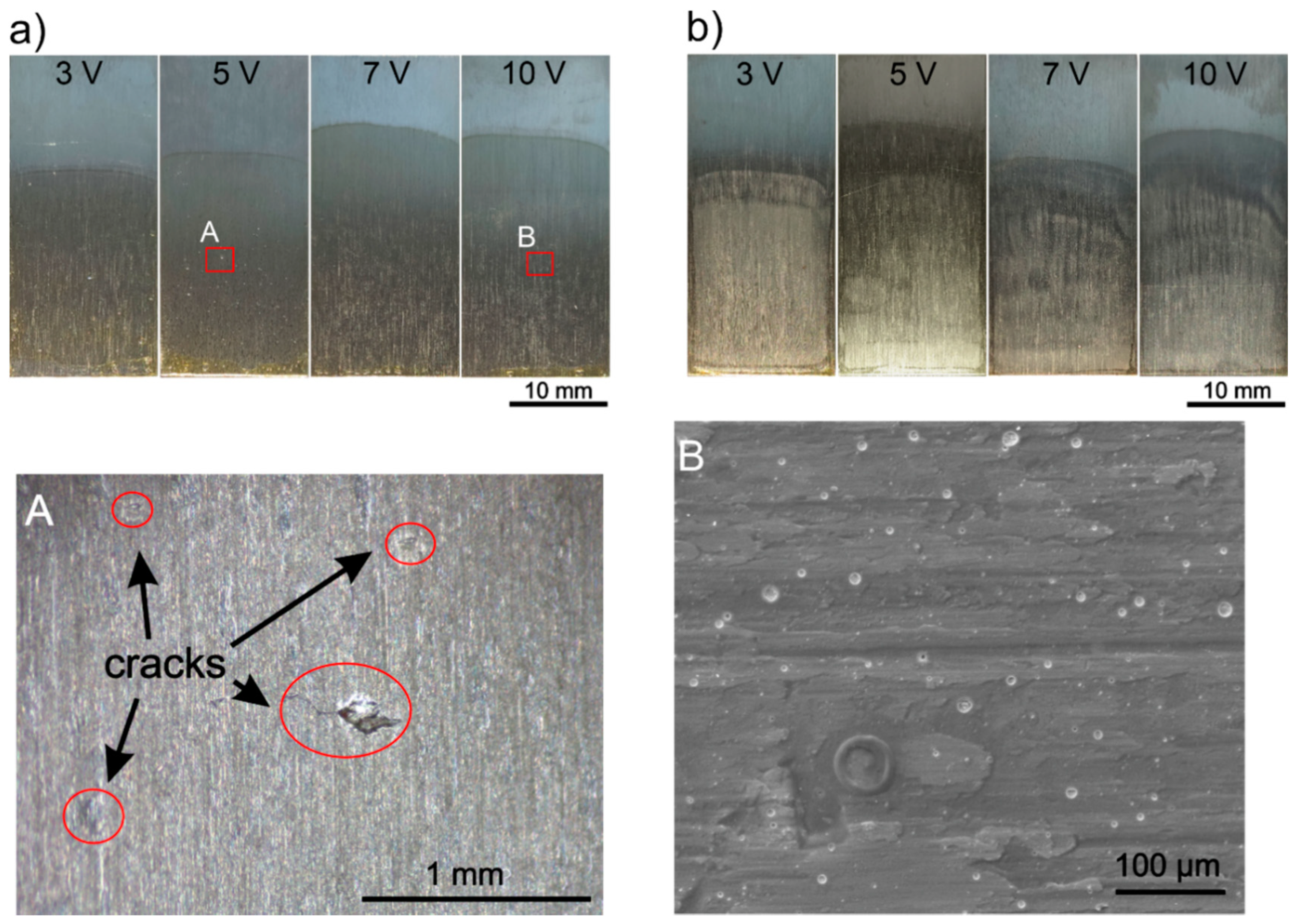
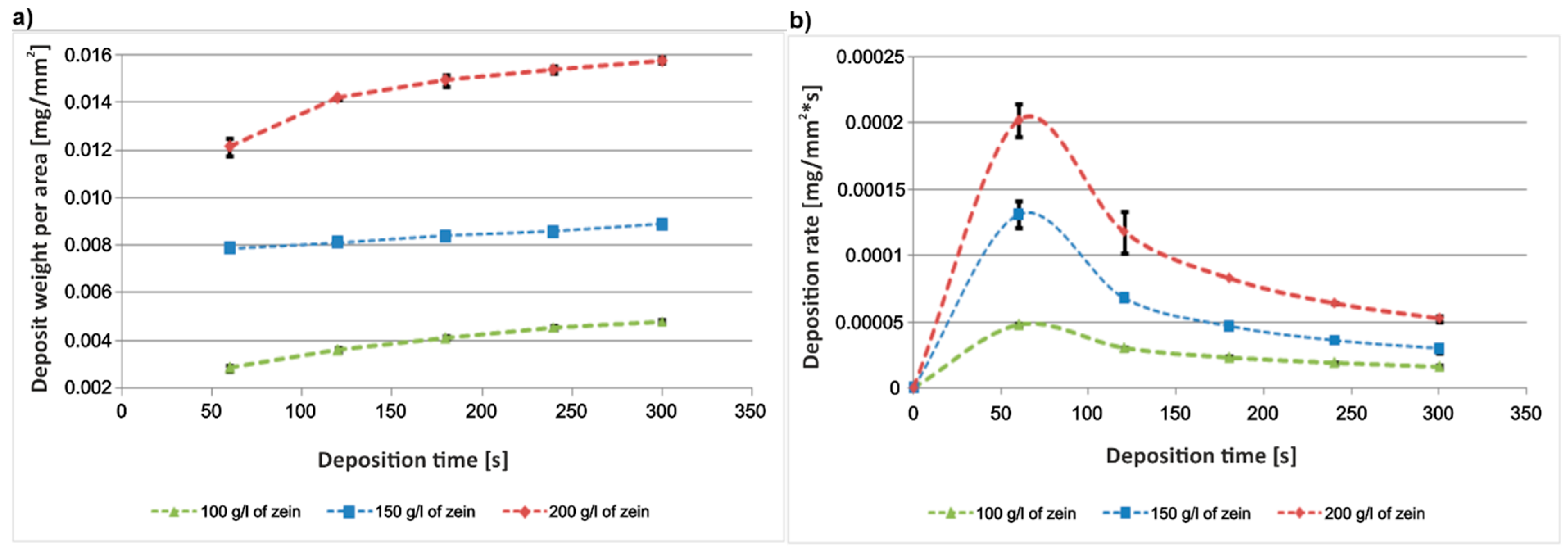
3.1. Optimization of Zein Solution Composition and EPD Parameters
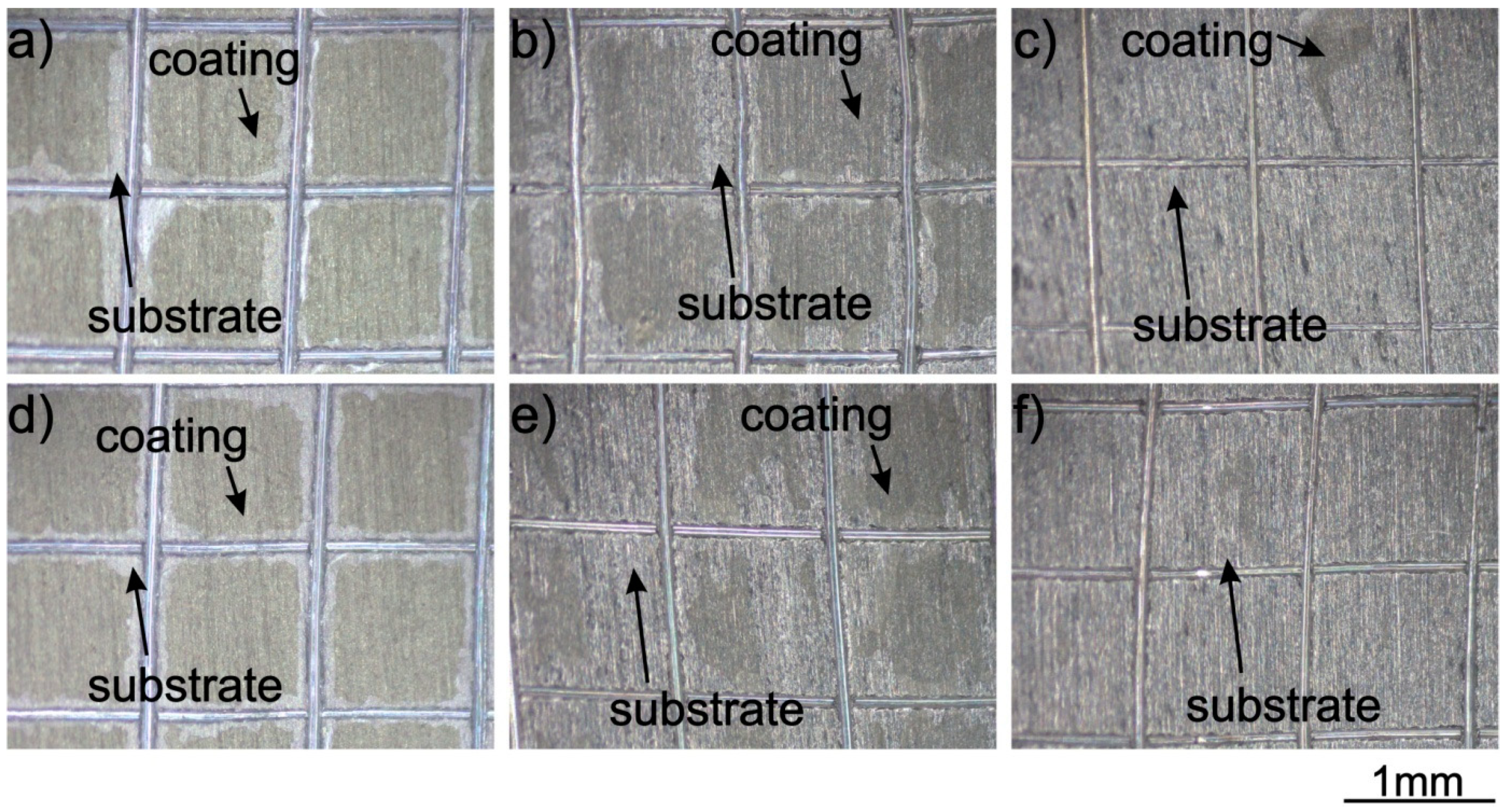
3.2. Adhesion of the Coating to the Titanium Substrates
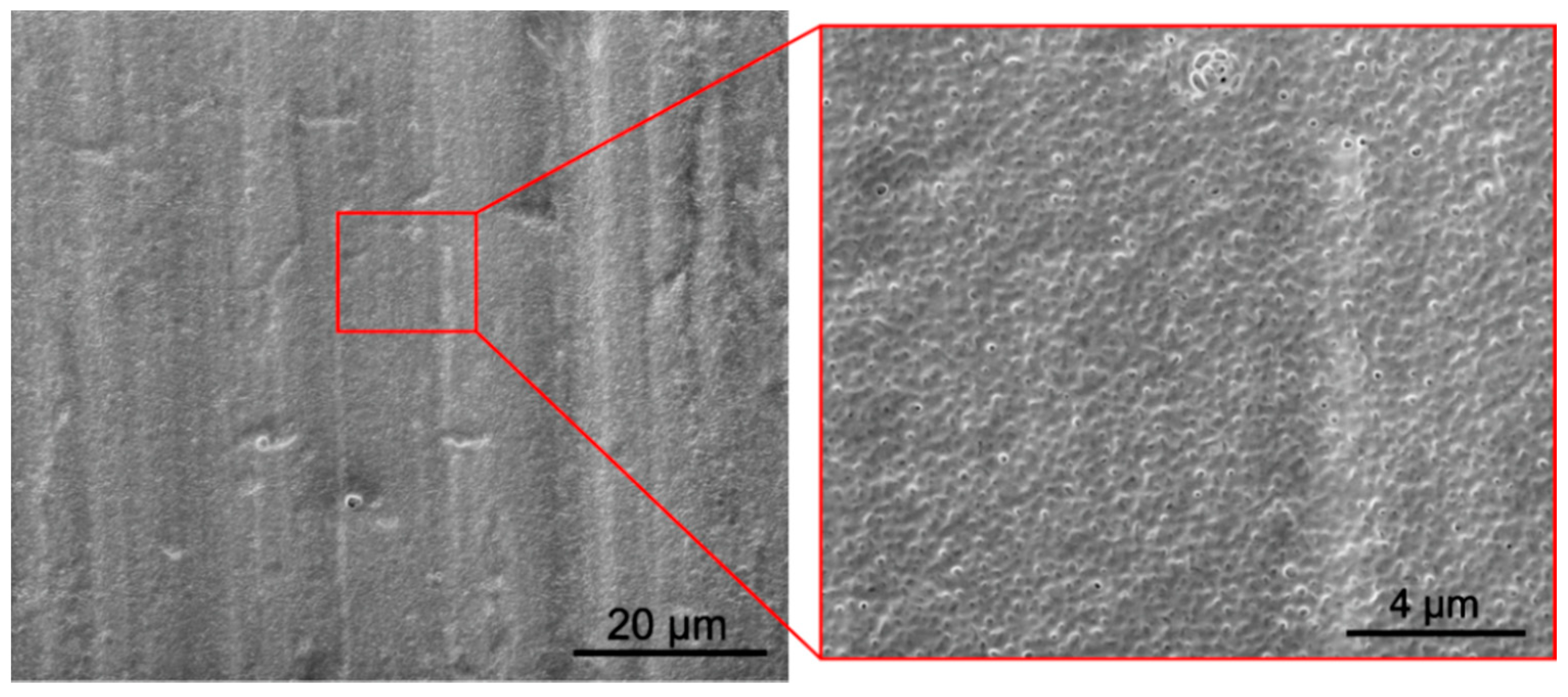
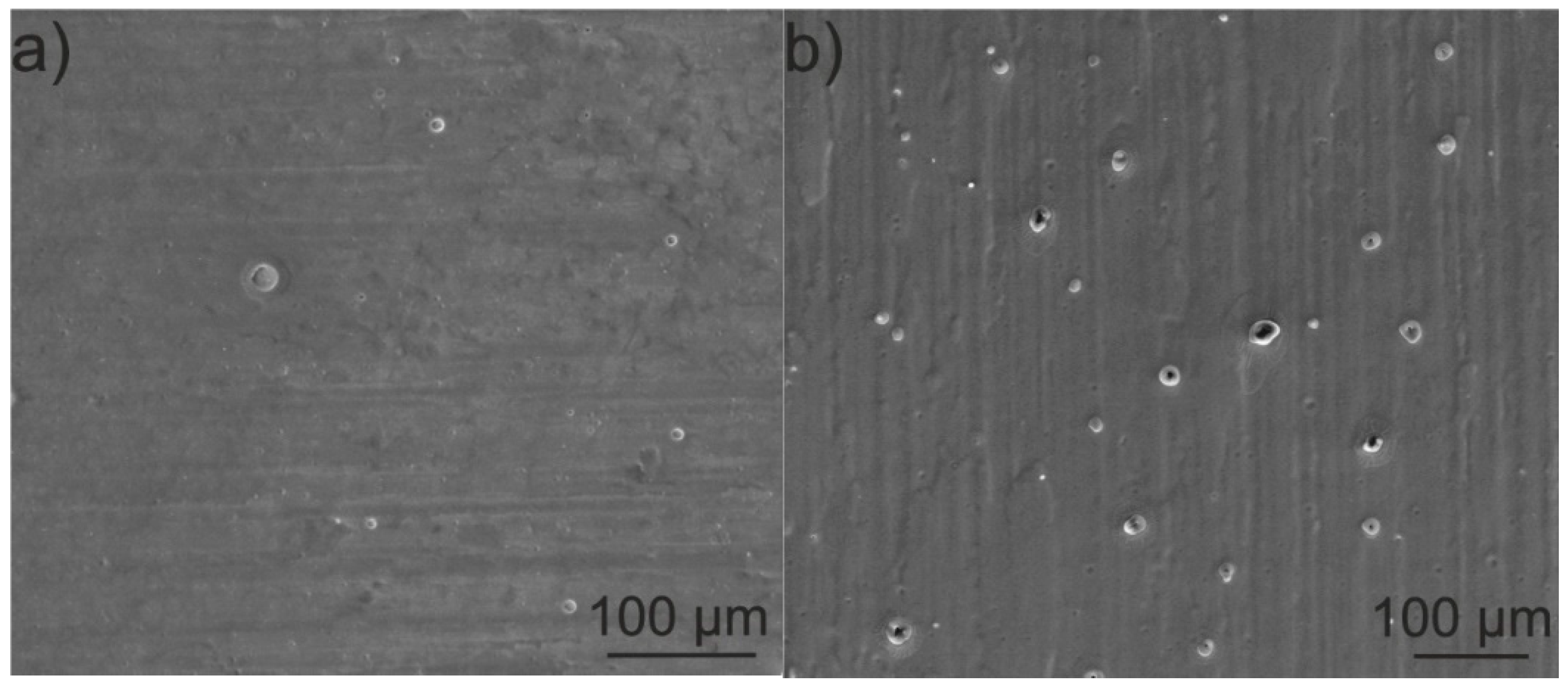
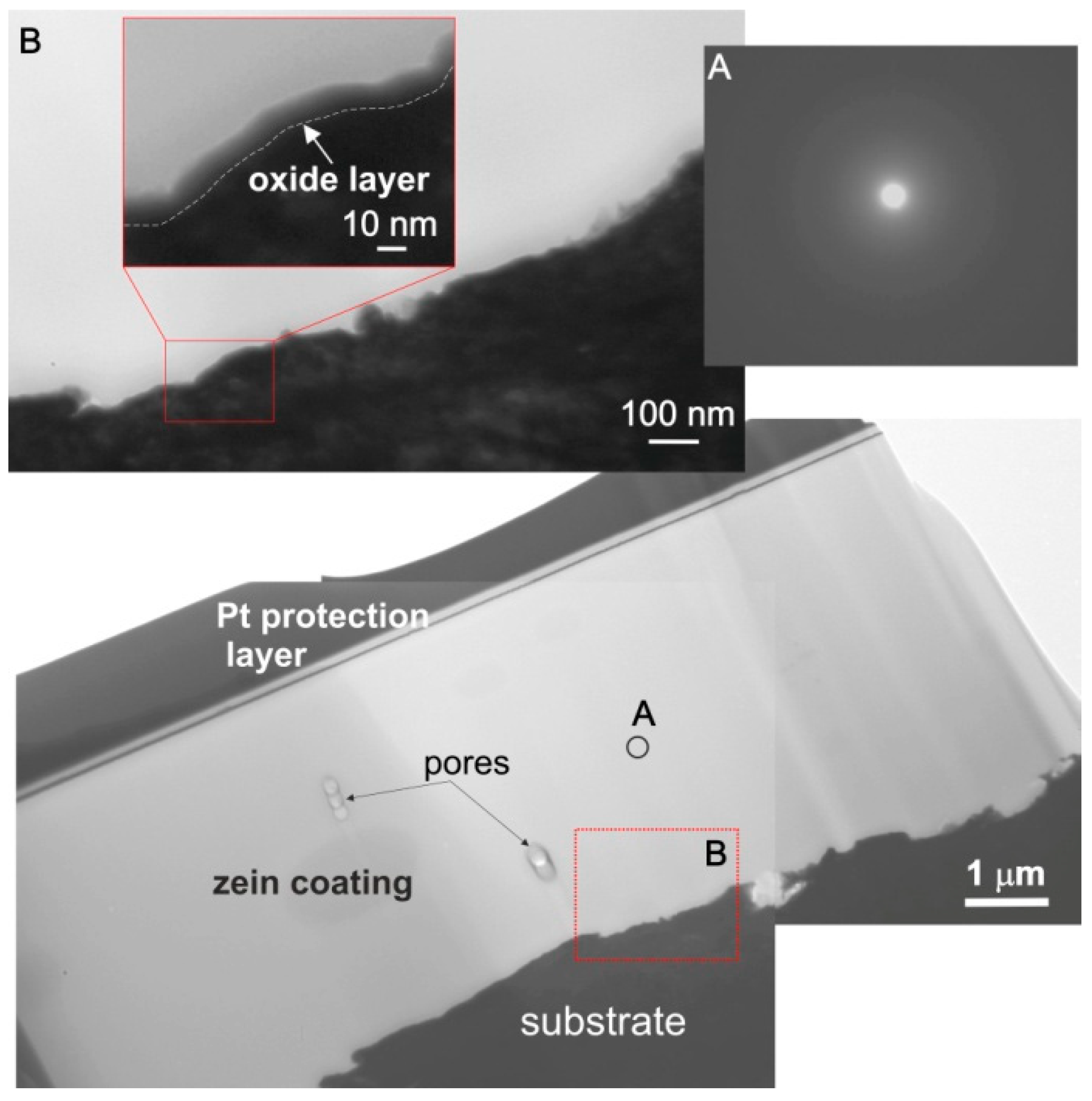
3.3. Morphology, Microstructure and Surface Topography of Coatings
3.4. Wettability
3.5. Electrochemical Corrosion Resistance
4. Conclusions
- (1)
- The EPD conditions for the deposition of pure zein coatings on the titanium substrates were established. To obtain homogeneous coatings, the ratio of water to ethanol in the zein solution should be approx. 10 vol % of water and 90 vol % of anhydrous ethanol. Higher water content resulted in nonuniform and cracked coatings, while higher ethanol content caused the precipitation of zein in the solution. The zein content in the solution should not be less than about 150 g/L and not higher than 200 g/L. Lower zein content led to inhomogeneous coatings, while higher content significantly increased the solution density;
- (2)
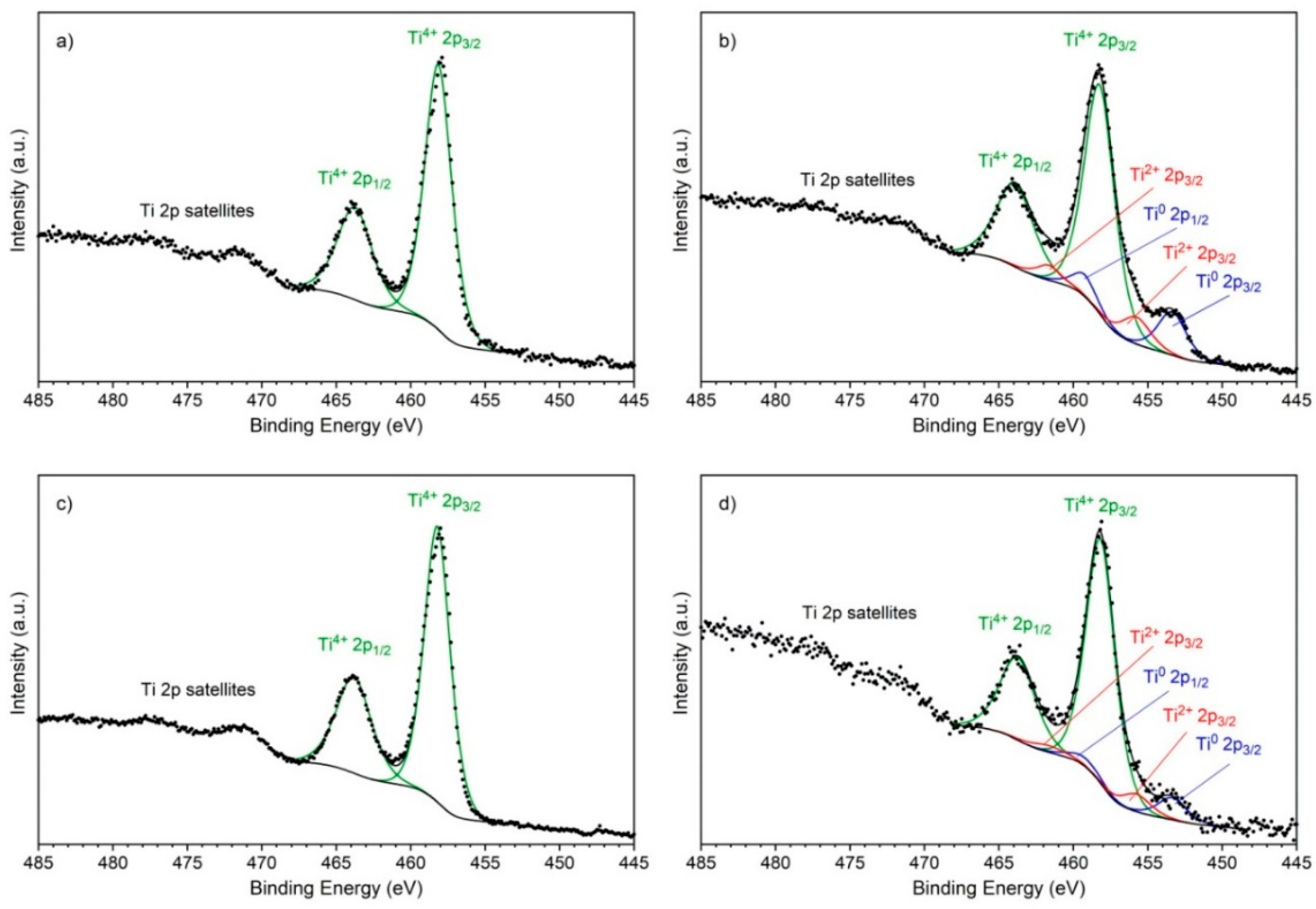
- The adhesion of zein coatings to the titanium substrates strongly depended on their surface preparation. It was found that surface chemistry and surface features have a greater influence on the coating adhesion than the surface roughness of the substrates. The coating exhibited very good adhesion to the as-received and anodic oxidized substrates due to the presence of a 10 nm thick TiO2 layer on the surface and the presence of specific surface features, such as deep scratches and open porosity, respectively. In contrast, the adhesion of zein coatings to substrates chemically treated with HF and HNO3 acids or with H2O2 and H2SO4 acid or HCl and H2SO4 acids was poor even though the surface roughness parameters, Ra and Rq, of these substrates were higher than that of the as-received titanium. In this case, the oxide layer present on the sample surface was thinner and consisted of different titanium oxides. The obtained results indicate that both adhesion mechanisms, mechanical interlocking and chemical bonding, are responsible for high coating adhesion to the underlying titanium substrates;
- (3)
- The zein coatings were dense and exhibited higher surface topography development than the as-received substrate material. Sporadically, open pores on the surface and closed pores in the middle part of the coatings were observed;
- (4)
- The zein coatings exhibited a hydrophilic nature and relatively high surface free energy (of 59.5 ± 3.8 mN/m), about 40% higher than that of the as-received substrate.
- (5)
- The zein coated titanium exhibited slightly lower resistance to corrosion in Ringer’s solution at the temperature of 37 °C compared to the as-received titanium;
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jugowiec, D.; Kot, M.; Moskalewicz, T. Electrophoretic deposition and characterisation of chitosan coatings on near-β titanium alloy. Arch. Metall. Mater. 2016, 61, 657–664. [Google Scholar] [CrossRef]
- Geetha, M.; Mudali, U.K.; Gogia, A.K.; Asokamani, R.; Raj, B. Influence of microstructure and alloying elements on corrosion behavior of Ti–13Nb–13Zr alloy. Corr. Sci. 2004, 46, 877–892. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. The corrosion behaviour of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-3Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar] [PubMed]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Tamaddon, M.; Samizadeh, S.; Wang, L.; Blunn, G.; Liu, C. Intrinsic Osteoinductivity of Porous Titanium Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Secinti, K.D.; Özalp, H.; Attar, A.; Sargon, M.F. Nanoparticle Silver Ion Coatings Inhibit Biofilm Formation on Titanium Implants. J. Clin. Neurosci. 2011, 18, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Hea, D.; Zheng, S.; Pub, J.; Zhang, G.; Hub, L. Improving tribological properties of titanium alloys by combining laser surface texturing and diamond-like carbon film. Tribol. Int. 2014, 82, 20–27. [Google Scholar] [CrossRef]
- Liua, X.; Chub, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.T.; Khan, Z.A.; Siddiquee, A.N. Surface Modifications of Titanium Materials for developing Corrosion Behavior in Human Body Environment: A Review. Procedia Mater. Sci. 2014, 6, 1610–1618. [Google Scholar] [CrossRef] [Green Version]
- Kaya, S.; Boccaccini, A.R. Electrophoretic deposition of zein coatings. J. Coat. Technol. Res. 2017, 14, 683–689. [Google Scholar] [CrossRef]
- Ramos-Rivera, L.; Dippel, J.; Boccaccini, A.R. Formation of zein/bioactive glass layers using electrophoretic deposition technique. ECS Trans. 2018, 82, 73–80. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Zein-based micro- and nano-particles for drug and nutrient delivery: A review. J. Appl. Polym. Sci. 2014, 131, 40696. [Google Scholar] [CrossRef]
- Panchapakesan, C.; Sozer, N.; Dogan, H.; Huang, Q.; Kokini, J.L. Effect of different fractions of zein on the mechanical and phase properties of zein films at nano-scale. J. Cereal. Sci. 2012, 55, 174–182. [Google Scholar] [CrossRef]
- Forato, L.A.; Bicudo, T.D.C.; Colnago, L.A. Conformation of alpha zeins in solid state by Fourier transform IR. Biopolymers 2003, 72, 421–426. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.; Kaur, A.; Bakshi, M.S. Zein: Structure, Production, Film Properties and Applications, Natural Polymers: Volume 1: Composites. R. Soc. Chem. 2012, 204–218. [Google Scholar] [CrossRef]
- Meyer, N.; Ramos-Rivera, L.; Ellis, T.; Qi, J.; Ryan, M.P.; Boccaccini, A.R. Bioactive and Antibacterial Coatings Based on Zein/Bioactive Glass Composites by Electrophoretic Deposition. Coatings 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Jiang, H.; Reddy, N.; Yang, Y. Hollow nanoparticles from zein for potential medical applications. J. Mater. Chem. 2011, 21, 18227–18235. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Potential of plant proteins for medical applications. Trends Biotechnol. 2011, 29, 490–498. [Google Scholar] [CrossRef]
- Han, Y.L.; Xu, Q.; Lu, Z.Q.; Wang, J.Y. Preparation of Transparent Zein Films for Cell Culture Applications. Colloids Surf. B 2014, 120, 55–62. [Google Scholar] [CrossRef]
- Demir, M.; Ramos-Rivera, L.; Silva, R.; Nazhat, S.N.; Boccaccini, A.R. Zein-based composites in biomedical applications. J. Biomed. Mater. Res. 2017, 105, 1656–1665. [Google Scholar] [CrossRef]
- Yong, Z.; Lili, C.; Xiaoxia, C.; Heng, Z.; Nianqiu, S.; Chunlei, L.; Yan, C.; Wei, K. Zein-based films and their usage for controlled delivery: Origin, classes and current landscape. J. Control Release 2015, 206, 206–219. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of Electrophoretic Deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7, S581–S613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikkema, R.; Baker, K.; Zhitomirsky, I. Electrophoretic deposition of polymers and proteins for biomedical applications. Adv. Colloid Interface Sci. 2020, 284, 102272. [Google Scholar] [CrossRef] [PubMed]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Van der Biest, O.O.; Vandeperre, L.J. Electrophoretic deposition of materials. Annu. Rev. Mater. Res. 1999, 29, 327–352. [Google Scholar] [CrossRef]
- Verné, E.; Fernández Vallés, C.; Vitale Brovarone, C.; Spriano, S.; Moisescu, C. Double-layer glass-ceramic coatings on Ti6Al4V for dental implants. J. Eur. Ceram. Soc. 2004, 24, 2699–2705. [Google Scholar] [CrossRef]
- Selimin, M.A.; Idrus, N.H.M.; Abdullah, H.Z.I. Anodic Oxidation of Titanium for Biomedical Application. Adv. Mat. Res. 2015, 1087, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Casagrande, R.B.; Kunst, S.R.; Beltrami, L.V.R.; Aguzzoli, C.; Brandalise, R.N.; Fraga Malfatti, C. Pretreatment effect of the pure titanium surface on hybrid coating adhesion based on tetraethoxysilane and methyltriethoxysilane. J. Coat. Technol. Res. 2018, 15, 1089–1106. [Google Scholar] [CrossRef]
- Wen, H.B.; Liu, Q.; Wijn, J.R.; Groot, K.; Cui, F.Z. Preparation of bioactive microporous titanium surface by a new two-step chemical treatment. J. Mater. Sci. Mater. Med. 1998, 9, 121–128. [Google Scholar] [CrossRef]
- Standard Test Methods for Rating Adhesion by Tape Test, ASTM D3359–17. Available online: https://www.astm.org/Standards/D3359 (accessed on 8 January 2021).
- Cafferty, E.; Hubler, G.K. Electrochemical behavior of palladium-implanted titanium. J. Electrochem. Soc. 1978, 125, 1892–1893. [Google Scholar] [CrossRef]
- Aziz, S.A.A.; Amirnordin, S.H.; Rahman, H.A.; Dan, H.Z.A.; Taib, H. Effect of Zeta Potential of Stanum Oxide (SnO2) on Electrophoretic Deposition (EPD) on Porous Alumina. AMR 2013, 795, 334–337. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, B.; Moreno, R. EPD kinetics: A review. J. Eur. Ceram. Soc. 2010, 30, 1069–1078. [Google Scholar] [CrossRef]
- Ferrari, B.; Moreno, R. Electrophoretic deposition of aqueous alumina slips. J. Eur. Ceram. Soc. 1997, 17, 549–556. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Wang, Q. Effect of acid and base treatments on structural, rheological, and antioxidant properties of α-zein. Food Chem. 2011, 124, 210–220. [Google Scholar] [CrossRef]
- Bouman, J.; Belton, P.; Venema, P.; Linden, E.; Vries, R.; Qi, S. Controlled Release from Zein Matrices: Interplay of Drug Hydrophobicity and pH. Pharm. Res. 2016, 33, 673–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabra, V.; Arreguin, R.; Vazquez-Duhalt, R.; Farres, A. Effect of temperature and pH on the secondary structure and processes of oligomerization of 19 kDa alpha-zein. Biochim. Biophys. Acta Proteins Proteom. 2006, 1764, 1110–1118. [Google Scholar] [CrossRef]
- Simchi, A.; Pishbin, F.; Boccaccini, A.R. Electrophoretic deposition of chitosan. Mater. Lett. 2009, 63, 2253–2256. [Google Scholar] [CrossRef]
- Mishra, M.; Sakka, Y.; Uchikoshi, T.; Besra, L. pH localization: A case study during electrophoretic deposition of ternary MAX phase carbide-Ti3SiC2. J. Ceram. Soc. Jpn. 2013, 121, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Xu, J. Aggregate formation of zein and its structural inversion in aqueous ethanol. J. Cereal Sci. 2008, 47, 1–5. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Xia, Q.; Zhang, B.; Wang, Q.; Huang, Q. Understanding the dissolution of α-zein in aqueous ethanol and acetic acid solutions. J. Phys. Chem. B 2012, 116, 12057–12064. [Google Scholar] [CrossRef] [PubMed]
- Bisharat, L.; Berardi, A.; Perinelli, D.R.; Bonacucina, G.; Casettari, L.; Cespi, M.; AlKhatiba, H.S.; Palmieri, G.F. Aggregation of zein in aqueous ethanol dispersions: Effect on cast film properties. Int. J. Biol. Macromol. 2016, 106, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, I.; Hashambhoy, A. Chitosan-mediated electrosynthesis of organic–inorganic nanocomposites. J. Mater. Process. Technol. 2007, 191, 68–72. [Google Scholar] [CrossRef]
- Watson, C.C.; Arrhenius, S.; Williams, J.W. Physical Chemistry of Zein. Nat. Publ. 1936, 137, 322–323. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Liu, J. Effects of different concentrations of ethanol and isopropanol on physicochemical properties of zein-based films. Ind. Crop. Prod. 2014, 53, 140–147. [Google Scholar] [CrossRef]
- Tabellion, J.; Clasen, R. Electrophoretic deposition from aqueous suspensions for near-shape manufacturing of advanced ceramics and glasses-applications. J. Mater. Sci. 2004, 39, 803–811. [Google Scholar] [CrossRef]
- Xua, L.C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [Green Version]
- Corradini, E.; Curti, P.; Meniqueti, A.; Martins, A.; Rubira, A.; Muniz, E. Recent advances in food-packing, pharmaceutical and biomedical applications of zein and zein-based materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [Green Version]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter. 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Trzaskowska, P.A.; Kuźmińska, A.; Butruk-Raszeja, B.; Rybak, E.; Ciach, T. Electropolymerized hydrophilic coating on stainless steel for biomedical applications. Colloids Surf. B 2018, 167, 499–508. [Google Scholar] [CrossRef]
- Ngo, B.; Grunlan, M. Protein Resistant Polymeric Biomaterials. ACS Macro Lett. 2017, 6, 992–1000. [Google Scholar] [CrossRef] [Green Version]
- Nouri, A.; Wen, C. Introduction to surface coating and modification for metallic biomaterials. Surf. Coat. Modif. Met. Biomater. 2015, 3–60. [Google Scholar] [CrossRef]
- Shukla, A.K.; Balasubramaniam, R.; Bhargava, S. Properties of passive film formed on CP titanium, Ti–6Al–4V and Ti–13.4Al–29Nb alloys in simulated human body conditions. Intermetallics 2005, 13, 631–637. [Google Scholar] [CrossRef]
- Gonzáles, J.E.G.; Mirza-Rosca, J.C. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Popa, M.V.; Demetrescu, I.; Vasilescu, E.; Drob, P.; Lopez, A.S.; Mirza-Rosca, J.; Vasilescu, C.; Ionita, D. Corrosion susceptibility of implant materials Ti–5Al–4V and Ti–6Al–4Fe in artificial extra-cellular fluids. Electrochim. Acta 2004, 49, 2113–2121. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Zhang, B.; Liu, C.; Lv, S.; Yang, S.; Qu, X. Effects of Porosity on Mechanical Properties and Corrosion Resistances of PM-Fabricated Porous Ti-10Mo Alloy. Metals 2018, 8, 188. [Google Scholar] [CrossRef] [Green Version]














| No | Zein (g/L) | Ethanol (vol %) | Distilled Water (vol %) | Glycerol (wt %) | pH |
|---|---|---|---|---|---|
| 1 | 100 | 90 | 10 | 20 | 5.88 |
| 2 | 150 | 90 | 10 | 20 | 5.68 |
| 3 | 150 | 90 | 10 | 20 | 5.79 |
| 4 | 150 | 85 | 15 | 20 | 5.74 |
| 5 | 150 | 75 | 25 | 20 | 5.59 |
| 6 | 200 | 90 | 10 | 20 | 5.83 |
| No | Stirring Time (min) | Dispersion Time (min) | Stirring Velocity (rpm) | Voltage (V) | Deposition Time (min) |
|---|---|---|---|---|---|
| 1 | 30 | 30 | 500 | 3, 5, 7, 10 | 5 |
| 2 | 30 | 30 | 500 | 3, 5, 7, 10 | 5 |
| 3 | 90 | 30 | 500 | 3, 5, 7, 10, 15, 20, 25, 30 | 5 |
| 4 | 90 | 30 | 500 | 3, 5, 7, 10, 15, 20, 25, 30 | 5 |
| 5 | 180 | 30 | 500 | 3, 5, 7, 10 | 5 |
| 6 | 90 | 30 | 1000 | 3, 5, 7 | 5 |
| Ethanol Content [vol %] | Zeta Potential [mV] |
|---|---|
| 75 | 0.03 ± 0.21 |
| 85 | 2.22 ± 0.57 |
| 90 | 6.09 ± 0.84 |
| Ti Surface Preparation | Coatings | Adhesion Class, According to ASTM D3359-17 | |
|---|---|---|---|
| Content of Zein in Solution (g/L) | Deposition Time (min) | ||
| As-received | 150 | 5 | 0B |
| As-received | 200 | 5 | 4B |
| Treatment A | 200 | 5 | 0B |
| Treatment B | 200 | 2.5 | 4B |
| Treatment B | 200 | 5 | 4B |
| Treatment C | 200 | 2.5 | 2B |
| Treatment C | 200 | 5 | 2B |
| Treatment D | 200 | 2.5 | 0B |
| Treatment D | 200 | 5 | 0B |
| Parameter | As-Received | A | B | C | D |
|---|---|---|---|---|---|
| Ra [μm] | 0.42 ± 0.10 | 0.46 ± 0.02 | 0.53 ± 0.04 | 0.51 ± 0.04 | 0.49 ± 0.04 |
| Rq [μm] | 0.57 ± 0.10 | 0.59 ± 0.02 | 0.76 ± 0.08 | 0.67 ± 0.06 | 0.67 ± 0.08 |
| Rmax [μm] | 22.8 ± 5.7 | 5.6 ± 0.9 | 10.8 ± 1.7 | 9.1 ± 1.6 | 8.0 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciąg, F.; Moskalewicz, T.; Kowalski, K.; Łukaszczyk, A.; Hadzhieva, Z.; Boccaccini, A.R. The Effect of Electrophoretic Deposition Parameters on the Microstructure and Adhesion of Zein Coatings to Titanium Substrates. Materials 2021, 14, 312. https://doi.org/10.3390/ma14020312
Maciąg F, Moskalewicz T, Kowalski K, Łukaszczyk A, Hadzhieva Z, Boccaccini AR. The Effect of Electrophoretic Deposition Parameters on the Microstructure and Adhesion of Zein Coatings to Titanium Substrates. Materials. 2021; 14(2):312. https://doi.org/10.3390/ma14020312
Chicago/Turabian StyleMaciąg, Filip, Tomasz Moskalewicz, Kazimierz Kowalski, Alicja Łukaszczyk, Zoya Hadzhieva, and Aldo Roberto Boccaccini. 2021. "The Effect of Electrophoretic Deposition Parameters on the Microstructure and Adhesion of Zein Coatings to Titanium Substrates" Materials 14, no. 2: 312. https://doi.org/10.3390/ma14020312





