A Phase-Change Mechanism of GST-SL Based Superlattices upon Sb Flipping
Abstract
:1. Introduction
2. Materials and Methods
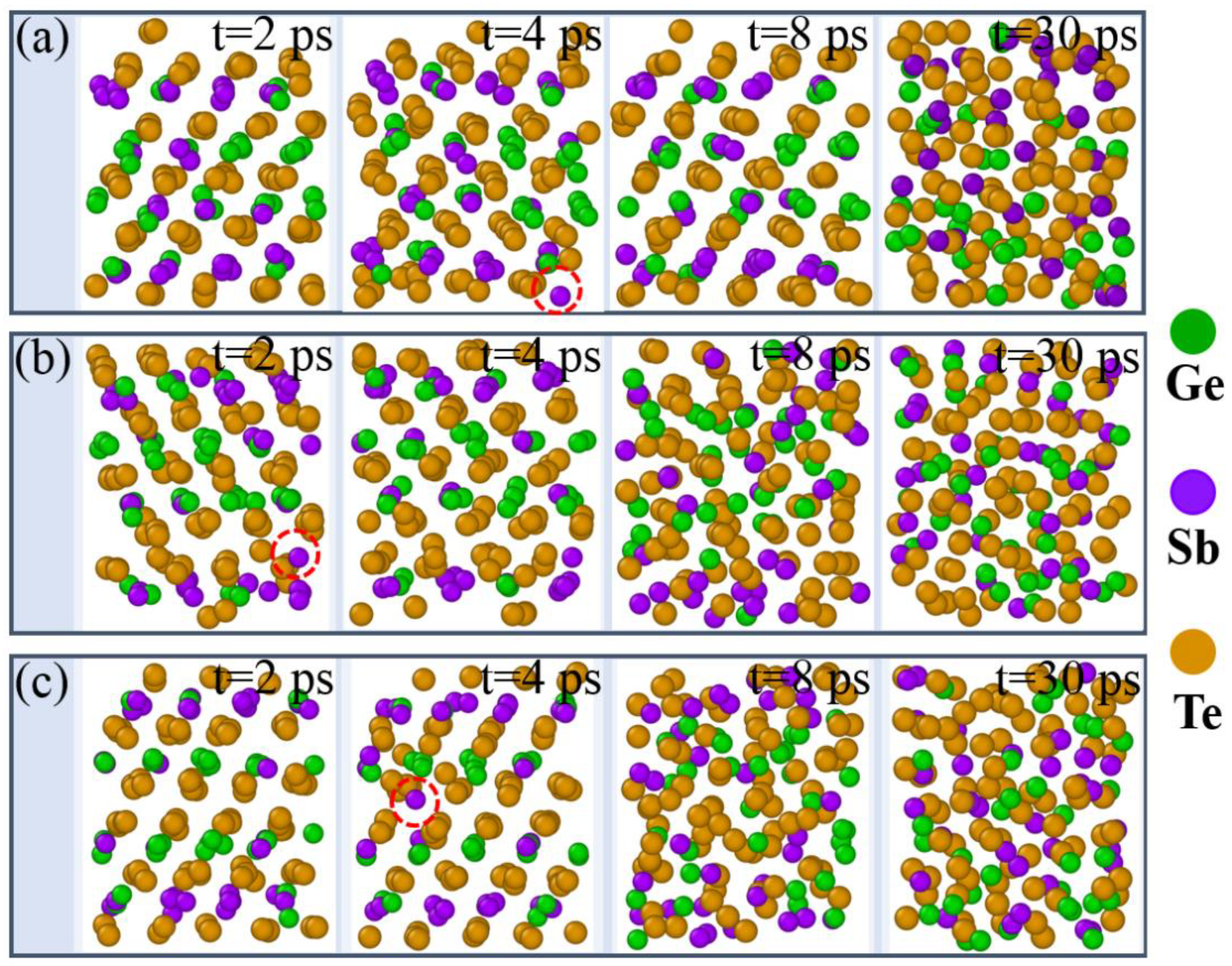
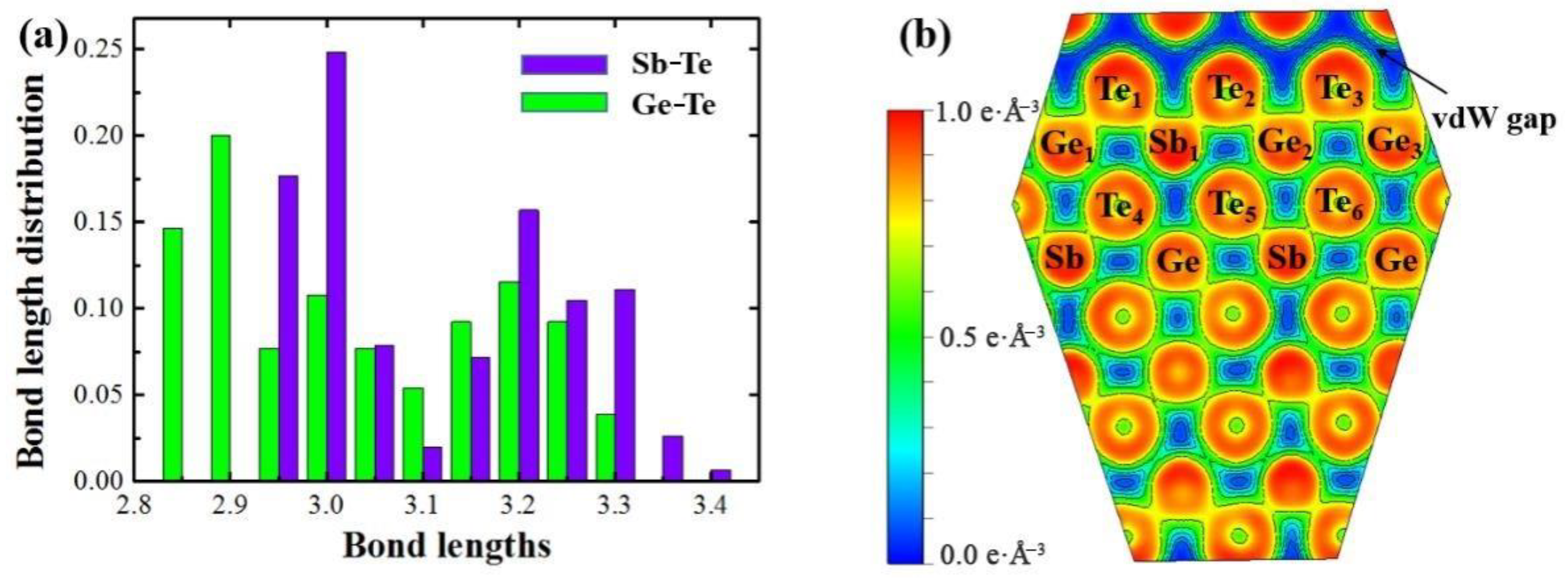
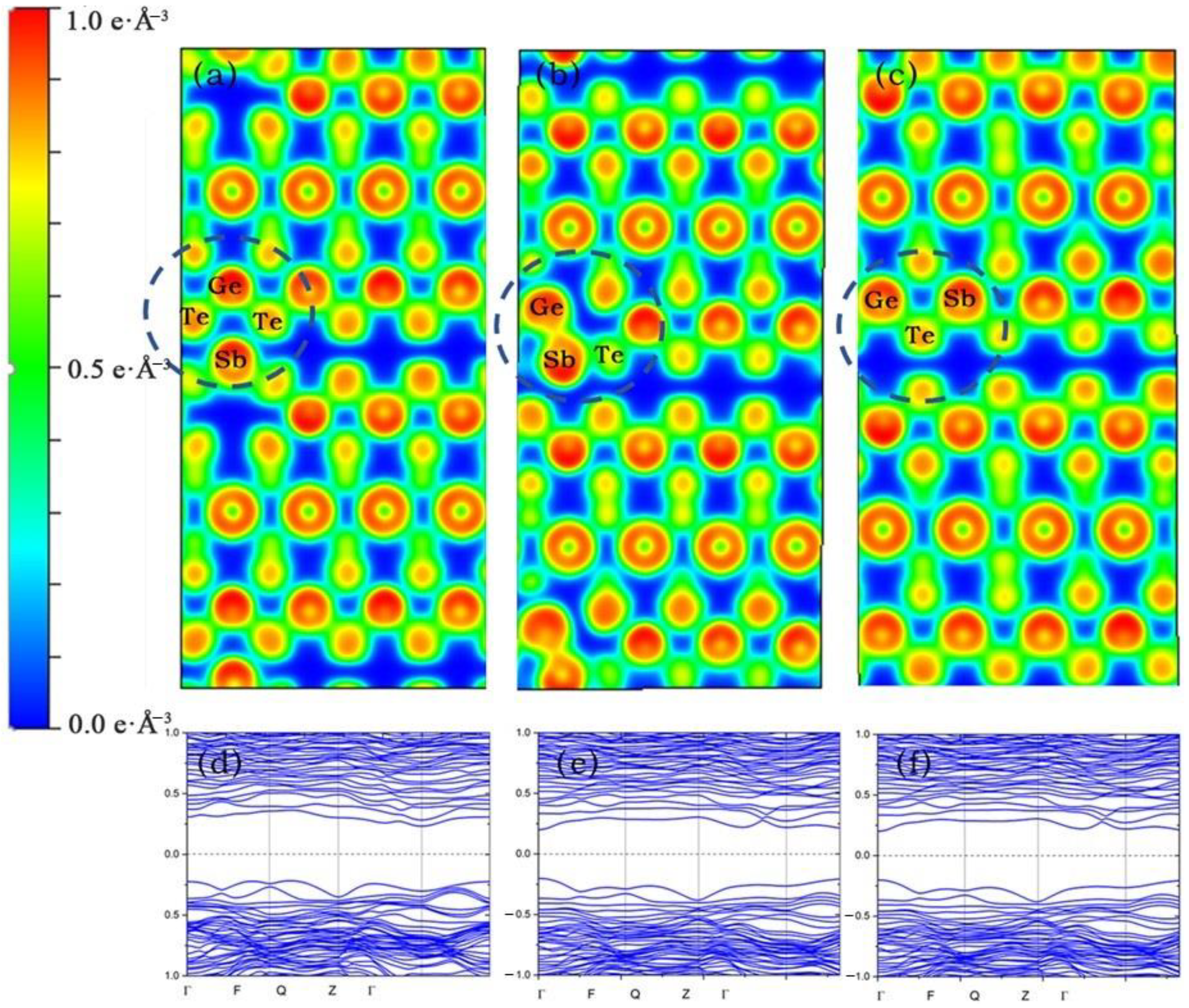
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 2007, 6, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.E.; Fons, P.; Kolobov, A.V.; Fukaya, M.; Krbal, M.; Yagi, T.; Tominaga, J. Interfacial phase-change memory. Nat. Nanotechnol. 2011, 6, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Kolobov, A.V.; Kim, D.J.; Giussani, A.; Fons, P.; Tominaga, J.; Calarco, R.; Gruverman, A. Ferroelectric switching in epitaxial GeTe films. APL Mater. 2014, 2, 753–755. [Google Scholar] [CrossRef]
- Tominag, J.; Saito, Y.; Mitrofanov, K.; Inoue, N.; Fons, P.; Kolobov, A.V.; Nakamura, H.; Miyata, N. A Magnetoresistance Induced by a Nonzero Berry Phase in GeTe/Sb2Te3 Chalcogenide Superlattices. Adv. Funct. Mater. 2017. [Google Scholar] [CrossRef]
- Li, X.-B.; Chen, N.-K.; Wang, X.-P.; Sun, H.-B. Phase-Change Superlattice Materials toward Low Power Consumption and High-Density Data Storage: Microscopic Picture, Working Principles, and Optimization. Adv. Funct. Mater. 2018, 28, 1803380. [Google Scholar] [CrossRef]
- Tominaga, J.; Kolobov, A.; Fons, P.; Nakano, T.; Murakami, S. Ferroelectric Order Control of the Dirac-Semimetal Phase in GeTe-Sb2Te3 Superlattices. Adv. Mater. Interfaces 2014, 1, 1300027. [Google Scholar] [CrossRef]
- Yu, X.; Robertson, J. Modeling of switching mechanism in GeSbTe chalcogenide superlattices. Sci. Rep. 2015, 5, 12612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalikka, J.; Zhou, X.; Dilcher, E.; Wall, S.; Li, J.; Simpson, R.E. Strain-engineered diffusive atomic switching in two-dimensional crystals. Nat. Commun. 2016, 7, 11983. [Google Scholar] [CrossRef] [Green Version]
- Casarinet, B.; Caretta, A.; Momand, J.; Kooi, B.J.; Verheijen, M.A.; Bragaglia, V.; Calarco, R.; Chukalina, M.; Yu, X.; Rpbertson, J.; et al. Revisiting the Local Structure in Ge-Sb-Te based Chalcogenide Superlattices. Sci. Rep. 2016, 6, 22353. [Google Scholar] [CrossRef] [Green Version]
- Lotnyk, A.; Hilmi, I.; Ross, U.; Rauschenbach, B. Van der Waals interfacial bonding and intermixing in GeTe-Sb2Te3-based superlattices. Nano Res. 2017, 11, 1676–1686. [Google Scholar] [CrossRef]
- Yu, X.; Robertson, J. Atomic Layering, Intermixing and Switching Mechanism in Ge-Sb-Te based Chalcogenide Superlattices. Sci. Rep. 2016, 6, 37325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Kolobov, A.V.; Fons, P.; Mitrofanov, K.V.; Makino, K.; Tominaga, J.; Robertson, J. Origin of resistivity contrast in interfacial phase-change memory: The crucial role of Ge/Sb intermixing. Appl. Phys. Lett. 2019, 114, 132102. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, X.B.; Chen, N.K.; Chen, Q.D.; Han, X.D.; Zhang, S.; Sun, H.B. Element-specific amorphization of vacancy-ordered GeSbTe for ternary-state phase change memory. Acta Mater. 2017, 136, 242. [Google Scholar] [CrossRef]
- Kolobov, A.V.; Fons, P.; Saito, Y.; Tominaga, J. Atomic Reconfiguration of van der Waals Gaps as the Key to Switching in GeTe/Sb2Te3 Superlattices. ACS Omega 2017, 2, 6223–6232. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Sun, J.; Subedi, A.; Siegrist, T.; Singh, D.J. Competing covalent and ionic bonding in Ge-Sb-Te phase change materials. Sci. Rep. 2016, 6, 25981. [Google Scholar] [CrossRef] [Green Version]
- Momand, J.; Wang, R.; Boschker, J.E.; Verheijen, M.A.; Calarcob, R.; Kooi, B.J. Interface formation of two-and three-dimensionally bonded materials in the case of Ge Te–Sb2Te3 superlattices. Nanoscale 2015, 7, 19136–19143. [Google Scholar] [CrossRef] [Green Version]
- Savin, P.A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. Engl. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Shportko, K.; Kremers, S.; Woda, M.; Lencer, D.; Robertson, J.; Wuttig, M. Resonant bonding in crystalline phase-change materials. Nat. Mater. 2008, 7, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Kalikka, J.; Zhou, X.; Behera, J.; Nannicinib, G.; Simpson, R.E. Evolutionary design of interfacial phase change van der Waals heterostructures. Nanoscale 2016, 8, 18212–18220. [Google Scholar] [CrossRef] [PubMed]
- Ohyanagi, T.; Kitamura, M.; Araidai, M.; Kato, S.; Takaura, N.; Shiraishi, K. Ge Te sequences in superlattice phase change memories and their electrical characteristics. Appl. Phys. Lett. 2014, 104, 5763. [Google Scholar] [CrossRef]
- Nakamura, H.; Rungger, I.; Sanvito, S.; Inoue, N.; Tominagad, J.; Asai, Y. Resistive switching mechanism of Ge Te-Sb2Te3 interfacial phase change memory and topological properties of embedded two-dimensional states. Nanoscale 2017, 9, 9386–9395. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Liu, F.; Guo, J.; Han, G.; Zhang, Y. A Phase-Change Mechanism of GST-SL Based Superlattices upon Sb Flipping. Materials 2021, 14, 360. https://doi.org/10.3390/ma14020360
Sun T, Liu F, Guo J, Han G, Zhang Y. A Phase-Change Mechanism of GST-SL Based Superlattices upon Sb Flipping. Materials. 2021; 14(2):360. https://doi.org/10.3390/ma14020360
Chicago/Turabian StyleSun, Teng, Furong Liu, Jicheng Guo, Gang Han, and Yongzhi Zhang. 2021. "A Phase-Change Mechanism of GST-SL Based Superlattices upon Sb Flipping" Materials 14, no. 2: 360. https://doi.org/10.3390/ma14020360




