Reduced Graphene Oxide Aerogel inside Melamine Sponge as an Electrocatalyst for the Oxygen Reduction Reaction
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
3.1. Elemental Analysis
3.2. SEM
3.3. Raman Spectra
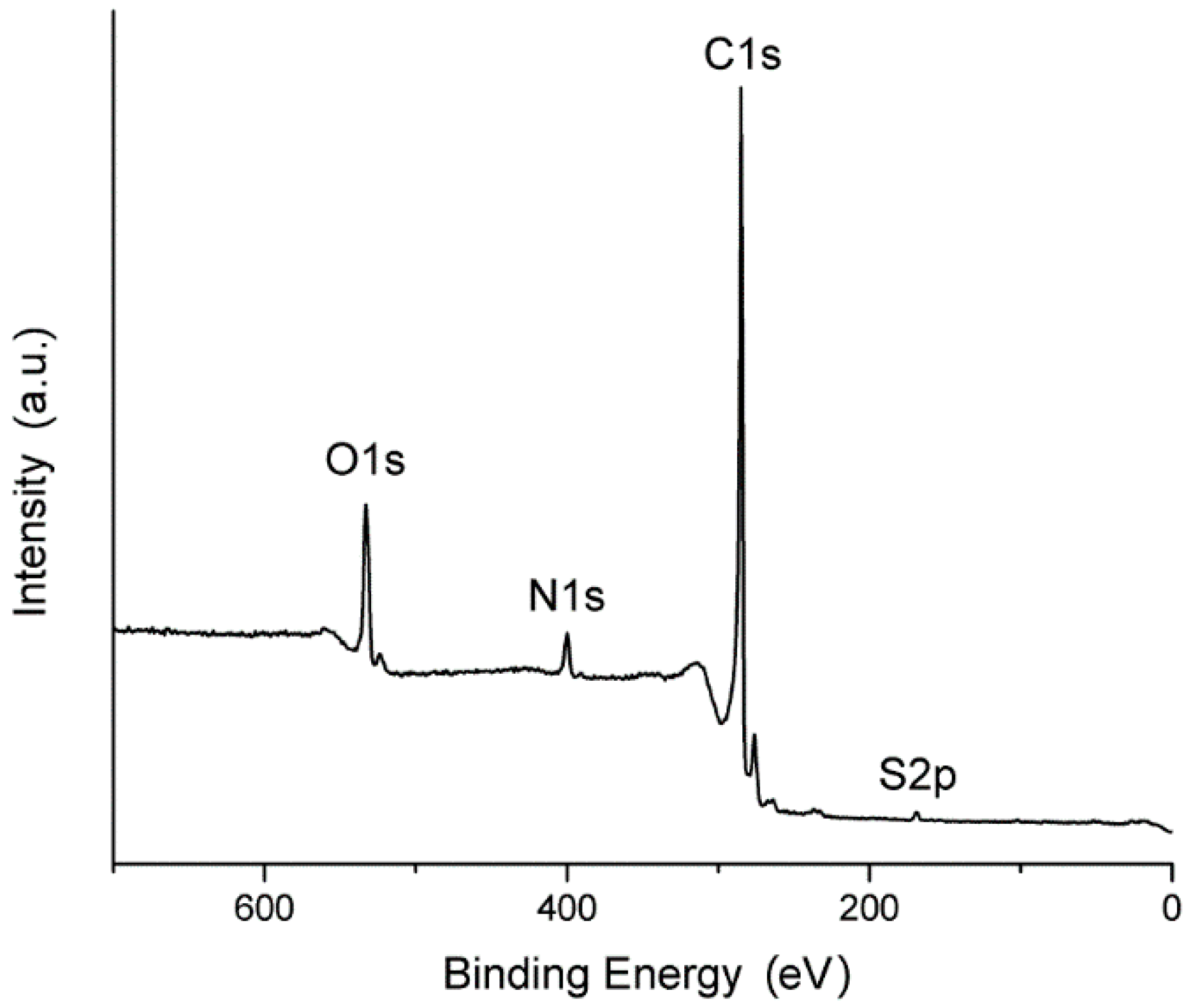
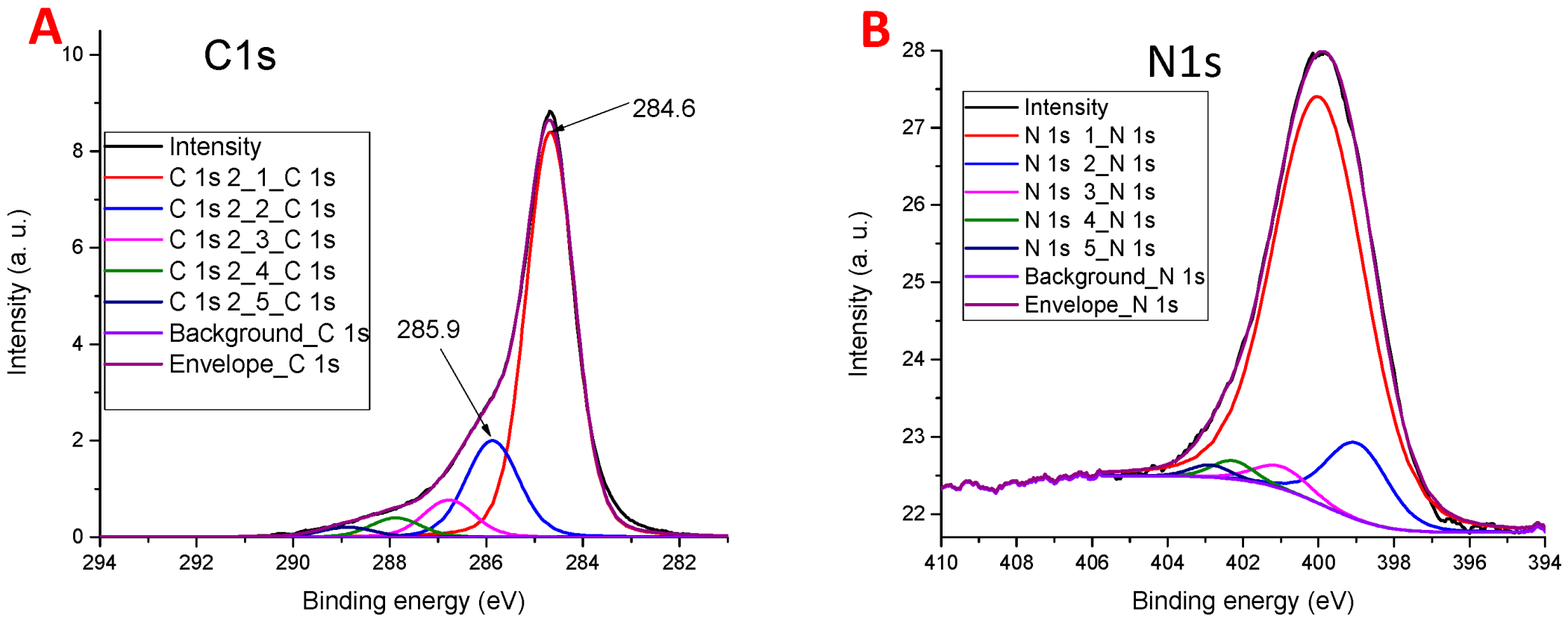
3.4. XPS
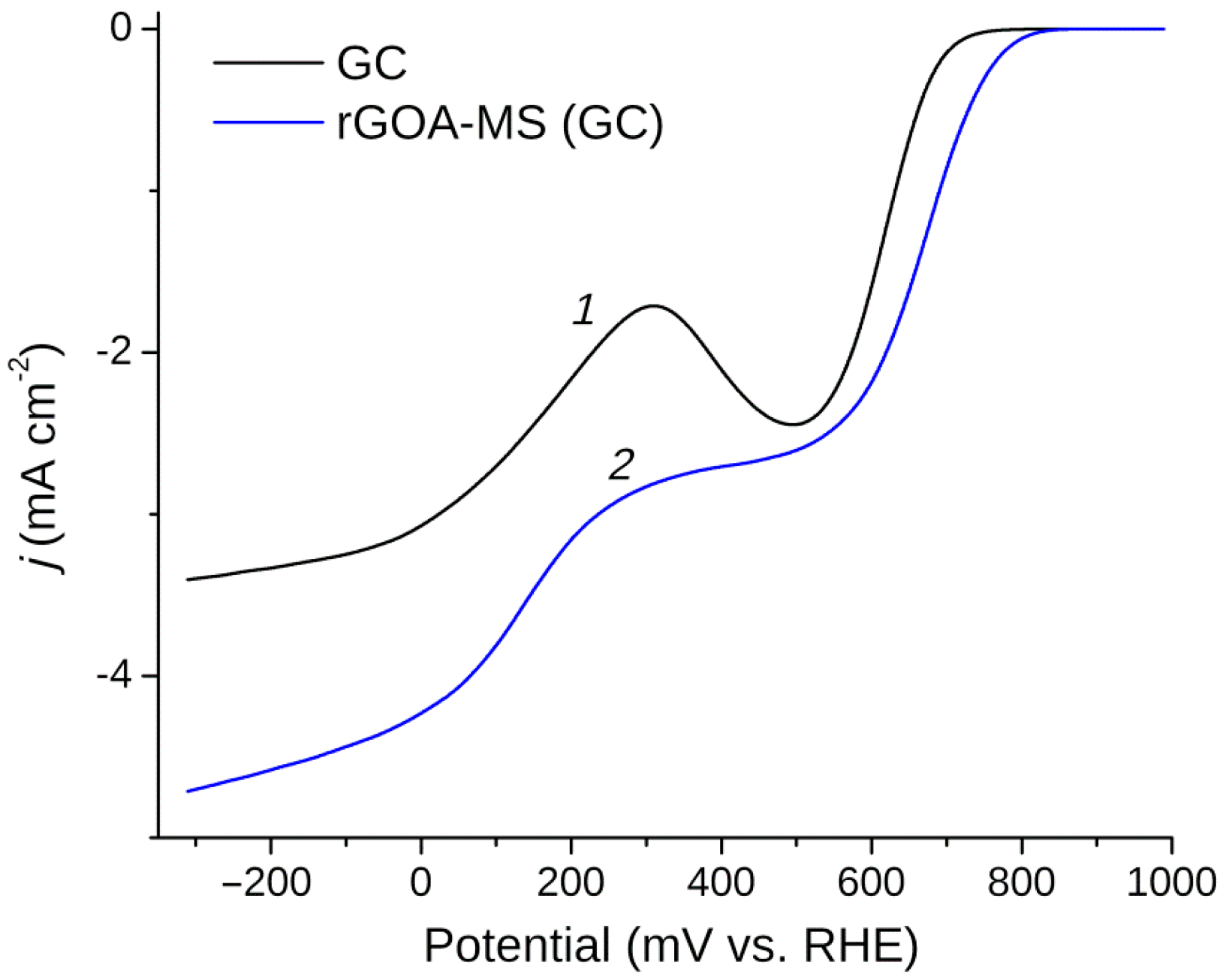
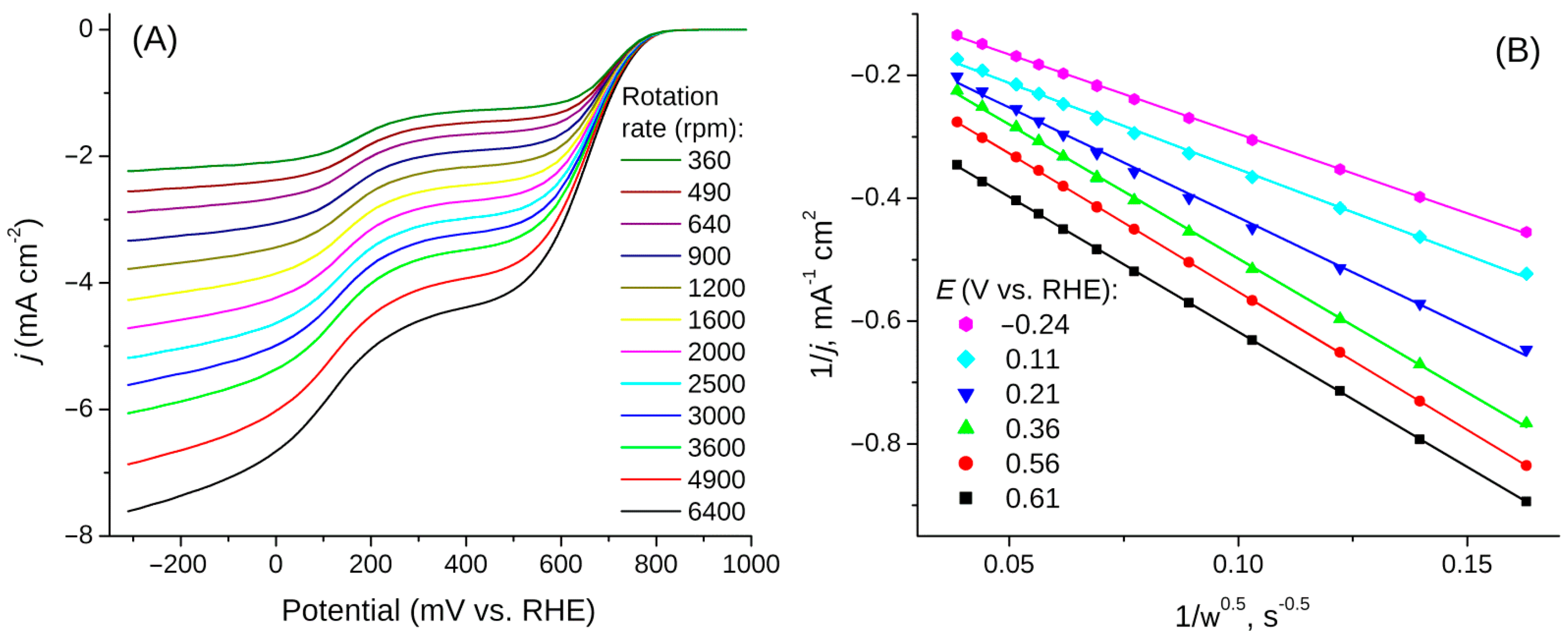
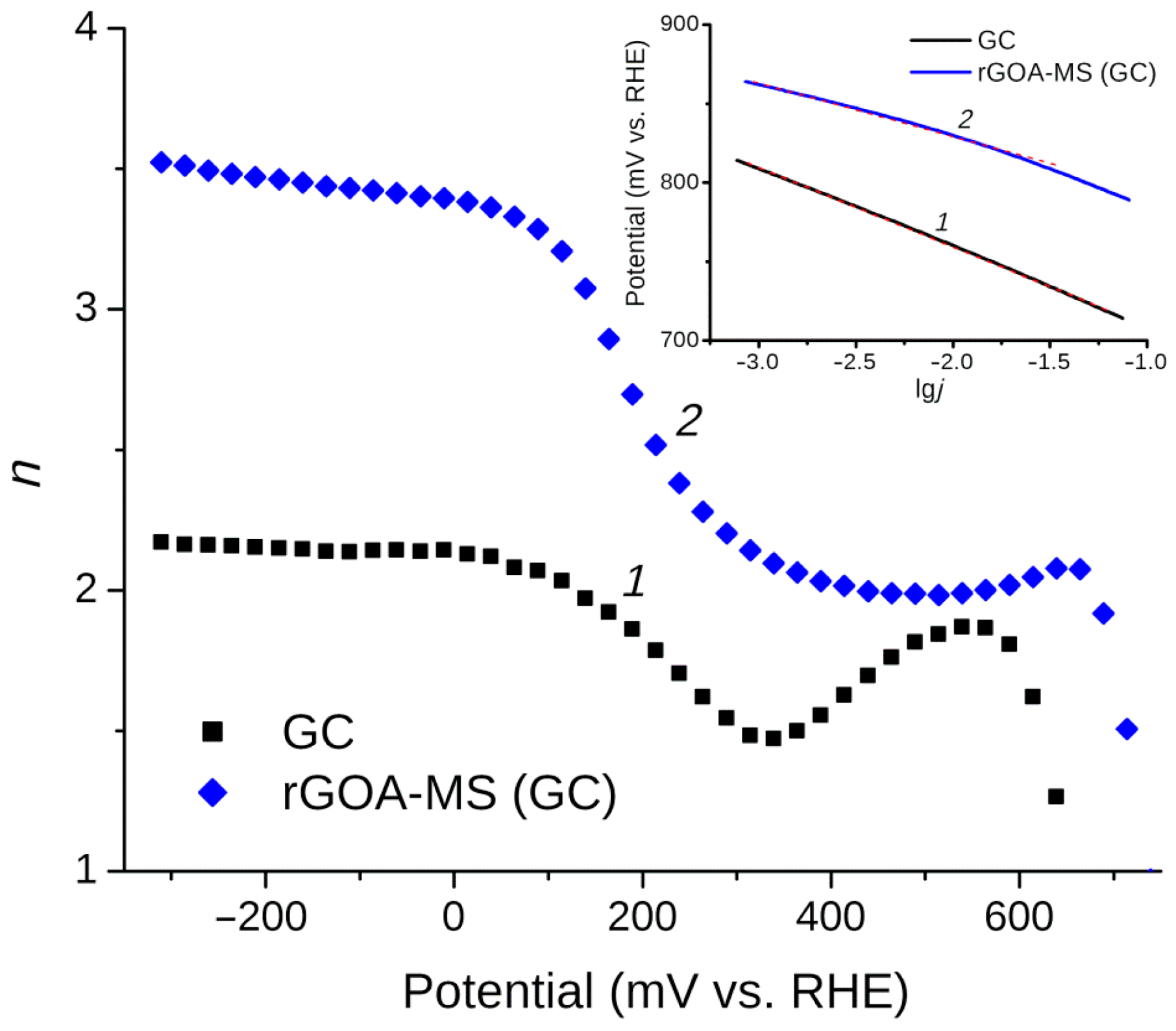
3.5. Oxygen Reduction Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Xu, Z.; Gao, C. Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, G. Preparation of Highly Conductive Graphene Hydrogels for Fabricating Supercapacitors with High-Rate Capability. J. Phys. Chem. C 2011, 115, 17206–17212. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis of graphene aerogel with high electrical conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sui, Z.; Xu, B.; Yue, S.; Luo, Y.; Zhan, W.; Liu, B. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J. Mater. Chem. 2011, 21, 6494–6497. [Google Scholar] [CrossRef]
- Pham, H.D.; Pham, V.H.; Cuong, T.V.; Nguyen-Phan, T.-D.; Chung, J.S.; Shin, E.W.; Kim, S. Synthesis of the chemically converted graphene xerogel with superior electrical conductivity. Chem. Commun. 2011, 47, 9672–9674. [Google Scholar] [CrossRef] [PubMed]
- Gorgolis, G.; Galiotis, C. Graphene aerogels: A review. 2D Mater. 2017, 4, 032001. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4265. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef]
- Wang, R.; Xu, C.; Sun, J.; Gao, L. Three-dimensional Fe2O3 nanocubes/nitrogen-doped graphene aerogels: Nucleation mechanism and lithium storage properties. Sci. Rep. 2014, 4, 7171. [Google Scholar] [CrossRef]
- Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Macroscopic multifunctional graphene-based hydrogels and aerogels by a metal ion induced self-assembly process. ACS Nano 2012, 6, 2693–2703. [Google Scholar] [CrossRef]
- Liu, X.; Cui, J.; Sun, J.; Zhang, X. 3D graphene aerogel-supported SnO2 nanoparticles for efficient detection of NO2. RSC Adv. 2014, 4, 22601–22605. [Google Scholar] [CrossRef]
- Lobach, A.S.; Kazakov, V.A.; Spitsyna, N.G.; Baskakov, S.A.; Dremova, N.N.; Shulga, Y.M. Comparative study of graphene aerogels synthesized using sol-gel method by reducing graphene oxide suspension. High Energy Chem. 2017, 51, 269–276. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, X.; Selvaraj, M.; Wei, W.; Jiang, Z.; Ullah, N.; Liu, J.; Xie, J. MXP (M= Co/Ni)@ carbon core–shell nanoparticles embedded in 3D cross-linked graphene aerogel derived from seaweed biomass for hydrogen evolution reaction. Nanoscale 2018, 10, 9698–9706. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shen, J.; Yang, X.; Zhang, T.; Tang, H. 3D reduced graphene oxide aerogel-mediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl. Catal. B 2018, 232, 562–573. [Google Scholar] [CrossRef]
- Gu, J.; Hu, C.; Zhang, W.; Dichiara, A.B. Reagentless preparation of shape memory cellulose nanofibril aerogels decorated with Pd nanoparticles and their application in dye discoloration. Appl. Catal. B 2018, 237, 482–490. [Google Scholar] [CrossRef]
- Ma, Y.; Yue, Y.; Zhang, H.; Cheng, F.; Zhao, W.; Rao, J.; Luo, S.; Wang, J.; Jiang, X.; Liu, Z.; et al. 3D Synergistical MXene/Reduced Graphene Oxide Aerogel for a Piezoresistive Sensor. ACS Nano 2018, 12, 3209–3216. [Google Scholar] [CrossRef]
- Nagy, B.; Bakos, I.; Bertóti, I.; Domán, A.; Menyhárd, A.; Mohai, M.; László, K. Synergism of nitrogen and reduced graphene in the electrocatalytic behavior of resorcinol-Formaldehyde based carbon aerogels. Carbon 2018, 139, 872–879. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, G.; Chen, C.; Li, R.; Mai, Y.; Sun, G.; Yan, D. Superhydrophobic and superoleophilic graphene aerogel prepared by facile chemical reduction. J. Mater. Chem. A 2015, 3, 7498–7504. [Google Scholar] [CrossRef]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Graphene: Spongy Graphene as a Highly Efficient and Recyclable Sorbent for Oils and Organic Solvents. Adv. Funct. Mater. 2012, 22, 4401. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Z.; Fan, J.; Ge, Y.; Yin, J.; Hu, G. Self-assembly of graphene into three-dimensional structures promoted by natural phenolic acids. J. Mater. Chem. 2012, 22, 22459–22466. [Google Scholar] [CrossRef]
- Wu, T.; Chen, M.; Zhang, L.; Xu, X.; Liu, Y.; Yan, J.; Wang, W.; Gao, J. Three-dimensional graphene-based aerogels prepared by a self-assembly process and its excellent catalytic and absorbing performance. J. Mater. Chem. A 2013, 1, 7612–7621. [Google Scholar] [CrossRef]
- Huang, X.; Sun, B.; Su, D.; Zhao, D.; Wang, G. Soft-template synthesis of 3D porous graphene foams with tunable architectures for lithium—O2 batteries and oil adsorption applications. J. Mater. Chem. A 2014, 2, 7973–7979. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V.; Kabachkov, E.N.; Dremova, N.N.; Michtchenko, A.; Shulga, Y.M. Novel Superhydrophobic Aerogel on the Base of Polytetrafluoroethylene. ACS Appl. Mater. Interfaces 2019, 11, 32517–32522. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Tan, C.; Zhang, X.; Chen, J.; Cao, X.; Li, B.; Zong, Y.; Huang, L.; Huang, X.; Wang, L.; et al. Preparation of Cobalt Sulfide Nanoparticle-Decorated Nitrogen and Sulfur Co-Doped Reduced Graphene Oxide Aerogel Used as a Highly Efficient Electrocatalyst for Oxygen Reduction Reaction. Small 2016, 12, 5920–5926. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, S.; Ren, J.; Jia, Y.A.; Chen, C.; Komarneni, S.; Yang, D.; Yao, X. Electronic Structure Tuning in Ni3FeN/r-GO Aerogel toward Bifunctional Electrocatalyst for Overall Water Splitting. ACS Nano 2018, 12, 245–253. [Google Scholar] [CrossRef]
- Xiong, C.; Yang, Q.; Dang, W.; Li, M.; Li, B.; Su, J.; Liu, Y.; Zhao, W.; Duan, C.; Dai, L.; et al. Fabrication of eco-friendly carbon microtubes@ nitrogen-doped reduced graphene oxide hybrid as an excellent carbonaceous scaffold to load MnO2 nanowall (PANI nanorod) as bifunctional material for high-performance supercapacitor and oxygen reduction reaction catalyst. J. Power Sources 2020, 447, 227387. [Google Scholar]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- He, Q.; Cairns, E.J. Recent progress in electrocatalysts for oxygen reduction suitable for alkaline anion exchange membrane fuel cells. J. Electrochem. Soc. 2015, 162, F1504. [Google Scholar] [CrossRef]
- Shah, A.; Zahid, A.; Subhan, H.; Munir, A.; Iftikhar, F.J.; Akbar, M. Heteroatom-doped carbonaceous electrode materials for high performance energy storage devices. Sustain. Energy Fuels 2018, 2, 1398–1429. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhu, E.; McLouth, T.; Chiu, C.-Y.; Huang, X.; Huang, Y. Stabilization of high-performance oxygen reduction reaction Pt electrocatalyst supported on reduced graphene oxide/carbon black composite. J. Am. Chem. Soc. 2012, 134, 12326–12329. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.H.; Choi, S.M.; Lee, D.U.; Kim, W.B.; Chen, Z. Correlation between theoretical descriptor and catalytic oxygen reduction activity of graphene supported palladium and palladium alloy electrocatalysts. J. Power Sources 2015, 300, 1–9. [Google Scholar] [CrossRef]
- Lüsi, M.; Erikson, H.; Treshchalov, A.; Rähn, M.; Merisalu, M.; Kikas, A.; Kisand, V.; Sammelselg, V.; Tammeveski, K. Oxygen reduction reaction on Pd nanocatalysts prepared by plasma-assisted synthesis on different carbon nanomaterials. Nanotechnology 2021, 32, 035401. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Manzhos, R.A.; Lobach, A.S.; Baskakova, Y.V.; Kulikov, A.V.; Martynenko, V.M.; Milovich, F.O.; Kumar, Y.; Michtchenko, A.; Kabachkov, E.N.; et al. Properties of a granulated nitrogen-doped graphene oxide aerogel. J. Non-Cryst. Solids 2018, 498, 236–243. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Huang, L.; Li, C.; Hong, J.-D.; Shi, G. Highly compressible macroporous graphene monoliths via an improved hydrothermal process. Adv. Mater. 2014, 26, 4789–4793. [Google Scholar] [CrossRef]
- Feng, Y.; Yao, J. Design of Melamine Sponge-Based Three-Dimensional Porous Materials toward Applications. Ind. Eng. Chem. Res. 2018, 57, 7322–7330. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, Y.; Zhang, X.-F.; Yao, J. In-situ gelation of sodium alginate supported on melamine sponge for efficient removal of copper ions. J. Colloid Interface Sci. 2018, 512, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Melamine Sponge. Available online: https://www.melaminefoamtech.com/applications/Melamine-spongeMagic-eraserCleaning-Sponge.html (accessed on 7 January 2021).
- Feng, Y.; Cao, M.; Yang, L.; Zhang, X.-F.; Wang, Y.; Yu, D.; Gu, X.; Yao, J. Bilayer N-doped carbon derived from furfuryl alcohol-wrapped melamine sponge as high-performance supercapacitor. J. Electroanal. Chem. 2018, 823, 633–637. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, K.; Li, L.; Chen, X.; Hu, J.; Yan, D.; Xiao, G.; He, X. High performance graphene-based foam fabricated by a facile approach for oil absorption. J. Mater. Chem. A 2017, 5, 11263–11270. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Yang, Y.; Chen, Z.; Jia, G.; Zhang, L. Silk fibroin-graphene oxide functionalized melamine sponge for efficient oil absorption and oil/water separation. Appl. Surf. Sci. 2019, 497, 143762. [Google Scholar] [CrossRef]
- Feng, T.; Xu, J.; Yu, C.; Cheng, K.; Wu, Y.; Wang, Y.; Li, F. Graphene oxide wrapped melamine sponge as an efficient and recoverable adsorbent for Pb(II) removal from fly ash leachate. J. Hazard. Mater. 2019, 367, 26–34. [Google Scholar] [CrossRef]
- Song, S.; Yang, H.; Su, C.; Jiang, Z.; Lu, Z. Ultrasonic-microwave assisted synthesis of stable reduced graphene oxide modified melamine foam with superhydrophobicity and high oil adsorption capacities. Chem. Eng. J. 2016, 306, 504–511. [Google Scholar] [CrossRef]
- Saha, P.; Dashairya, L. Reduced graphene oxide modified melamine formaldehyde (rGO@ MF) superhydrophobic sponge for efficient oil–water separation. J. Porous Mater. 2018, 25, 1475–1488. [Google Scholar] [CrossRef]
- Sun, S.; Tang, S.; Chang, X.; Wang, N.; Wang, D.; Liu, T.; Lei, Y.; Zhu, Y. A bifunctional melamine sponge decorated with silver-reduced graphene oxide nanocomposite for oil-water separation and antibacterial applications. Appl. Surf. Sci. 2019, 473, 1049–1061. [Google Scholar] [CrossRef]
- William, S.; Hummers, J.R.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Shulga, Y.M.; Baskakov, S.A.; Smirnov, V.A.; Shulga, N.Y.; Belay, K.G.; Gutsev, G.L. Graphene Oxide Films as Separators of Polyaniline-Based Supercapacitors. J. Power Sources 2014, 245, 33–36. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V.; Lyskov, N.V.; Dremova, N.N.; Irzhak, A.V.; Kumar, Y.; Michtchenko, A.; Shulga, Y.M. Fabrication of Current Collector Using a Composite of Polylactic Acid and Carbon Nano-Material for Metal-Free Supercapacitors with Graphene Oxide Separators and Microwave Exfoliated Graphite Oxide Electrodes. Electrochim. Acta 2018, 260, 557–563. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; John Wiley and Sons: Chichester, UK, 1983. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Fundamentals and Applications: Electrochemical Methods, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Jürmann, G.; Tammeveski, K. Electroreduction of oxygen on multi-walled carbon nanotubes modified highly oriented pyrolytic graphite electrodes in alkaline solution. J. Electroanal. Chem. 2006, 597, 119–126. [Google Scholar] [CrossRef]
- Qu, L.T.; Liu, Y.; Baek, J.-B.; Dai, L.M. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Shulga, Y.M.; Kabachkov, E.N.; Baskakov, S.A.; Baskakova, Y.V. Doping Graphene Oxide Aerogel with Nitrogen during Reduction with Hydrazine and Low Temperature Annealing in Air. Russ. J. Phys. Chem. A 2019, 93, 296–300. [Google Scholar] [CrossRef]
- Komarova, N.S.; Konev, D.V.; Kotkin, A.S.; Kochergin, V.K.; Manzhos, R.A.; Krivenko, A.G. Effect of graphene surface functionalization on the oxygen reduction reaction in alkaline media. Mendeleev Commun. 2020, 30, 472–473. [Google Scholar] [CrossRef]
- Meier, R.J.; Maple, J.R.; Hwang, M.-J.; Hagler, A.T. Molecular modeling urea-and melamine-formaldehyde resins. 1. A force field for urea and melamine. J. Phys. Chem. 1995, 99, 5445–5456. [Google Scholar] [CrossRef]
- Koglin, E.; Kip, B.J.; Meier, R.J. Adsorption and displacement of melamine at the Ag/electrolyte interface probed by surface-enhanced Raman microprobe spectroscopy. J. Phys. Chem. 1996, 100, 5078–5089. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Lin, M.; Awika, J.; Ledoux, D.R.; Li, H.; Mustapha, A. A new approach to measure melamine, cyanuric acid, and melamine cyanurate using surface enhanced Raman spectroscopy coupled with gold nanosubstrates. Sens. Instrum. Food Qual. Saf. 2008, 2, 66–71. [Google Scholar] [CrossRef]
- Huang, H.; Shende, C.; Sengupta, A.; Inscore, F.; Brouillette, C.; Smith, W.; Farquharson, S. Surface-enhanced Raman spectra of melamine and other chemicals using a 1550 nm (retina-safe) laser. J. Raman Spectrosc. 2012, 43, 701–705. [Google Scholar] [CrossRef]
- Mircescu, N.E.; Oltean, M.; Chiş, V.; Leopold, N. FTIR, FT-Raman, SERS and DFT study on melamine. Vib. Spectrosc. 2012, 62, 165–171. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- López-Díaz, D.; Holgado, M.L.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Korepanov, V.I. Asymmetric least-squares baseline algorithm with peak screening for automatic processing of the Raman spectra. J. Raman Spectrosc. 2020, 51, 2061–2065. [Google Scholar] [CrossRef]
- Korepanov, V.I.; Sedlovets, D.M. An asymmetric fitting function for condensed-phase Raman spectroscopy. Analyst 2018, 143, 2674–2679. [Google Scholar] [CrossRef]
- Daems, N.; Sheng, X.; Vankelecom, I.F.J.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Mathumba, P.; Fernandes, A.J.S.; Iwuoha, E.I.; Freire, C. Towards efficient oxygen reduction reaction electrocatalysts through graphene doping. Electrochim. Acta 2019, 319, 72–81. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Q.; Su, F.; Zhao, X.S. Replicating novel carbon nanostructures with 3D macroporous silica template. J. Mater. Chem. 2005, 15, 2569–2574. [Google Scholar] [CrossRef]


| Sample | C | H | N | S | O * |
|---|---|---|---|---|---|
| MS | 35.40 ± 0.22 | 5.164 ± 0.039 | 43.84 ± 0.28 | 1.325 ± 0.021 | 14.27 |
| GOA-MS | 41.65 ± 0.18 | 3.750 ± 0.074 | 17.40 ± 0.36 | 1.727 ± 0.058 | 35.47 |
| rGOA-MS | 53.34 ± 0.76 | 3.179 ± 0.061 | 27.50 ± 0.92 | 1.500 ± 0.106 | 14.48 |
| Peak | Pos, cm−1 | FWHM, cm−1 | Int, % |
|---|---|---|---|
| D* | 1148 | 227 | 10 |
| D | 1348 | 138 | 46 |
| D″ | 1500 | 140 | 18 |
| G | 1581 | 62 | 14 |
| D′ | 1612 | 58 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzhos, R.A.; Baskakov, S.A.; Kabachkov, E.N.; Korepanov, V.I.; Dremova, N.N.; Baskakova, Y.V.; Krivenko, A.G.; Shulga, Y.M.; Gutsev, G.L. Reduced Graphene Oxide Aerogel inside Melamine Sponge as an Electrocatalyst for the Oxygen Reduction Reaction. Materials 2021, 14, 322. https://doi.org/10.3390/ma14020322
Manzhos RA, Baskakov SA, Kabachkov EN, Korepanov VI, Dremova NN, Baskakova YV, Krivenko AG, Shulga YM, Gutsev GL. Reduced Graphene Oxide Aerogel inside Melamine Sponge as an Electrocatalyst for the Oxygen Reduction Reaction. Materials. 2021; 14(2):322. https://doi.org/10.3390/ma14020322
Chicago/Turabian StyleManzhos, Roman A., Sergey A. Baskakov, Evgeny N. Kabachkov, Vitaly I. Korepanov, Nadezhda N. Dremova, Yulia V. Baskakova, Alexander G. Krivenko, Yury M. Shulga, and Gennady L. Gutsev. 2021. "Reduced Graphene Oxide Aerogel inside Melamine Sponge as an Electrocatalyst for the Oxygen Reduction Reaction" Materials 14, no. 2: 322. https://doi.org/10.3390/ma14020322
APA StyleManzhos, R. A., Baskakov, S. A., Kabachkov, E. N., Korepanov, V. I., Dremova, N. N., Baskakova, Y. V., Krivenko, A. G., Shulga, Y. M., & Gutsev, G. L. (2021). Reduced Graphene Oxide Aerogel inside Melamine Sponge as an Electrocatalyst for the Oxygen Reduction Reaction. Materials, 14(2), 322. https://doi.org/10.3390/ma14020322








