Effects of Co Addition on the Properties and Microstructure of Cu-Ni-Si-P-Mg Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
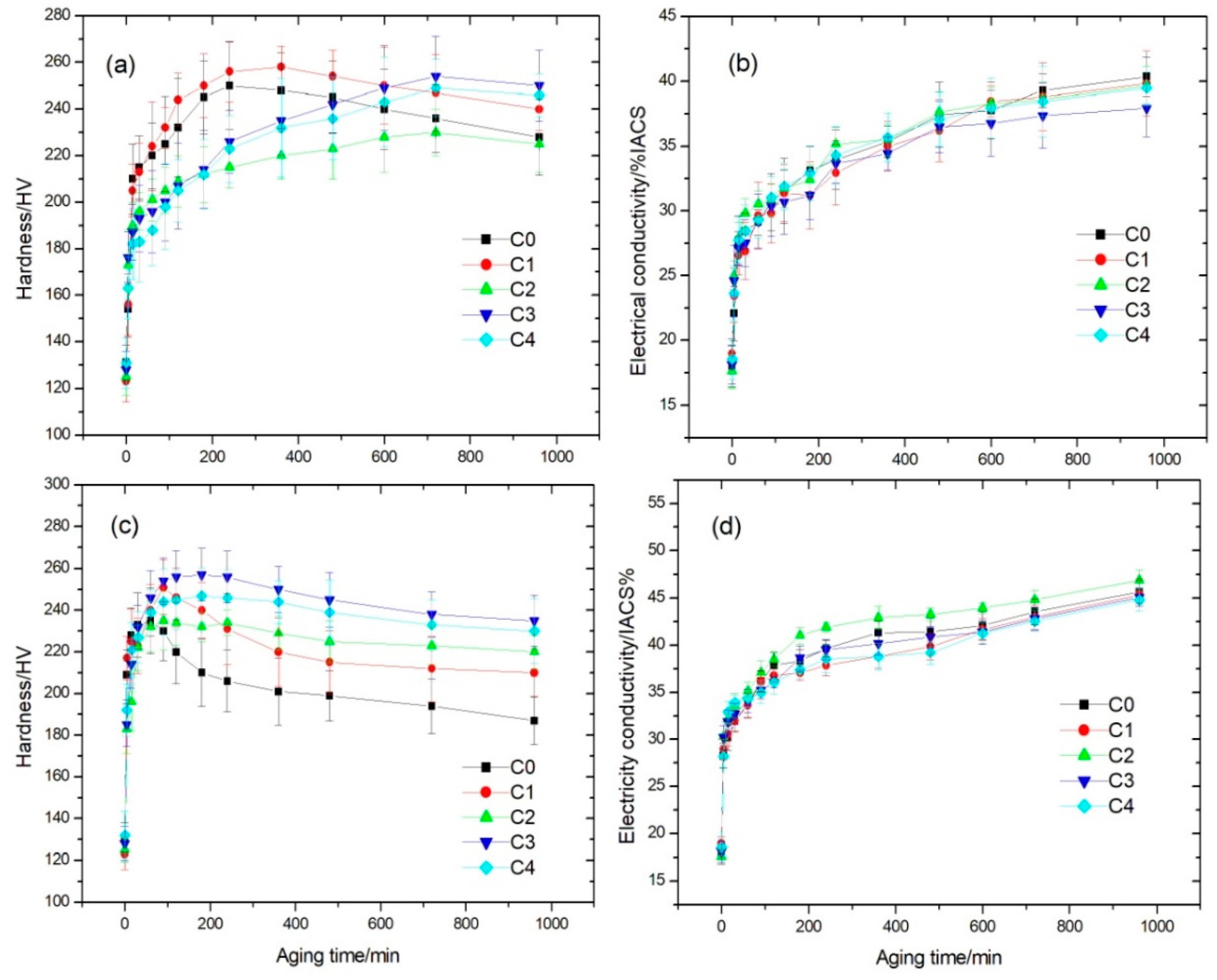
3.1. Effects of Co Addition on Hardness and Electrical Conductivity of Cu-Ni-Si-P-Mg Alloys
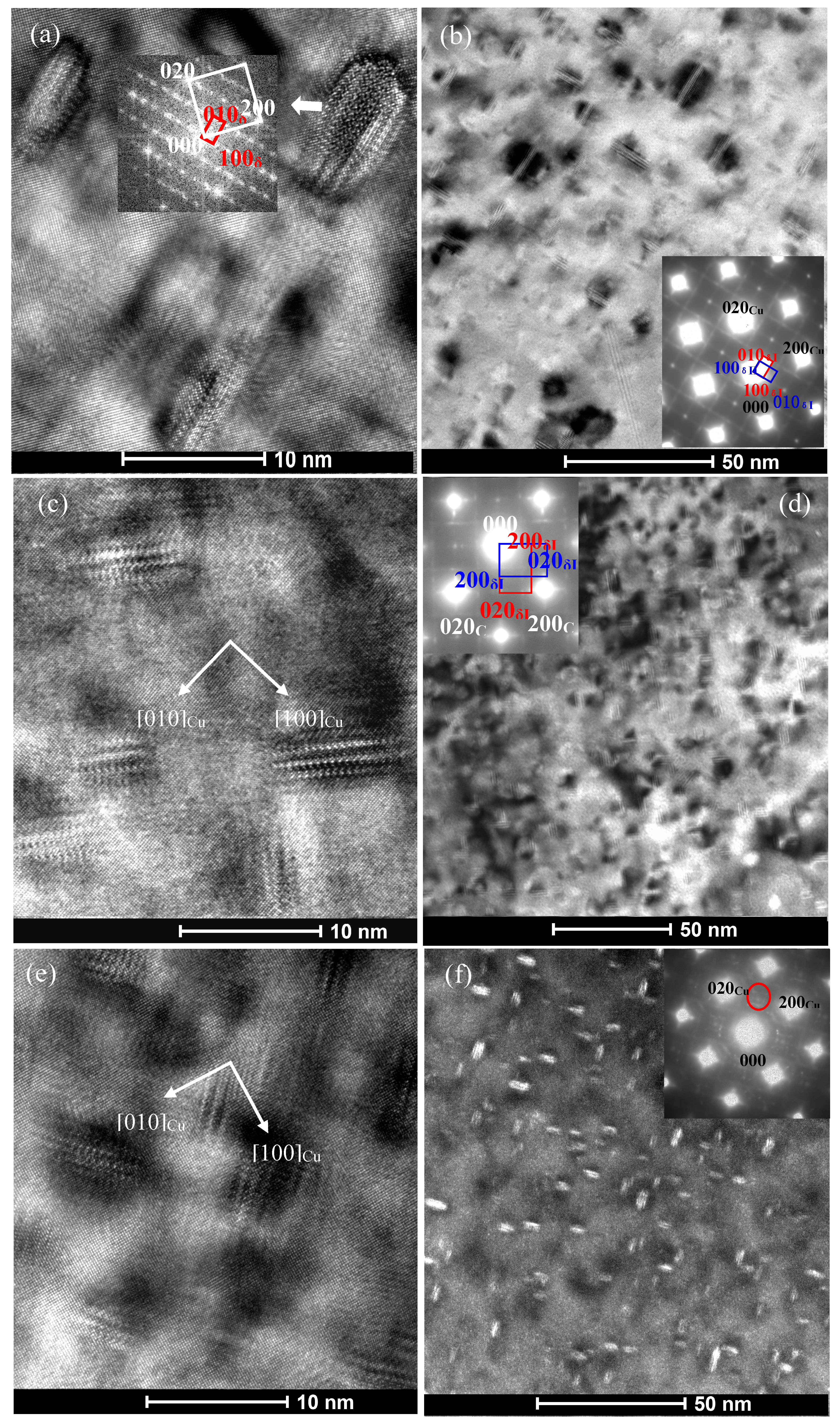
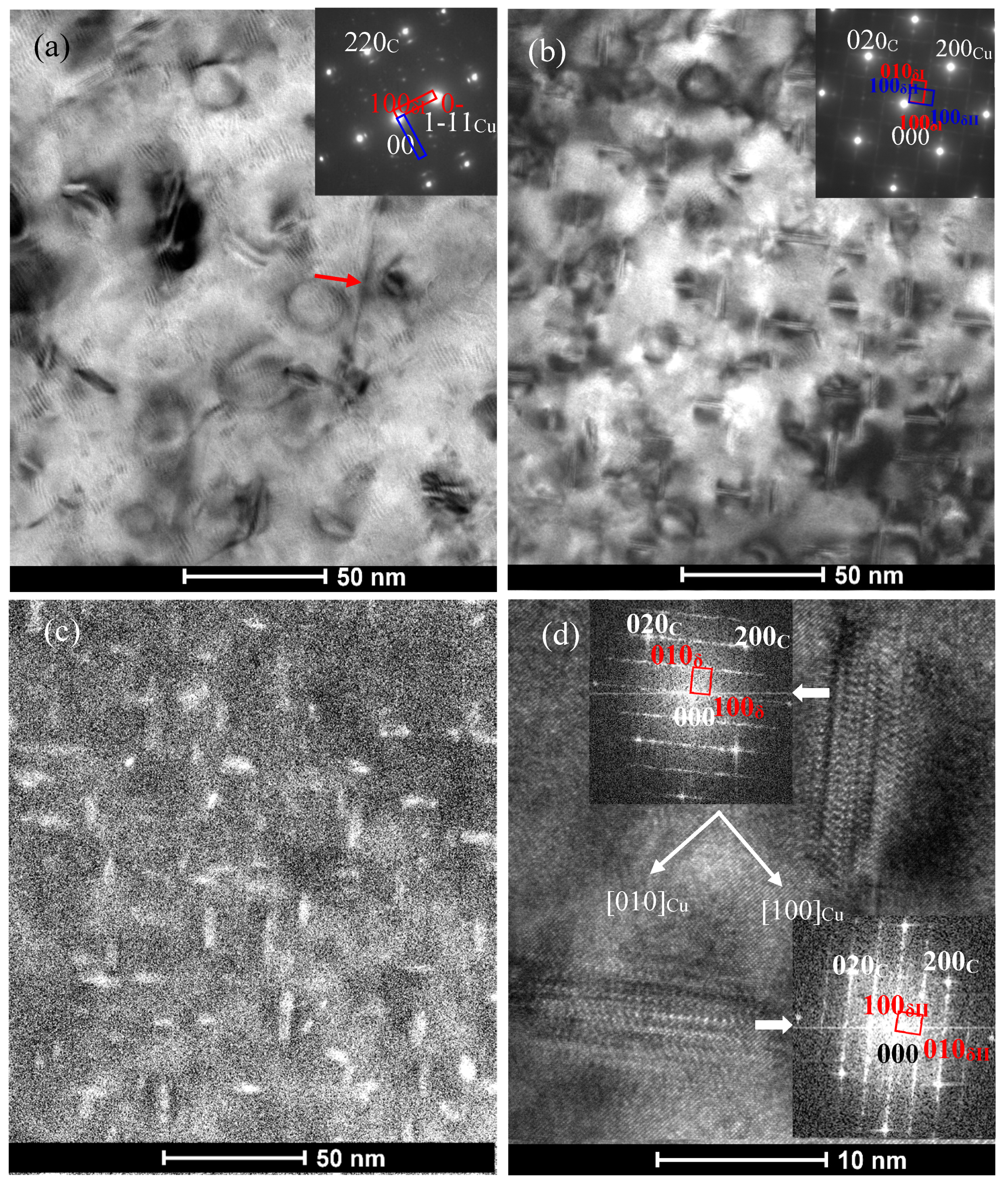
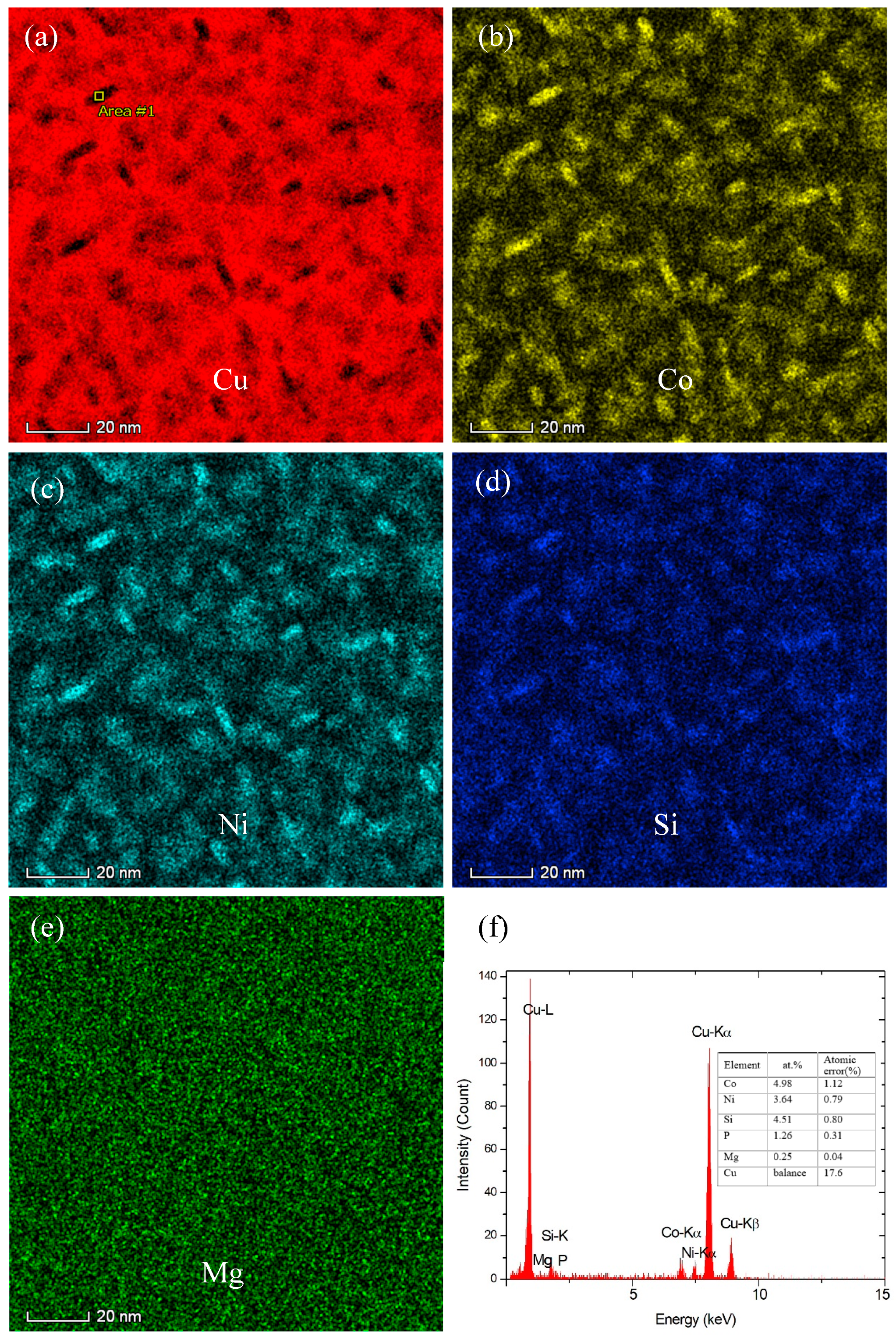
3.2. Effect of Co Addition on Microstructure of Cu-Ni-Si-P-Mg Alloys
4. Conclusions
- According the aging behavior, the studied alloys can be divided into two groups. Cu-2.8Ni-0.65Si-0.1P-0.05Mg and Cu-2.2Ni-0.6Co-0.65Si-0.1P-0.05Mg with Co/Ni weight ratio of 0.27 have the same aging behavior, but Cu-2.2Ni-0.6Co-0.65Si-0.1P-0.05Mg can obtain higher peak hardness than the former and almost the same electrical conductivity. For another group of Cu-(2.8 − x)Ni-xCo-0.65Si-0.1P-0.05Mg (x ≥ 1 or Co/Ni weight ratio ≥0.56) alloys, the aging time to reach the peak hardness can be postponed during aging. However, its electrical conductivity decreases slightly with the increase of Co content.
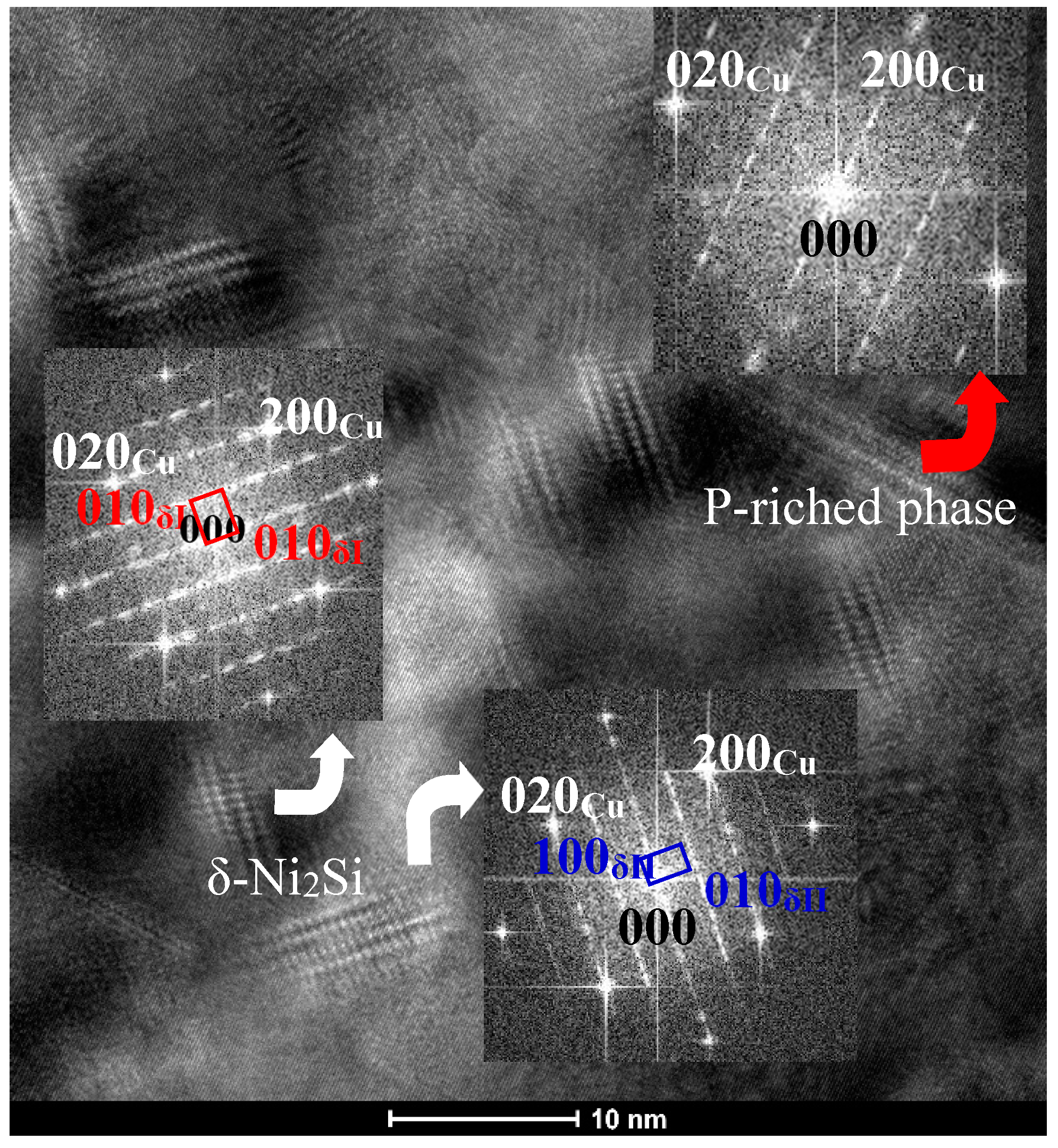
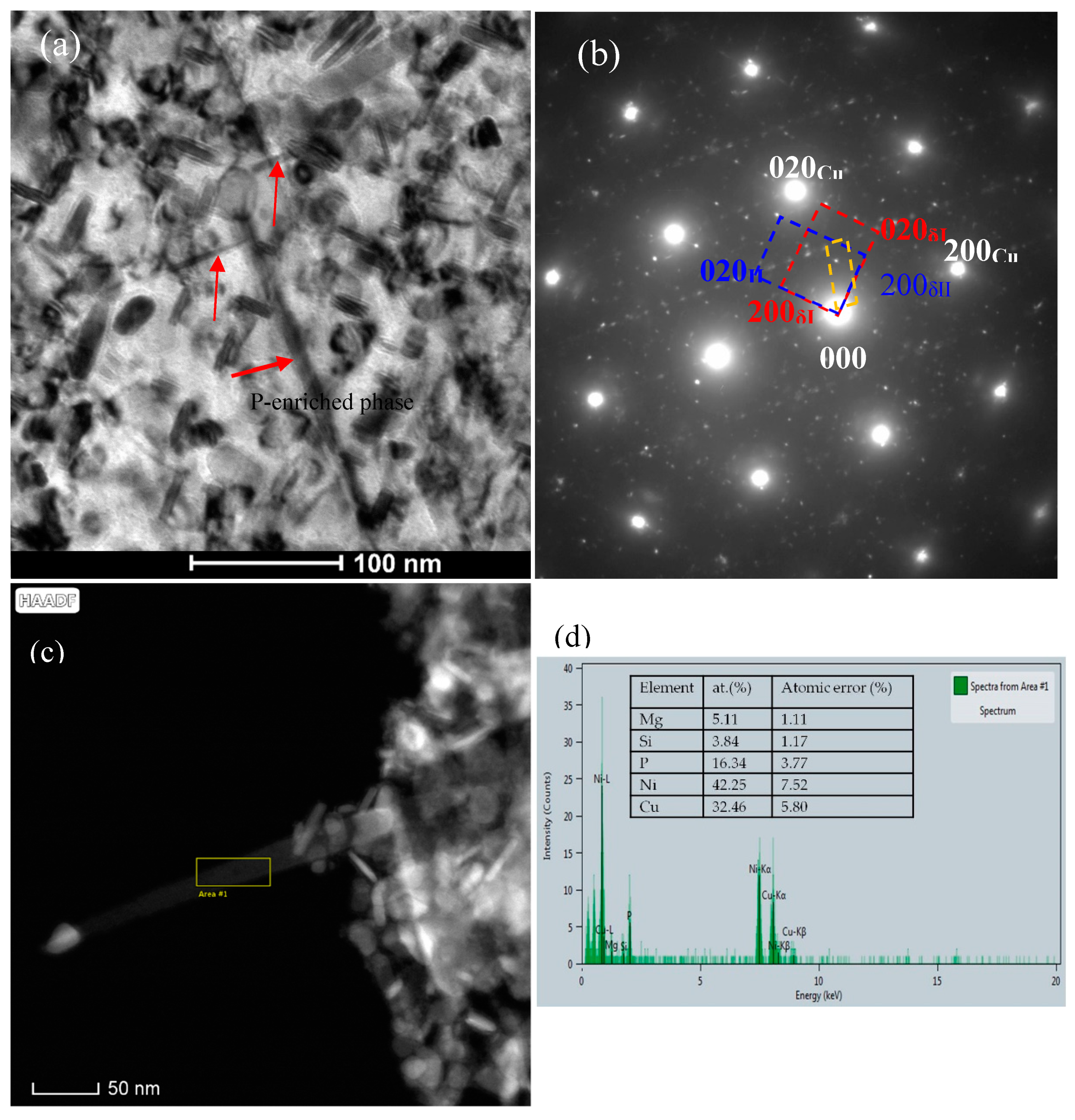
- The addition of Co can reduce the size of precipitates. The larger the amount of Co in the alloys is, the smaller the precipitates formed is. Meanwhile, the addition of Co in the Cu-Ni-Si-P-Mg alloy can also inhibit the formation of P-enriched phase in the Co-containing alloys during aging. The P-enriched phase has a definite orientation relationship with the matrix, which habit plane is parallel to the {001} planes of Cu matrix. The P-enriched precipitate phase is obviously easier to coarsen than the δ-Ni2Si and δ-(Ni, Co)2Si, which results in a greater decrement in hardness for Co-free alloy during over-aging.
- The optimal addition amounts of Ni and Co are 1.6 wt.% Ni and 1.2% Co. The hardness and electrical conductivity of the as-quenched Cu-1.6Ni-1.2Co-0.65Si-0.1P-0.05Mg alloy increase to 257 HV and 38.7 %IACS after aging at 500 °C for 3 h, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, Q.; Li, Z.; Gao, Y.; Peng, X.; Derby, B. Microstructure and mechanical properties of a high strength Cu-Ni-Si alloy treated by combined aging processes. J. Alloy Compd. 2017, 695, 2413–2423. [Google Scholar] [CrossRef]
- Jia, Y.L.; Wang, M.P.; Chen, C.; Dong, Q.Y.; Wang, S.; Li, Z. Orientation and diffraction patterns of δ-Ni2Si precipitates in Cu-Ni-Si alloy. J. Alloy Compd. 2013, 557, 147–153. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Tang, B.B.; Yu, F.X.; Shen, B. Evaluation of nanoscaled precipitates in a Cu-Ni-Si-Cr alloy during aging. J. Alloy Compd. 2014, 614, 189–195. [Google Scholar] [CrossRef]
- Kim, Y.; Seong, T.Y.; Han, J.H.; Ardell, A.J. Effect of heat treatment on precipitation behaviour in a Cu-Ni-Si-P alloy. J. Mater. Sci. 1986, 21, 1357–1362. [Google Scholar] [CrossRef]
- Sun, W.H.; Xu, H.H.; Liu, S.H.; Du, Y.; Yuan, Z.H.; Huang, B.Y. Phase equilibria of the Cu-Ni-Si system at 700 °C. J. Alloy Compd. 2011, 509, 9776–9781. [Google Scholar] [CrossRef]
- Lockyer, S.A.; Noble, F.W. Precipitate structure in a Cu-Ni-Si alloy. J. Mater. Sci. 1994, 29, 218–226. [Google Scholar] [CrossRef]
- Monzen, R.; Watanabe, C. Microstructure and mechanical properties of Cu–Ni–Si alloys. Mater. Sci. Eng. A 2008, 483–484, 117–119. [Google Scholar] [CrossRef]
- Zhao, D.M.; Dong, Q.M.; Liu, P.; Kang, B.X.; Huang, J.L.; Jin, Z.H. Structure and strength of the age hardened Cu-Ni-Si alloy. Mater. Chem. Phys. 2003, 79, 81–86. [Google Scholar] [CrossRef]
- Suzuki, S.; Shubutani, N.; Mimura, K.; Isshiki, M.; Waseda, Y. Improvement in strength and electrical conductivity of Cu-Ni-Si alloys by aging and cold rolling. J. Alloy Compd. 2006, 417, 116–120. [Google Scholar] [CrossRef]
- Lee, S.W.; Matsunaga, H.; Sauvage, X.; Horuta, Z. Strengthening of Cu–Ni–Si alloy using high-pressure torsion and aging. Mater. Charact. 2014, 90, 62–70. [Google Scholar] [CrossRef]
- Izawa, K.; Ozawa, A.; Kita, K.; Watanabe, C.; Monzen, R. Influence of Co on Strength and Microstructure of Cu-Ni-Co-Si Alloy. J. Soc. Mater. Sci. Jpn. 2014, 63, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, C.; Takeshita, S.; Monzen, R. Effects of Small Addition of Ti on Strength and Microstructure of a Cu-Ni-Si Alloy. Metall. Mater. Trans. A 2015, 46, 2469–2475. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Y.; Han, S.Z.; Euh, K.J.; Lim, S.H.; Kim, S.S. Effect of Ti Addition on Tensile Properties of Cu-Ni-Si Alloys. Met. Mater. Int. 2011, 17, 569–576. [Google Scholar] [CrossRef]
- Kim, H.G.; Lee, T.W.; Kim, S.M.; Han, S.Z.; Euh, K.; Kim, W.Y.; Lim, S.H. Effects of Ti addition and heat treatments on mechanical and electrical properties of Cu-Ni-Si alloys. Met. Mater. Int. 2013, 19, 61–65. [Google Scholar] [CrossRef]
- Han, S.Z.; Gu, J.H.; Lee, J.H.; Que, Z.P.; Shiin, J.H.; Lim, S.H.; Kim, S.S. Effect of V addition on hardness and electrical conductivity in Cu-Ni-Si alloys. Met. Mater. Int. 2013, 19, 637–641. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Dai, C.; Wang, J.; Chen, X.; Xie, J.M.; Yang, W.W.; Chen, D.L. Effect of aluminum on microstructure and property of Cu-Ni-Si alloys. Mater. Sci. Eng. A 2013, 572, 65–74. [Google Scholar] [CrossRef]
- Watanabe, C.; Nishijima, F.; Monzen, R.; Tazaki, K. Mechanical properties of Cu-4.0wt% Ni-0.95wt% Si alloys with and without P and Cr addition. Mater. Sci. Forum 2007, 561–565, 2321–2324. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Kang, H.J.; Chen, Z.N.; Chen, Z.J.; Zou, C.L.; Li, R.G.; Yin, G.M.; Wang, T.M. Effects of Cr and Zr additions on microstructure and properties of Cu-Ni-Si alloys. Mater. Sci. Eng. A 2016, 673, 378–390. [Google Scholar] [CrossRef]
- Lei, Q.; Li, Z.; Xiao, T.; Pang, Y.; Xiang, Z.Q.; Qiu, W.T.; Xiao, Z. A new ultrahigh strength Cu-Ni-Si alloy. Intermet 2013, 42, 77–84. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Xiao, Z.; Li, Z.; Qiu, W.T.; Jiang, H.Y.; Lei, Q.; Liu, Z.R.; Jiang, Y.B.; Zhang, S.J. Microstructure and properties of a Cu–Ni–Si–Co–Cr alloy with high strength and high conductivity. Mater. Sci. Eng. A 2019, 759, 396–403. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.J.; Mi, X.J.; Peng, L.J.; Xie, H.F.; Kang, Y.L. Microstructure evolution and properties of a quaternary Cu–Ni–Co–Si alloy with high strength and conductivity. Mater. Sci. Eng. A 2019, 766, 138390. [Google Scholar] [CrossRef]
- Xiao, X.P.; Yi, Z.Y.; Chen, T.T.; Liu, R.Q.; Wang, H. Suppressing spinodal decomposition by adding Co into Cu-Ni-Si alloy. J. Alloy Compd. 2016, 660, 178–183. [Google Scholar] [CrossRef]
- Hu, T.; Chen, J.H.; Liu, J.Z.; Liu, Z.R.; Wu, C.L. The crystallographic and morphological evolution of the strengthening precipitates in Cu-Ni-Si alloys. Acta Mater. 2013, 61, 1210–1219. [Google Scholar] [CrossRef]
- Hu, T.; Chen, W.L.; Ning, Y.; Liang, N.N.; Liu, J.Z. On the morphology and crystallography of the strengthening precipitates in an aged Cu-Ni-P alloy. J. Alloy Compd. 2017, 729, 84–88. [Google Scholar] [CrossRef]
- Byun, J.S.; Choi, J.H.; Lee, D.N. Analysis of precipitate structure in a Cu-Ni-P Alloy. Scripta. Mater. 2000, 42, 637–643. [Google Scholar] [CrossRef]
- Murayama, M.; Belyakov, A.; Hara, T.; Sakai, Y.; Tsuzaki, K.; Okubo, M.; Eto, M.; Kimura, T. Development of a high-strength high-conductivity Cu-Ni-P alloy. Part. I: Characterization of precipitation products. J. Electron. Mater. 2006, 35, 1787–1792. [Google Scholar]
- Mokles, V. Orowan process controlled dislocation glide in materials containing incoherent particles. Mater. Sci. Eng. A 2001, 309–310, 265–269. [Google Scholar]
- Gladman, T. Precipitation hardening in metals. Mater. Sci. Tech. Lond. 1999, 15, 30–36. [Google Scholar] [CrossRef]
- Seidman, D.N.; Marquis, E.A.; Dunand, D.C. Precipitation strengthening at ambient and elevated temperatures of heat-treatable Al(Sc) alloys. Acta Mater. 2002, 50, 4021–4035. [Google Scholar] [CrossRef]
- Brown, L.M.; Ham, R.K. Strengthening Methods in Crystals; Kelly, A., Nicholson, R.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1971; pp. 9–135. [Google Scholar]
- Watanbe, D.; Watanabe, C.; Monzen, R. Determination of the interface energies of spherical, cuboidal and octahedral face-centered cubic precipitates in Cu-Co, Cu-Co-Fe and Cu-Fe alloys. Acta Mater. 2009, 57, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Dohl, R.; Macht, M.P.; Naundorf, V. Measurement of the diffusion coefficient of cobalt in copper. Phys. Status Solidi. A 1984, 86, 603–612. [Google Scholar] [CrossRef]
- Almazouzi, A.; Macht, M.P.; Naundor, V.; Neumann, G. Diffusion of iron and nickel in single-crystalline copper. Phys. Rev. B 1996, 54, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, C.; Monzen, R. Coarsening of δ-Ni2Si precipitates in a Cu-Ni-Si alloy. J. Mater. Sci. 2011, 46, 4327–4335. [Google Scholar] [CrossRef] [Green Version]



| Process | Route |
|---|---|
| I | Aged at 400, 450 and 500 °C for different time, respectively. |
| II | Cold rolled and then aged at 450 and 500 °C for different times, respectively. |
| Alloy No. | Ni | Co | Si | P | Mg | Cu |
|---|---|---|---|---|---|---|
| C0 | 2.8 | 0 | 0.65 | 0.1 | 0.05 | balance |
| C1 | 2.2 | 0.6 | 0.65 | 0.1 | 0.05 | balance |
| C2 | 1.8 | 1.0 | 0.65 | 0.1 | 0.05 | balance |
| C3 | 1.6 | 1.2 | 0.65 | 0.1 | 0.05 | balance |
| C4 | 1.2 | 1.6 | 0.65 | 0.1 | 0.05 | balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Yu, F.; Huang, F. Effects of Co Addition on the Properties and Microstructure of Cu-Ni-Si-P-Mg Alloys. Materials 2021, 14, 368. https://doi.org/10.3390/ma14020368
Cheng J, Yu F, Huang F. Effects of Co Addition on the Properties and Microstructure of Cu-Ni-Si-P-Mg Alloys. Materials. 2021; 14(2):368. https://doi.org/10.3390/ma14020368
Chicago/Turabian StyleCheng, Jianyi, Fangxin Yu, and Fu Huang. 2021. "Effects of Co Addition on the Properties and Microstructure of Cu-Ni-Si-P-Mg Alloys" Materials 14, no. 2: 368. https://doi.org/10.3390/ma14020368





