Properties and Classification of Diamond-Like Carbon Films
Abstract
1. Introduction
2. Method of Characterization
2.1. Density and Film Thickness
2.2. Hydrogen Content
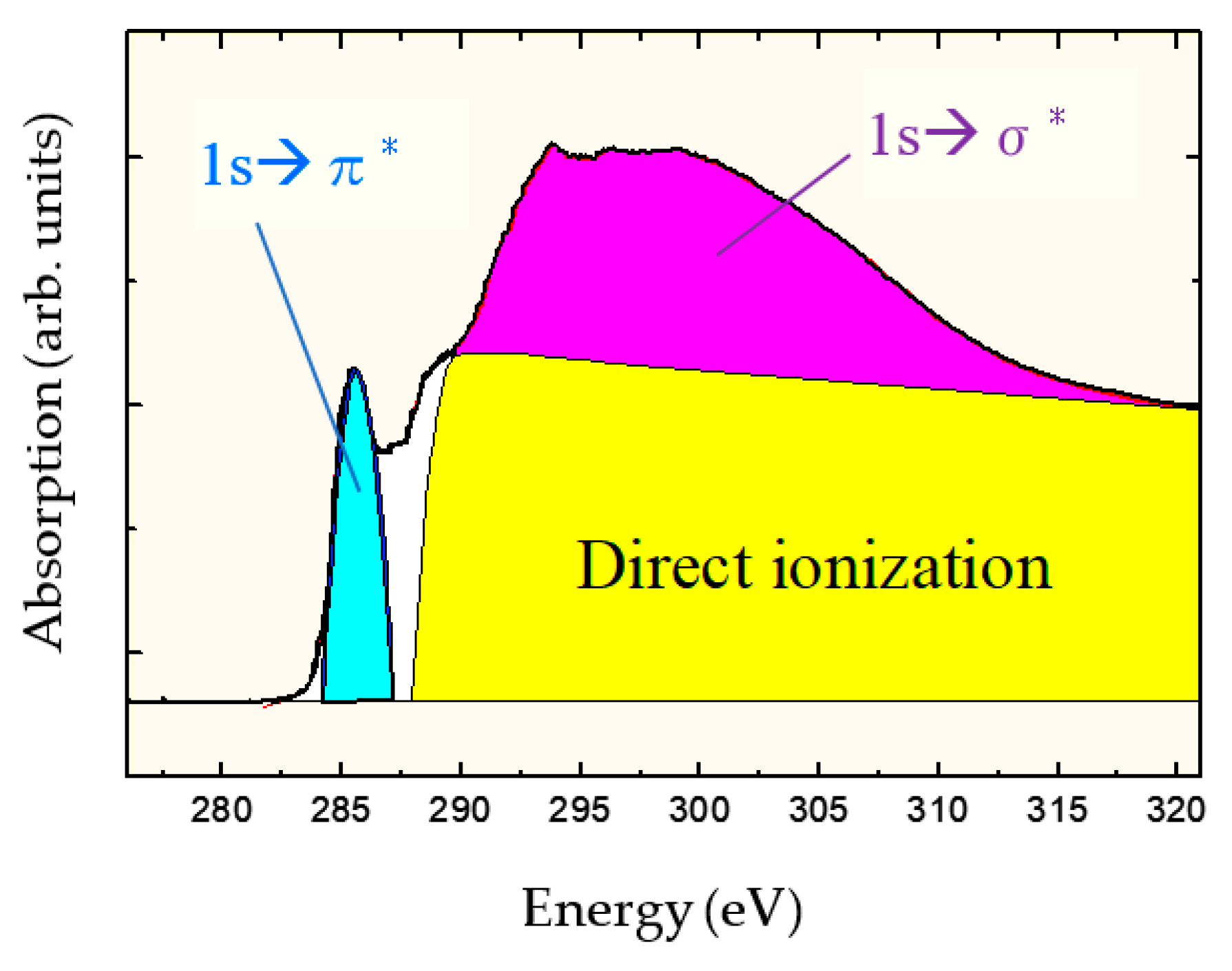
2.3. sp3/(sp2 + sp3) Structure (sp3 Ratio)
2.4. Refractive Index, Extinction Coefficient, and Optical Band Gap
2.5. Nano-Indentation Hardness
- Load: The maximum load was set at 1.0 mN.
- Load application speed: 2.0 mN/min.
- Test atmosphere: Atmospheric and room temperature. The temperature was maintained within 20 ± 2 °C.
- Indentation hardness (HIT) was calculated from the projected contact area (Ap) and the maximum load (Fmax) using the following equation (see ISO14577):
2.6. Raman Spectrum
2.6.1. Visible Raman Scattering Spectroscopy
2.6.2. Ultraviolet Raman Scattering Spectroscopy
2.7. Static Contact Angle of Water Drop and Corrosion Characteristics
3. Analysis and Testing Results
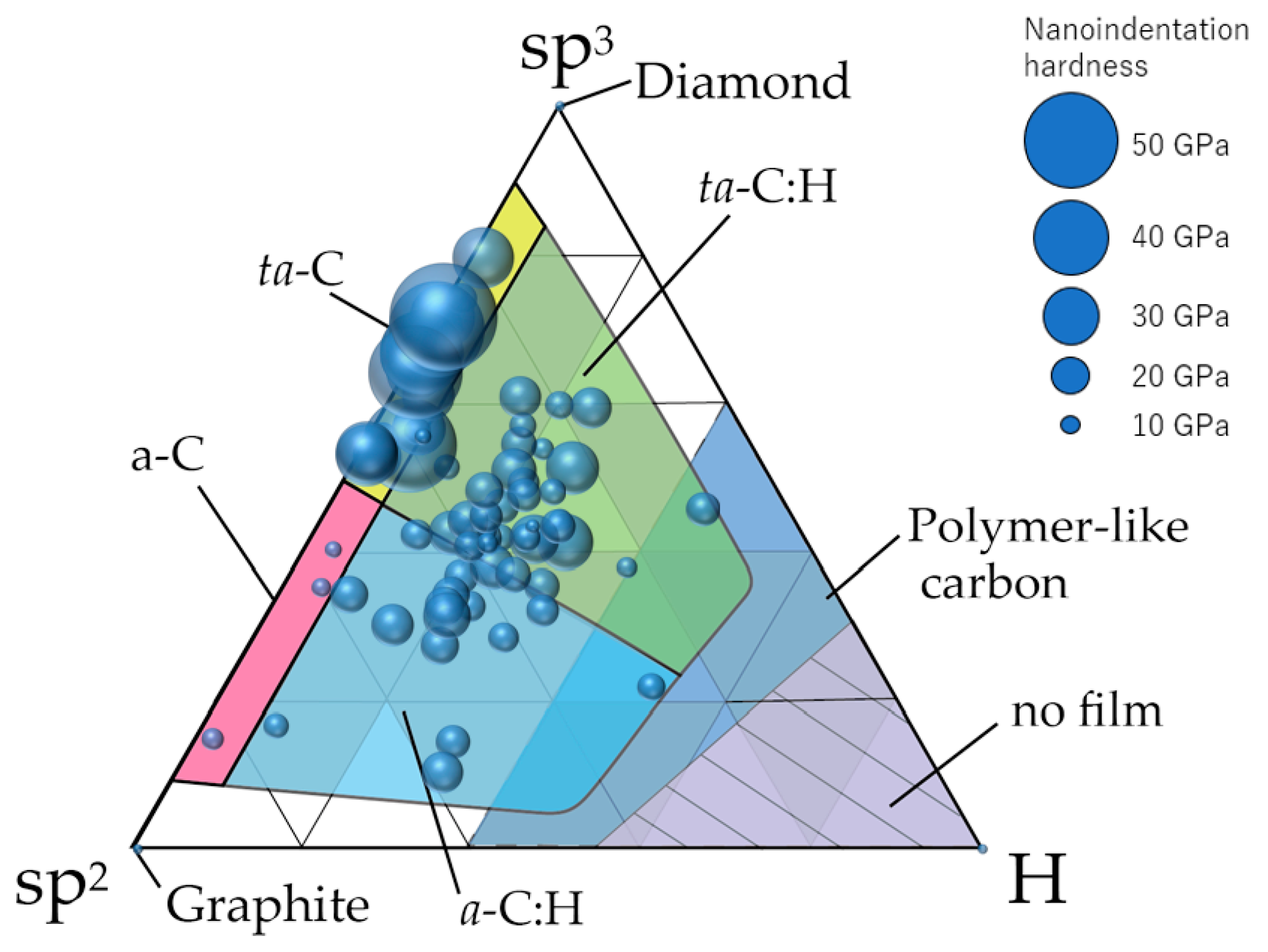
4. sp3 Ratio-Hydrogen Content-Based Classification of Amorphous Carbon
4.1. Classification of Amorphous Carbon
4.2. Use of NMR Measurement Results
5. DLC Characteristics and Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. 2002, R 37, 129–281. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. 2000, B 61, 14095–14107. [Google Scholar] [CrossRef]
- McKenzie, D.R. Tetrahedral bonding in amorphous carbon. Rep. Prog. Phys. 1996, 59, 1611–1664. [Google Scholar] [CrossRef]
- Erdemir, A.; Donnet, C. Tribology of diamond-like carbon films: Recent progress and future prospects. J. Phys. D Appl. Phys. 2006, 39, R311. [Google Scholar] [CrossRef]
- Aisenberg, S.; Chabot, R. Ion-beam deposition of thin films of diamondlike carbon. J. Appl. Phys. 1971, 42, 2953–2958. [Google Scholar] [CrossRef]
- Gupta, B.K.; Bhushan, B. Micromechanical properties of amorphous carbon coatings deposited by different deposition techniques. Thin Solid Film. 1995, 270, 391–398. [Google Scholar] [CrossRef]
- Neuville, S. New application perspective for tetrahedral amorphous carbon coatings. Sci. Connect. 2014, 8, 1–27. [Google Scholar] [CrossRef]
- Donnet, C.; Fontaine, J.; Le Mogne, T.; Belin, M.; Heau, C.; Terrat, J.P.; Vaux, F.; Pont, G. Diamond-like carbon-based functionally gradient coatings for space tribology. Surf. Coat. Technol. 1999, 120–121, 548–554. [Google Scholar] [CrossRef]
- Kano, M. Super low friction of DLC applied to engine cam follower lubricated with ester-containing oil. Tribo. Int. 2006, 39, 1682–1685. [Google Scholar] [CrossRef]
- Weiler, M.; Sattel, S.; Jung, K.; Ehrhardt, H.; Veerasamy, V.S.; Robertson, J. Highly tetrahedral, diamond-like amorphous hydrogenated carbon prepared from a plasma beam source. Appl. Phys. Lett. 1994, 64, 2797–2799. [Google Scholar] [CrossRef]
- Angus, J.C.; Jansen, F. Dense "diamondlike" hydrocarbons as random covalent networks. J. Vat. Sci. Technol. 1988, A 6, 1778–1782. [Google Scholar] [CrossRef]
- Angus, J.C. Diamond and diamond-like films. Thin Solid Film. 1992, 216, 126–133. [Google Scholar] [CrossRef]
- Jacob, W.; Muller, W. On the structure of thin hydrocarbon films. Appl. Phys. Lett. 1993, 63, 1771–1773. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Reinke, P.; Jacob, W.; Möller, W. Influence of the ion energy on the growth and structure of thin hydrocarbon films. J. Appl. Phys. 1993, 74, 1354–1361. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X.; Lin, Y.; Wang, F. A ternary phase diagram for amorphous carbon. Carbon 2015, 94, 202–213. [Google Scholar] [CrossRef]
- Vetter, J. 60 years of DLC coatings: historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like carbon. Pure Appl. Chem. 1994, 66, 1789–1796. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Philos. Trans. R. Soc. Lond. 2004, A 362, 2477–2512. [Google Scholar] [CrossRef]
- Erdemir, A. The role of hydrogen in tribological properties of diamond-like carbon films. Surf. Coat. Technol. 2001, 146–147, 292–297. [Google Scholar] [CrossRef]
- Hirakuri, K.K.; Minorikawa, T.; Friedbacher, G.; Grasserbauer, M. Thin film characterization of diamond-like carbon films prepared by r.f. plasma chemical vapor deposition. Thin Solid Film. 1997, 302, 5–11. [Google Scholar] [CrossRef]
- Caschera, D.; Cossari, P.; Federici, F.; Kaciulis, S.; Mezzi, A.; Padeletti, G.; Trucchi, D. Influence of PE-CVD parameters on the properties of diamond-like carbon films. Thin Solid Film. 2001, 519, 4087–4091. [Google Scholar] [CrossRef]
- Isono, Y.; Namazu, T.; Terayama, N.; Tanaka, T. Mechanical characterization of sub-micrometer Thick DLC Films by AFM Tensile Testing for Surface Modification in MEMS. Proc. IEEE Micro Electro. Mech. Syst. 2002, 431–434. [Google Scholar] [CrossRef]
- Kim, Y.T.; Cho, S.M.; Choi, W.S.; Hong, B.; Yoon, D.H. Dependence of the bonding structure of DLC thin film on the deposition condition of PECVD method. Surf. Coat. Technol. 2003, 169, 291–294. [Google Scholar] [CrossRef]
- Nakahigashi, T.; Tanaka, Y.; Miyake, K.; Oohara, H. Properties of flexible DLC film deposited by amplitude-modulated RF P-CVD. Tribo. Int. 2004, 37, 907–912. [Google Scholar] [CrossRef]
- Ohtake, N.; Saito, T.; Kondo, Y.; Hosono, S.; Nakamura, Y.; Imanishi, Y. Synthesis of Diamond-like Carbon Films by Nanopulse Plasma Chemical Vapor Deposition at Subatmospheric Pressure. Jpn. J. Appl. Phys. 2004, 43, L1406–L1408. [Google Scholar] [CrossRef]
- Ohana, T.; Nakamura, T.; Suzuki, M.; Tanaka, A.; Koga, Y. Tribological properties and characterization of DLC films deposited by pulsed bias CVD. Diam. Relat. Mater. 2004, 13, 1500–1504. [Google Scholar] [CrossRef]
- Nakanishi, K.; Mori, H.; Tachikawa, H.; Itou, K.; Fujioka, M.; Funaki, Y. Investigation of DLC-Si coatings in large-scale production using DC-PACVD equipment. Surf. Coat. Technol. 2006, 200, 4277–4281. [Google Scholar] [CrossRef]
- Fujimoto, S.; Ohtake, N.; Takai, O. Mechanical properties of silicon-doped diamond-like carbon films prepared by pulse-plasma chemical vapor deposition. Surf. Coat. Technol. 2011, 206, 1011–1015. [Google Scholar] [CrossRef]
- Seker, Z.; Ozdamar, H.; Esen, M.; Esen, R.; Kavak, H. The effect of nitrogen incorporation in DLC films deposited by ECR Microwave Plasma CVD. Appl. Surf. Sci. 2014, 314, 46–51. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, L.P.; Fu, K.Y.; Huang, N.; Leng, Y.; Leng, Y.X.; Yang, P.; Wang, J.; Wan, G.J.; Sun, H.; et al. Blood compatibility and sp3/sp2 contents of diamond-like carbon (DLC) synthesized by plasma immersion ion implantation-deposition. Surf. Coat. Technol. 2002, 156, 289–294. [Google Scholar] [CrossRef]
- Flege, S.; Hatada, R.; Ensinger, W.; Baba, K. Improved adhesion of DLC films on copper substrates by preimplantation. Surf. Coat. Technol. 2014, 256, 37–40. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Nakamori, H.; Kogo, Y.; Sakurai, M.; Ohtake, N.; Saitoh, H. Correlation between Optical Properties and Hardness of Diamond-Like Carbon Films. Solid Mech. Mater. Eng. 2013, 7, 187–198. [Google Scholar] [CrossRef]
- Bewilogua, K.; Wittorf, R.; Thomsen, H.; Weber, M. DLC based coatings prepared by reactive d.c. magnetron sputtering. Thin Solid Film. 2004, 447–448, 142–147. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Ou, K.-L.; Huang, W.-C.; Chu, K.-T.; Ou, S.-F. Phase transformation of diamond-like carbon/silver composite films by sputtering deposition. Ceram. Int. 2013, 39, 2575–2580. [Google Scholar] [CrossRef]
- Fujimaki, S.; Kashiwase, H.; Kokaku, Y. New DLC coating method using magnetron plasma in an unbalanced magnetic field. Vacuum 2000, 59, 657–664. [Google Scholar] [CrossRef]
- Amanovn, A.; Watabe, T.; Tsuboi, R.; Sasaki, S. Fretting wear and fracture behaviors of Cr-doped and non-doped DLC films deposited on Ti–6al–4V alloy by unbalanced magnetron sputtering. Tribo. Int. 2013, 62, 49–57. [Google Scholar] [CrossRef]
- Kamijo, E.; Nakamura, T.; Tani, Y. AFM observations of DLC films prepared by the ECR sputtering method. Nucl. Instr. Methods Phys. Res. 1997, B 121, 110–115. [Google Scholar] [CrossRef]
- Pang, J.; Lu, W.; Xin, Y.; Wang, H.; He, J.; Xu, J. Plasma Diagnosis for Microwave ECR Plasma Enhanced Sputtering Deposition of DLC Films. Plasma Sci. Technol. 2012, 14, 172–176. [Google Scholar] [CrossRef]
- Münz, W.-D.; Schenkel, M.; Kunkel, S.; Paulitsch, J.; Bewilogua, K. Industrial Applications of HIPIMS. J. Phys. Conf. Ser. 2008, 100, 082001. [Google Scholar] [CrossRef]
- Evaristo, M.; Fernandes, F.; Cavaleiro, A. Room and High Temperature Tribological Behaviour of W-DLC Coatings Produced by DCMS and Hybrid DCMS-HiPIMS Configuration. Coatings 2020, 10, 319. [Google Scholar] [CrossRef]
- Xu, S.; Tay, B.K.; Tan, H.S.; Zhong, L.; Tu, Y.Q.; Silva, S.R.P.; Milne, W.I. Properties of carbon ion deposited tetrahedral amorphous carbon films as a function of ion energy. J. Appl. Phys. 1996, 79, 7234–7240. [Google Scholar] [CrossRef]
- Inaba, H.; Fujimaki, S.; Sasaki, S.; Hirano, S.; Todoroki, S.; Furusawa, K.; Yamasaka, M.; Shi, X. Properties of Diamond-Like Carbon Films Fabricated by the Filtered Cathodic Vacuum Arc Method. Jpn. J. Appl. Phys. 2002, 41, 5730–5733. [Google Scholar] [CrossRef]
- Takikawa, H.; Izumi, K.; Miyano, R.; Sakakibara, T. DLC thin film preparation by cathodic arc deposition with a super droplet-free system. Surf. Coat. Technol. 2003, 163–164, 368–373. [Google Scholar] [CrossRef]
- Kondo, S.; Liza, S.; Ohtake, N.; Akasaka, H.; Matsuo, M.; Iwamoto, Y. Mechanical characterization of segment-structured hydrogen-free a-C films fabricated by filtered cathodic vacuum arc method. Surf. Coat. Technol. 2015, 278, 71–79. [Google Scholar] [CrossRef]
- Weissmantel, C.; Reisse, G. Preparation of hard coatings by ionbeam methods. Thin Solid Film. 1979, 63, 315–325. [Google Scholar] [CrossRef]
- Akita, N.; Konishi, Y.; Ogura, S.; Imamura, M.; Hu, Y.H.; Shi, X. Comparison of deposition methods for ultra thin DLC overcoat film for MR head. Diam. Relat. Mater. 2001, 10, 1017–1023. [Google Scholar] [CrossRef]
- Roy, R.K.; Ahmed, S.F.; Yi, J.W.; Moon, M.-W.; Lee, K.-R.; Jun, Y. Improvement of adhesion of DLC coating on nitinol substrate by hybrid ion beam deposition technique. Vacuum 2009, 83, 1179–1183. [Google Scholar] [CrossRef]
- Oohira, K. Characteristics and Applications of DLC films. NTN Tech. Rev. 2009, 77, 90–95. [Google Scholar]
- Lan, R.; Ma, Z.; Wang, C.; Lu, G.; Yuan, Y.; Shi, C. Microstructural and tribological characterization of DLC coating by in-situ duplex plasma nitriding and arc ion plating. Diam. Relat. Mater. 2019, 98, 107473. [Google Scholar] [CrossRef]
- Panda, M.; Krishnan, R.; Krishna, N.G.; Amirthapandian, S.; Magudapathy, P.; Kamruddin, M. Tuning the tribological property of PLD deposited DLC-Au nanocomposite thin films. Ceram. Int. 2019, 45, 8847–8855. [Google Scholar] [CrossRef]
- Scheibe, H.-J.; Schultrich, B. DLC film deposition by Laser-Arc and study of properties. Thin Solid Film. 1994, 246, 92–102. [Google Scholar] [CrossRef]
- Wu, J.-B.; Chen, C.-Y.; Shin, C.-T.; Li, M.-Y.; Leu, M.-S.; Li, A.-K. Microstructure and physical properties of DLC films deposited by laser induced high current pulsed arc deposition. Thin Solid Film. 2008, 517, 1141–1145. [Google Scholar] [CrossRef]
- VDI 2840 Carbon Films—Basic Knowledge, Film Types and Properties; Verein Deuttscher Ingennieure: Dusseldorf, Germany, 2005.
- Grill, A.; Patel, V.V. Characterization of diamondlike carbon by infrared spectroscopy? Appl. Phys. Lett. 1992, 60, 2089–2091. [Google Scholar] [CrossRef]
- Grill, A.; Patel, V.V.; Meyerson, B.S. Optical and tribological properties of heat-treated diamond-like carbon. J. Mater. Res. 1990, 5, 2531–2537. [Google Scholar] [CrossRef]
- Jacob, W.; Dürbeck, T.; Schwarz-Selinger, T.; Toussaint, U. Bonding States of Hydrogen in Plasma-Deposited Hydrocarbon Films. J. Carbon Res. 2020, 6, 3. [Google Scholar] [CrossRef]
- Kanda, K.; Shimizugawa, Y.; Haruyama, Y.; Yamada, I.; Matsui, S.; Kitagawa, T.; Tsubakino, H.; Gejo, T. NEXAFS study on substrate temperature dependence of DLC films formed by Ar cluster ion beam assisted deposition. Nucl. Instr. Methods Phys. Res. 2003, B 206, 880–883. [Google Scholar] [CrossRef]
- Kanda, K.; Suzuki, S.; Niibe, M.; Hasegawa, T.; Suzuki, T.; Saitoh, H. Local Structure Analysis on Si-Containing DLC Films Based on the Measurement of C K-Edge and Si K-Edge X-ray Absorption Spectra. Coatings 2020, 10, 330. [Google Scholar] [CrossRef]
- Grill, A.; Meyerson, B.S.; Patel, V.V.; Reimer, J.A.; Petrich, M.A. Inhomogeneous carbon bonding in hydrogenated amorphous carbon films. J. Appl. Phys. 1987, 61, 2874–2877. [Google Scholar] [CrossRef]
- DeLongchamp, D.M.; Kline, R.J.; Lin, E.K.; Fischer, D.A.; Richter, L.J.; Lucas, L.A.; Heeney, M.; McCulloch, I.; Northrup, J.E. High Carrier Mobility Polythiophene Thin Films: Structure Determination by Experiment and Theory. Adv. Mater. 2007, 19, 833–837. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Lee, C.S.; Lee, Y.J.; Lee, K.-R.; Chae, K.-H.; Oh, K.H. Reduction of the residual compressive stress of tetrahedral amorphous carbon film by Ar background gas during the filtered vacuum arc process. J. Appl. Phys. 2007, 101, 023504. [Google Scholar] [CrossRef]
- Ohmagari, S.; Yoshitake, T.; Nagano, A.; Al-Riyami, S.; Ohtani, R.; Setoyama, H.; Kobayashi, E.; Nagayama, K. Near-Edge X-Ray Absorption Fine Structure of Ultrananocrystalline Diamond/Hydrogenated Amorphous Carbon Films Prepared by Pulsed Laser Deposition. J. Nanomater. 2009, 2009, 876561. [Google Scholar] [CrossRef]
- Al-Riyami, S.; Ohmagari, S.; Yoshitake, T. Nitrogen-Doped Ultrananocrystalline Diamond/Hydrogenated Amorphous Carbon Composite Films Prepared by Pulsed Laser Deposition. Appl. Phys. Exp. 2010, 3, 115102. [Google Scholar] [CrossRef]
- Hoffman, A.; Heiman, A.; Strunk, H.P.; Christiansen, S.H. Microstructure and phase composition evolution of nanocrystalline carbon films: dependence on deposition temperature. J. Appl. Phys. 2002, 91, 3336–3344. [Google Scholar] [CrossRef]
- Bustillo, K.C. Characterization of Amorphous Hydrogenated Carbon Using Solid-State Nuclear Magnetic Resonance Spectroscopy. Chem. Mater. 1990, 2, 202–205. [Google Scholar] [CrossRef]
- Metallic Materials—Instrumented Indentation Test for Hardness and Materials Parameters—Part 1: Test Method ISO 14577-1:2015; The International Organization for Standardization; Vernier: Geneva, Switzerland, 2015.
- Ferris, L.M. Mellitic Acid from the Oxidation of Graphite with 90% Nitric Acid. J. Chem. Eng. Data 1964, 9, 387–388. [Google Scholar] [CrossRef]
- Sato, Y.; Kamo, M.; Setaka, N. Raman Spectra of Carbons at 2600-3300 cm-1 Region. Carbon 1978, 16, 279–280. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. 1979, B 20, 392–401. [Google Scholar]
- Thomsen, C.; Reich, S. Double resonant Raman scattering in graphite. Phys. Rev. 2000, 85, 5214–5217. [Google Scholar] [CrossRef]
- Castiglioni, C.; Tommasini, M.; Zerbi, G. Raman spectroscopy of polyconjugated molecules and materials: confinement effect in one and two dimensions. Phil. Trans. R. Soc. Lond. 2004, A 362, 2425–2459. [Google Scholar] [CrossRef]
- Okubo, H.; Tadokoro, C.; Hirata, Y.; Sasaki, S. In situ Raman observation of the graphitization process of tetrahedral amorphous carbon under boundary lubrication in poly-alpha-olefin with an organic friction modifier. Tribo. Online 2017, 12, 229–237. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Nagai, N.; Matsuki, M.; Fukuda, H.; Katagiri, G.; Ishida, H.; Ishitani, A.; Nagai, I. Raman scattering from sp carbon clusters. Phys. Rev. 1992, B 46, 7169–7174. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Rodil, S.E.; Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. 2003, B 67, 155306. [Google Scholar] [CrossRef]
- Racine, B.; Ferrari, A.C.; Morrison, N.A.; Hutchings, I.; Milne, W.I.; Robertson, J. Properties of amorphous carbon–silicon alloys deposited by a high plasma density source. J. Appl. Phys. 2001, 90, 5002–5012. [Google Scholar] [CrossRef][Green Version]
- Gilkes, K.W.R.; Prawer, S.; Nugent, K.W.; Robertson, J.; Sands, H.S.; Lifshitz, Y.; Shi, X. Direct quantitative detection of the sp3 bonding in diamond-like carbon films using ultraviolet and visible Raman spectroscopy. J. Appl. Phys. 2000, 87, 7283. [Google Scholar] [CrossRef]
- Hasebe, T.; Nagashima, S.; Kamijo, A.; Moon, M.-W.; Kashiwagi, Y.; Hotta, A.; Lee, K.-R.; Takahashi, K.; Yamagami, T.; Suzuki, T. Hydrophobicity and non-thrombogenicity of nanoscale dual rough surface coated with fluorine-incorporated diamond-like carbon films: Biomimetic surface for blood-contacting medical devices. Diam. Relat. Mater. 2013, 38, 14–18. [Google Scholar] [CrossRef]
- Ha, S.-W.; Hauert, R.; Ernst, K.-H.; Wintermantel, E. Surface analysis of chemically-etched and plasma-treated polyetheretherketone (PEEK) for biomedical applications. Surf. Coat. Technol. 1997, 96, 293–299. [Google Scholar] [CrossRef]
- Roy, R.K.; Choi, H.-W.; Park, S.-J.; Lee, K.-R. Surface energy of the plasma treated Si incorporated diamond-like carbon films. Diam. Relat. Mater. 2007, 16, 1732–1738. [Google Scholar] [CrossRef]
- Jongwannasiri, C.; Watanabe, S. Surface energy of the plasma treated Si incorporated diamond-like carbon films. J. Surf. Finish. Soc. Jpn. 2014, 65, 77–80. [Google Scholar] [CrossRef]
- Karan, S.; Samitsu, S.; Peng, X.; Kurashima, K.; Ichinose, I. Ultrafast Viscous Permeation of Organic Solvents Through Diamond-Like Carbon Nanosheets. Science 2012, 335, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Maerten, T.; Jaoul, C.; Oltra, R.; Duport, P.; Niniven, C.; Tristant, P.; Meunier, F.; Jarry, O. Micrometric Growth Defects of DLC Thin Films. J. Carbon Res. 2019, 5, 73. [Google Scholar] [CrossRef]
- Maerten, T.; Oltra, R.; Jaoul, C.; Le Niniven, C.; Tristant, P.; Meunier, F.; Jarry, O. Investigation of diamond-like carbon coated steel corrosion: Enhancing the optical detection of defects by a controlled electrochemical activation. Surf. Coat. Technol. 2019, 363, 334–351. [Google Scholar] [CrossRef]
- Silva, R.S.R.P.; Amaratunga, G.A.J. The optical properties of band-gap-modulated diamond-like carbon thin films. Diam. Relat. Mater. 1994, 3, 817–820. [Google Scholar]
- Dai, H.Y.; Cheng, X.R.; Wang, C.F.; Xue, Y.C.; Chen, Z.P. Structural, optical and electrical properties of amorphous carbon filmsdeposited by pulsed unbalanced magnetron sputtering. Optik 2015, 126, 861–864. [Google Scholar] [CrossRef]
- Filik, J.; May, P.W.; Pearce, S.R.J.; Wild, R.K.; Hallam, K.R. XPS and laser Raman analysis of hydrogenated amorphous carbon films. Diam. Relat. Mater. 2003, 12, 974–978. [Google Scholar] [CrossRef]
- Yasui, N.; Inaba, H.; Sasaki, S. XPS study of ultrathin carbon films prepared by filtered cathodic vacuum arc. Surf. Sci. Nanotechnol. 2006, 4, 129–132. [Google Scholar] [CrossRef]
- Lesiak, B.; Kövér, L.; Tóth, J.; Zemek, J.; Jiricek, P.; Kromka, A.; Rangam, N. sp2/sp3 hybridisations in carbon nanomaterials – XPS and (X)AES study. Appl. Surf. Sci. 2018, 452, 223–231. [Google Scholar] [CrossRef]
- Diociaiuti, M.; Casciardi, S.; Sisto, R. The sp2 and sp3 fractions of unknown carbon materials: electronenergy-loss near-edge structure analysis of C-K spectra acquired under the magic-angle condition by the electron nanobeam technique. Micron. 2016, 90, 97–107. [Google Scholar] [CrossRef]
- Tamor, M.A.; Vassell, W.C.; Cardune, K.R. Atomic constraint in hydrogenated "diamond-like" carbon. Appl. Phys. Lett. 1991, 58, 592–594. [Google Scholar] [CrossRef]
- Takabayashi, S.; Okamoto, K.; Shimada, K.; Motomitsu, K.; Motoyama, H.; Nakatani, T.; Sakaue, H.; Suzuki, H.; Takahagi, T. Chemical Structural Analysis of Diamondlike Carbon Films with Different Electrical Resistivities by X-ray Photoelectron Spectroscopy. Jpn. J. Appl. Phys. 2008, 47, 3376–3379. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Y.; He, C.; Gao, Y.; Jia, N.; Chen, Z.; Song, W. The role of sp2/sp3 hybrid carbon regulation in the nonlinear optical properties of graphene oxide materials. RSC Adv. 2017, 7, 53643–53652. [Google Scholar] [CrossRef]
- Tsugawa, K.; Ishihara, M.; Kim, J.; Hasegawa, M.; Koga, Y. Large-area and low-temperature nanodiamond coating by microwave plasma chemical vapor deposition. New Diam. Front. Carbon Technol. 2006, 16, 337–346. [Google Scholar]
- Shenderova, O.A.; Vlasov, I.I.; Turner, S.; Tendeloo, G.V.; Orlinskii, S.B.; Shiryaev, A.A.; Khomich, A.A.; Sulyanov, S.N.; Jelezko, F.; Wrachtrup, J. Nitrogen Control in Nanodiamond Produced by Detonation Shock-Wave-Assisted Synthesis. J. Phys. Chem. 2011, C 115, 14014–14024. [Google Scholar] [CrossRef]
- Isobe, F.; Ohfuji, H.; Sumiya, H.; Irifune, T. Nanolayered Diamond Sintered Compact Obtained by Direct Conversion from Highly Oriented Graphite under High Pressure and High Temperature. J. Nanomater. 2013, 380165. [Google Scholar] [CrossRef]
- Sumiya, H.; Irifune, T. Indentation hardness of nano-polycrystalline diamond prepared from graphite by direct conversion. Diam. Relat. Mater. 2004, 13, 1771–1776. [Google Scholar] [CrossRef]
- Schultrich, B. Tetrahedrally Bonded Amorphous Carbon Films I: Basics, Structure and Preparation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 236–239. [Google Scholar]
- Zhou, X.L.; Tunmee, S.; Suzuki, T.; Phothongkam, P.; Kanda, K.; Komatsu, K.; Kawahara, S.; Ito, H.; Saitoh, H. Quantitative NEXAFS and solid-state NMR studies of sp3/(sp2+sp3) ratio in the hydrogenated DLC films. Diam. Relat. Mater. 2017, 73, 232–240. [Google Scholar] [CrossRef]
- Pardanaud, C.; Cartry, G.; Lajaunie, L.; Arenal, R.; Buijnsters, J.G. Investigating the Possible Origin of Raman Bands in Defective sp2/sp3 Carbons below 900 cm-1:Phonon Density of States or Double Resonance Mechanism at Play? J. Carbon Res. 2019, 5, 79. [Google Scholar] [CrossRef]
- Puech, P.; Plewa, J.M.; Mallet-Ladeira, P.; Monthioux, M. Spatial confinement model applied to phonons in disordered graphene-based carbons. Carbon 2016, 105, 275–281. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Carbon Based Films—Classification and Designations ISO 20523:2017; The International Organization for Standardization; Vernier: Geneva, Switzerland, 2017.
- Ohgoe, Y.; Hirakuri, K.K.; Saitoh, H.; Nakahigashi, T.; Ohtake, N.; Hirata, A.; Kanda, K.; Hiratsuka, M.; Fukui, Y. Classification of DLC films in terms of biological response. Surf. Coat. Technol. 2012, 207, 350–354. [Google Scholar] [CrossRef]















| Temperature, Humidity | 20 °C, 32% |
|---|---|
| Liquid | Ultra-pure water |
| Drop Type | Normal Sessile Drop |
| Water-drop Volume | 2 μL |
| Baseline Type | Linear |
| Evaluation method | Circle Fitting |
| Sample Number | Deposition Method | True Density (g/cm3) | Hydrogen (at. %) | NEXAFS sp3/(sp2 + sp3) | Nano-Indentation Hardness (GPa) | NMR sp3/(sp2 + sp3) | Refractive Index n | Extinction Coefficient k | Optical Band Gap (eV) | Water Contact Angle (deg.) | Thickness (nm) in True Density Measurement |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PECVD | 2 | 22 | 0.536 | 13.8 | NA | 2.19 | 0.02 | 1.35 | 71.5 | NA |
| 2 | PECVD | 1.85 | 50 | 0.436 | 14 | NA | 1.46 | 0.25 | NA | 97.9 | NA |
| 3 | PECVD | 1.96 | 23 | 0.425 | 15.8 | NA | 1.22 | 0.19 | 0.86 | 84.4 | NA |
| 4 | PBII&D | 2 | 28 | 0.613 | 11.9 | NA | 3.59 | 0.56 | NA | 83.4 | NA |
| 5 | PBII&D | 1.77 | 12 | 0.48 | 15.8 | NA | 2.04 | 0.37 | 0.8 | 74.8 | 635 |
| 6 | PBII&D | 1.95 | 21 | 0.623 | 16 | NA | 2.12 | 0.38 | 0.69 | 80.2 | 596 |
| 7 | PECVD | 1.51 | 11 | 0.577 | 13 | NA | 1.84 | 0.37 | 0.76 | 88.7 | NA |
| 8 | PBII&D | 1.86 | 8 | 0.181 | 12.8 | NA | 2.09 | 0.42 | 0.53 | 74 | NA |
| 9 | PECVD | 1.71 | 21 | 0.681 | 11.2 | NA | 2.08 | 0.33 | 1.57 | 94.3 | 1622 |
| 10 | PECVD | 1.95 | 20 | 0.746 | 14.3 | NA | 2.3 | 0.13 | 1.21 | 77.7 | 590 |
| 11 | PECVD | 2.1 | 20 | 0.338 | 20.1 | NA | 1.99 | 0.24 | 0.77 | 75.2 | NA |
| 12 | PECVD | 2.09 | 20 | 0.459 | 17 | NA | 1.98 | 0.24 | 0.98 | 77.2 | 559 |
| 13 | PECVD | 1.7 | 30 | 0.506 | 17.6 | NA | 2.14 | 0.17 | 1.21 | 89.8 | 838 |
| 14 | PECVD | 2.3 | 22 | 0.351 | 19.1 | NA | 3.2 | 0.6 | NA | 79.6 | NA |
| 15 | PECVD | 1.59 | 29 | 0.401 | 15.3 | NA | 2.04 | 0.21 | 1.07 | 78.9 | 597 |
| 16 | PECVD | 1.52 | 8 | 0.373 | 19 | NA | 2.05 | 0.23 | 1.57 | 77.7 | 479 |
| 17 | PECVD | 2.04 | 20 | 0.576 | 17.8 | NA | 2.1 | 0.49 | 0.97 | 79.9 | NA |
| 18 | PECVD | 2.04 | 20 | 0.428 | 18.6 | NA | 1.89 | 0.52 | 0.7 | 74.7 | NA |
| 19 | PECVD | 1.83 | 32 | 0.473 | 16.5 | NA | 2.16 | 0.12 | NA | 88.7 | 504 |
| 20 | CVD | 1.86 | 26 | 0.562 | 24.8 | NA | 2.33 | 0.3 | NA | NA | 723 |
| 21 | CVD | 1.77 | 28 | 0.602 | 17.3 | NA | 2.18 | 0.19 | NA | NA | 474 |
| 22 | CVD | 1.71 | 17 | 0.531 | 20.3 | NA | 2.25 | 0.52 | NA | NA | 557 |
| 23 | CVD | 1.85 | 25 | 0.558 | 14.1 | NA | 2.27 | 0.22 | NA | NA | 639 |
| 24 | CVD | 1.91 | 18 | 0.664 | 17.8 | NA | 2.41 | 0.4 | NA | NA | 996 |
| 25 | CVD | 1.69 | 19 | 0.502 | 15.5 | NA | 2.29 | 0.47 | NA | NA | 524 |
| 26 | CVD | 1.73 | 44 | 0.816 | 17.1 | NA | 2.22 | 0.1 | NA | NA | 1215 |
| 27 | CVD | 1.73 | 26 | 0.499 | 17.9 | NA | 2.25 | 0.2 | NA | NA | 733 |
| 28 | CVD | 1.85 | 19 | 0.552 | 16.6 | NA | 2.32 | 0.42 | NA | NA | 567 |
| 29 | CVD | 1.78 | 17 | 0.686 | 13.7 | NA | 2.07 | 0.74 | NA | NA | 647 |
| 30 | CVD | 1.89 | 15 | 0.716 | 20.9 | NA | 2.37 | 0.46 | NA | NA | 506 |
| 31 | CVD | 1.93 | 24 | 0.781 | 20.8 | NA | 2.25 | 0.09 | NA | NA | 161 |
| 32 | CVD | 1.99 | 26 | 0.692 | 26.6 | NA | 2.35 | 0.44 | NA | NA | 571 |
| 33 | CVD | 1.78 | 25 | 0.578 | 7.2 | NA | NA | NA | NA | NA | 583 |
| 34 | CVD | 1.93 | 21 | 0.52 | 10.5 | NA | NA | NA | NA | NA | 1000 |
| 35 | CVD | 2.09 | 19 | 0.628 | 22.5 | NA | NA | NA | NA | NA | 403 |
| 36 | CVD | 2.1 | 22 | 0.61 | 16.9 | 0.192 | 2.41 | 0.32 | NA | NA | 300 |
| 37 | CVD | 1.85 | 25 | 0.64 | 13.4 | NA | 2.28 | 0.27 | NA | NA | 211 |
| 38 | CVD | 2.06 | 17 | 0.58 | 19.2 | NA | 2.33 | 0.53 | NA | NA | 500 |
| 39 | CVD | 1.09 | 38 | 0.61 | 0.83 | NA | 1.57 | 0 | NA | NA | 260 |
| 40 | AIP | 2 | 1.4 | 0.559 | 17.1 | NA | 2.04 | 0.04 | 0.96 | 74.4 | NA |
| 41 | IE | 1.92 | 2 | 0.539 | 24.3 | NA | 1.29 | 0.14 | 1.15 | 75.3 | 532 |
| 42 | SP | 1.81 | 4 | 0.367 | 9.7 | NA | 1.64 | 0.34 | 1.18 | 78.1 | NA |
| 43 | AIP | 2.81 | 5 | 0.595 | 26.6 | NA | 2.08 | 0.04 | NA | 76.9 | NA |
| 44 | IE | 2.37 | 20 | 0.409 | 19.7 | NA | 2.14 | 0.52 | 0.69 | 71.4 | NA |
| 45 | IE | 2.25 | 15 | 0.355 | 21.3 | NA | 1.21 | 0.25 | 0.72 | 73.1 | 435 |
| 46 | SP | 1.7 | 39 | 0.621 | 10.2 | NA | 1.99 | 0.08 | 1.34 | 96.2 | 588 |
| 47 | AIP | 3.32 | 4 | 0.636 | 26.1 | NA | 2.07 | 0.06 | NA | 79.5 | NA |
| 48 | AIP | 2.15 | 20 | 0.522 | 19.9 | NA | 2.87 | 0.44 | 0.44 | 102.2 | 438 |
| 49 | AIP | 2.03 | 30 | 0.207 | 17.2 | NA | 2.54 | 0.05 | 1.78 | 71.1 | 571 |
| 50 | SP | 2.33 | 16 | 0.506 | 22.4 | NA | 2.34 | 0.85 | NA | 85.2 | NA |
| 51 | SP | 1.8 | 20 | 0.532 | 9.3 | NA | 1.37 | 0.25 | NA | 78.8 | NA |
| 52 | PLD | 2.23 | 23 | 0.493 | 22.4 | NA | 2.18 | 0.33 | 0.78 | 79.8 | NA |
| 53 | SP | 1.89 | 3 | 0.415 | 8.6 | NA | 1.48 | 0.38 | 0.5 | 83.8 | NA |
| 54 | AIP | 2 | 5 | 0.591 | 13.4 | NA | 2.49 | 0.14 | 0.72 | 90.3 | NA |
| 55 | AIP | 3.15 | 5 | 0.572 | 47.1 | NA | 2.65 | 0.1 | 1.07 | 75 | 356 |
| 56 | SP | 1.39 | 1.5 | 0.151 | 11.2 | NA | 2.05 | 0.76 | 0.17 | 82.1 | NA |
| 57 | IBD | 2.12 | 31 | 0.151 | 20.9 | NA | NA | NA | NA | 82.7 | NA |
| 58 | IE | 2.03 | 21 | 0.397 | 23.8 | NA | 2.33 | 0.28 | NA | 75.6 | NA |
| 59 | IE | 1.85 | 30 | 0.59 | 28 | NA | 1.25 | 0.16 | 1.34 | 86.8 | NA |
| 60 | PVD | 2.37 | 1 | 0.804 | 30.6 | NA | 2.66 | 0.4 | NA | NA | 321 |
| 61 | PVD | 2.68 | 0.5 | 0.703 | 25.9 | NA | 2.59 | 0.18 | NA | NA | 189 |
| 62 | PVD | 2.2 | 2 | 0.664 | 19.8 | NA | NA | NA | NA | NA | 256 |
| 63 | PVD | 2.51 | 0.75 | 0.646 | 28 | NA | 2.74 | 0.3 | NA | NA | 246 |
| 64 | PVD | 2.88 | 0.3 | 0.718 | 32.6 | NA | 2.71 | 0.1 | NA | NA | 195 |
| 65 | PVD | 3.04 | 0.7 | 0.674 | 37.9 | NA | NA | NA | NA | NA | 290 |
| 66 | PVD | 3.12 | 0.2 | 0.669 | 41.4 | NA | 2.64 | 0.04 | NA | NA | 500 |
| 67 | PVD | 2.95 | 0.5 | 0.532 | 31 | NA | 2.71 | 0.27 | NA | NA | 415 |
| 68 | PVD | 2.99 | 0.3 | 0.535 | 30.9 | NA | 2.73 | 0.24 | NA | NA | 553 |
| 69 | PVD | 2.98 | 1.3 | 0.75 | 15.6 | NA | NA | NA | NA | NA | 68 |
| 70 | PVD | 1.76 | 6 | 0.59 | 8.3 | 0.01 | 2.04 | 0.69 | NA | NA | 250 |
| 71 | PVD | 3.12 | 0.2 | 0.67 | 41.3 | 0.901 | NA | NA | NA | NA | 464 |
| 72 | PVD | NA | 0.5 | 0.703 | 48 | 0.773 | NA | NA | NA | NA | NA |
| 73 | PVD | NA | 0.75 | 0.646 | 47.1 | 0.692 | NA | NA | NA | NA | NA |
| 74 | FCVA | NA | 0.3 | 0.718 | 54 | 0.955 | NA | NA | NA | NA | NA |
| Sample Number | Deposition Method | D-Peak Position (λ = 514 nm) | G-Peak Position (λ = 514 nm) | D-Peak FWHM (λ = 514 nm) | G-Peak FWHM (λ = 514 nm) | I(D)/I(G) (λ = 514 nm) | D-Peak Position (λ = 325 nm) | G-Peak Position (λ = 325 nm) | D-Peak FWHM (λ = 325 nm) | G-Peak FWHM (λ = 325 nm) | I(D)/I(G) (λ = 325 nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PECVD | 1387.9 | 1559.6 | 305.9 | 122.1 | 0.771 | 1396.2 | 1584.8 | 299.1 | 99.3 | 0.422 |
| 2 | PECVD | 1379.7 | 1577.5 | 288.5 | 93.2 | 1.093 | 1400 | 1538 | 289.8 | 84.1 | 0.598 |
| 3 | PECVD | 1387.7 | 1559.1 | 302.3 | 137.5 | 0.706 | 1400.3 | 1582.5 | 337.2 | 115.2 | 0.523 |
| 5 | PBII&D | 1375.5 | 1549 | 308.7 | 143.4 | 0.724 | 1403.9 | 1575.2 | 378.7 | 116.1 | 0.649 |
| 6 | PBII&D | 1378.1 | 1546.2 | 321.3 | 145.6 | 0.739 | 1412.5 | 1573.6 | 380.5 | 122.1 | 0.71 |
| 7 | PECVD | 1371.6 | 1548.1 | 299.9 | 143.9 | 0.747 | 1394.1 | 1573.1 | 347.8 | 118.1 | 0.641 |
| 8 | PBII&D | 1372.3 | 1548 | 306.6 | 143 | 0.817 | 1394 | 1571.4 | 350 | 117.9 | 0.719 |
| 9 | PECVD | 1380 | 1553.3 | 302 | 135.9 | 0.705 | 1406.4 | 1578.2 | 351.7 | 106.9 | 0.539 |
| 10 | PECVD | 1349.8 | 1515.6 | 279.4 | 145.5 | 0.481 | 1381.5 | 1555 | 378.5 | 117.5 | 0.465 |
| 11 | PECVD | 1387.1 | 1560.4 | 304.5 | 129.7 | 0.849 | 1399.3 | 1581.5 | 403.3 | 121 | 0.796 |
| 12 | PECVD | 1387.6 | 1560.8 | 303.2 | 126.9 | 0.739 | 1385.4 | 1586.2 | 382.3 | 125.1 | 0.537 |
| 13 | PECVD | 1388.9 | 1560.7 | 303.5 | 124.6 | 0.691 | 1388.3 | 1590.6 | 374.2 | 121.6 | 0.416 |
| 14 | PECVD | 1381.3 | 1555.4 | 299.2 | 141.6 | 0.681 | 1392.3 | 1584.7 | 413.2 | 128.3 | 0.747 |
| 15 | PECVD | 1363.3 | 1545.6 | 292.3 | 144.5 | 0.506 | 1390.8 | 1587.5 | 381.4 | 116.8 | 0.476 |
| 16 | PECVD | 1352.9 | 1543.5 | 285.2 | 156.1 | 0.395 | 1373.9 | 1590 | 386.1 | 132.2 | 0.467 |
| 17 | PECVD | 1373.2 | 1555 | 307.5 | 159.6 | 0.465 | 1396.3 | 1589.2 | 389 | 117.6 | 0.685 |
| 18 | PECVD | 1391.2 | 1563.1 | 302.1 | 137.8 | 0.801 | 1400.9 | 1589.8 | 405.6 | 117 | 0.838 |
| 19 | PECVD | 1369.5 | 1548.9 | 300.5 | 142.9 | 0.472 | 1394.9 | 1593 | 363.9 | 115.7 | 0.382 |
| 40 | AIP | 1377.9 | 1545.7 | 329 | 154.5 | 0.595 | 1413.8 | 1578.5 | 356.4 | 125.8 | 0.611 |
| 41 | IE | 1361.1 | 1547.1 | 312.9 | 164 | 0.417 | 1381.1 | 1587.3 | 370.7 | 141.7 | 0.396 |
| 42 | SP | 1384.4 | 1558.6 | 292.7 | 129.5 | 0.656 | 1406 | 1596.2 | 354.5 | 94 | 0.53 |
| 43 | AIP | 1296.5 | 1549.9 | 444.6 | 206.6 | 0.205 | 1297.5 | 1610.5 | 416 | 203.2 | 0.248 |
| 44 | IE | 1385.4 | 1553 | 294.9 | 137.7 | 0.808 | 1403.9 | 1570.4 | 366.5 | 112.1 | 0.704 |
| 45 | IE | 1396.9 | 1566.9 | 317.1 | 138.1 | 0.852 | 1406.4 | 1584 | 352.4 | 120.3 | 0.686 |
| 46 | SP | 1380.3 | 1548.8 | 318.3 | 130.6 | 0.639 | 1396.2 | 1590 | 320.1 | 94.4 | 0.349 |
| 47 | AIP | 1247.8 | 1547.4 | 575 | 211.4 | 0.206 | 1297.5 | 1613.7 | 374 | 208.4 | 0.268 |
| 48 | AIP | 1383.2 | 1557.1 | 310.6 | 155.3 | 0.542 | 1406.7 | 1587.4 | 382.3 | 130.9 | 0.521 |
| 49 | AIP | PSF | PSF | PSF | PSF | PSF | 1400.5 | 1585.5 | 363.8 | 123.4 | 0.563 |
| 50 | SP | 1387.4 | 1561.6 | 321.3 | 133.7 | 1.067 | PSF | PSF | PSF | PSF | PSF |
| 51 | SP | 1392.4 | 1568.6 | 308.8 | 121.9 | 1.11 | 1412.9 | 1581.3 | 347.4 | 101.9 | 0.731 |
| 52 | PLD | 1367.2 | 1532.8 | 288.2 | 169.2 | 0.48 | 1410.2 | 1566.7 | 442.6 | 129.4 | 1.186 |
| 53 | SP | 1400.5 | 1590 | 325.2 | 86.8 | 1.277 | 1424.8 | 1599.1 | 377.9 | 86.4 | 0.907 |
| 54 | AIP | 1404.8 | 1560 | 212.9 | 176.7 | 0.324 | 1322.7 | 1614.6 | 370.3 | 189.1 | 0.273 |
| 55 | AIP | 1360.3 | 1562.2 | 391.5 | 199.6 | 0.223 | 1270.8 | 1608.8 | 334.8 | 240.6 | 0.508 |
| 56 | SP | 1390.3 | 1567.6 | 311.1 | 132.5 | 1.463 | 1401 | 1577.3 | 427.8 | 117.4 | 1.541 |
| 57 | IBD | 1385.1 | 1559.8 | 300.3 | 138.8 | 0.714 | 1394.7 | 1589.6 | 432.4 | 128.6 | 0.791 |
| 58 | IE | 1372.2 | 1554.5 | 302 | 157.1 | 0.502 | 1387.1 | 1593.8 | 407.2 | 135.9 | 0.616 |
| 59 | IE | 1368.8 | 1554.7 | 291.9 | 159.9 | 0.445 | 1395.6 | 1597.8 | 415.7 | 139.7 | 0.609 |
| Classification | DLC | DLC | DLC | DLC | not DLC |
|---|---|---|---|---|---|
| Short name | ta-C | ta-C:H | a-C | a-C:H | PLC |
| Designation | Tetrahedral hydrogen-free amorphous carbon film | Tetrahedral hydrogenated amorphous carbon film | Hydrogen-free amorphous carbon film | Hydrogenated amorphous carbon film | Polymer-like carbon |
| sp3/(sp3 + sp2) (%) | 50–90 | 50–90 | 10–50 | 10–50 | - |
| H (at%) | <5 | 5–50 | <5 | 5–50 | 40–70 |
| Deposition method | PVD | PVD, CVD, PVD + CVD | PVD | CVD, PVD + CVD | CVD, PVD |
| Deposition temperature (°C) | RT-300 | RT-500 | RT-200 | RT-500 | RT-100 |
| True density ρ (g/cm3) (as reference) | 3.5 > ρ > 2.6 | 2.6 > ρ > 2.0 | 1.7 > ρ > 1.4 | 2.0 > ρ > 1.4 | ρ < 1.4 |
| Coefficient of friction vs. steel (dry cond.) (as reference) | 0.1–0.2 | 0.08–0.2 | 0.1–0.2 | 0.08–0.2 | Unstable |
| Wear resistance vs. steel (dry cond.) | A | B–A | C | B–A | × |
| Nano-indentation hardness (HIT: GPa) | 25–90 | 9–35 | 9–25 | 9–25 | 0.5–9 |
| Young’s modulus (GPa) | 200–900 | 120–300 | 100–400 | 100–220 | ~100 |
| Corrosion resistance | B–A | C–B | ×–C | B | B |
| Water contact angle (°) (as reference) | 70–80 | 80–100 | 70–100 | 70–90 | ~70 |
| Refractive index n (λ = 550 nm) | 2.5–3.0 | 2.0–2.5 | 1.8–2.5 | 1.7–2.4 | 1.5–2.0 |
| Extinction coefficient k (λ = 550 nm) | 0.04–0.5 | 0.05–0.6 | 0.1–0.6 | 0.05–0.6 | ~0.1 |
| Color (500 nm in thick) | Transparent (Interference color) | Brown | Black | Light brown | Transparent |
| Optical band gap (eV) | 0.2–1.7 | 1.0–2.5 | 1.0–1.7 | 0.5–2.0 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. https://doi.org/10.3390/ma14020315
Ohtake N, Hiratsuka M, Kanda K, Akasaka H, Tsujioka M, Hirakuri K, Hirata A, Ohana T, Inaba H, Kano M, et al. Properties and Classification of Diamond-Like Carbon Films. Materials. 2021; 14(2):315. https://doi.org/10.3390/ma14020315
Chicago/Turabian StyleOhtake, Naoto, Masanori Hiratsuka, Kazuhiro Kanda, Hiroki Akasaka, Masanori Tsujioka, Kenji Hirakuri, Atsushi Hirata, Tsuguyori Ohana, Hiroshi Inaba, Makoto Kano, and et al. 2021. "Properties and Classification of Diamond-Like Carbon Films" Materials 14, no. 2: 315. https://doi.org/10.3390/ma14020315
APA StyleOhtake, N., Hiratsuka, M., Kanda, K., Akasaka, H., Tsujioka, M., Hirakuri, K., Hirata, A., Ohana, T., Inaba, H., Kano, M., & Saitoh, H. (2021). Properties and Classification of Diamond-Like Carbon Films. Materials, 14(2), 315. https://doi.org/10.3390/ma14020315





