Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Coating of Mg-Based Scaffolds and Collection of Mg2+ Conditioned Media
2.2. Surface Roughness of Mg-Based Scaffold
2.3. Cell Culture
2.4. Scanning Electronic Microscopy (SEM)
2.5. Cell Viability and Proliferation Assays
2.6. Cell Differentiation Assay
2.7. Quantitative Polymerase Chain Reaction (qPCR)
2.8. Statistical Analysis
3. Results
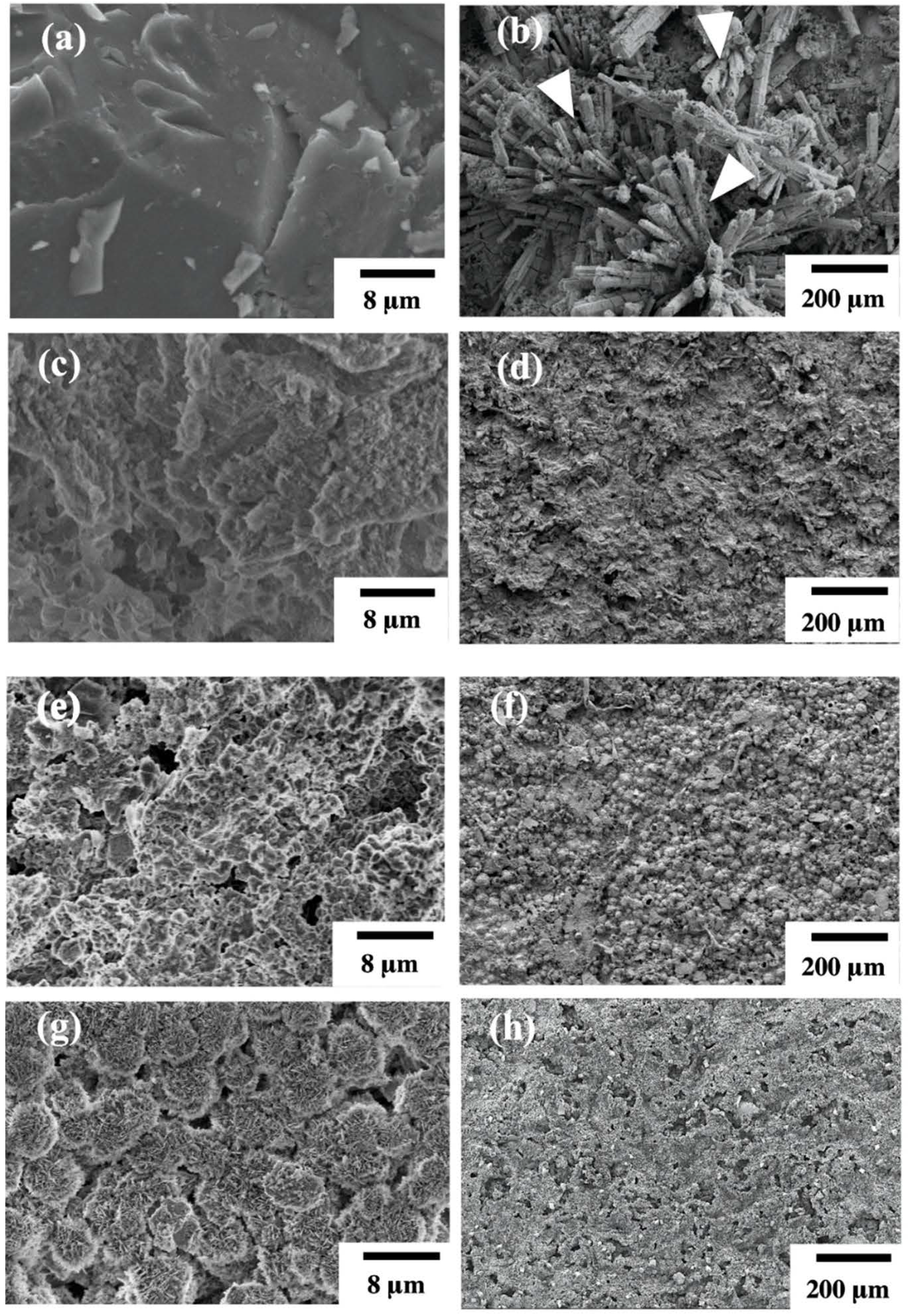
3.1. Structure and Surface Characterization of Hydrothermal Mg-Based Scaffolds
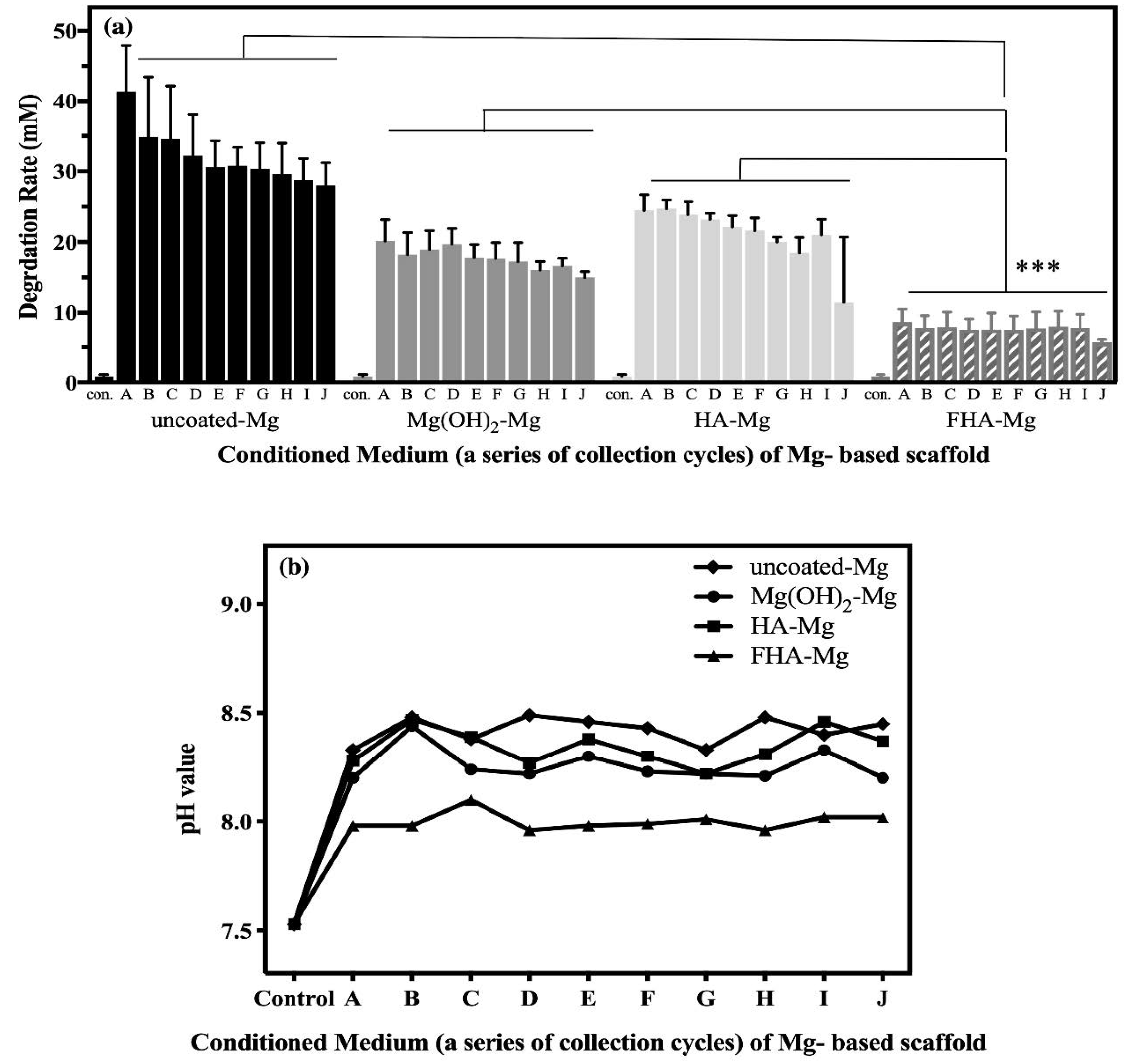
3.2. The Release of Mg2+ Ions
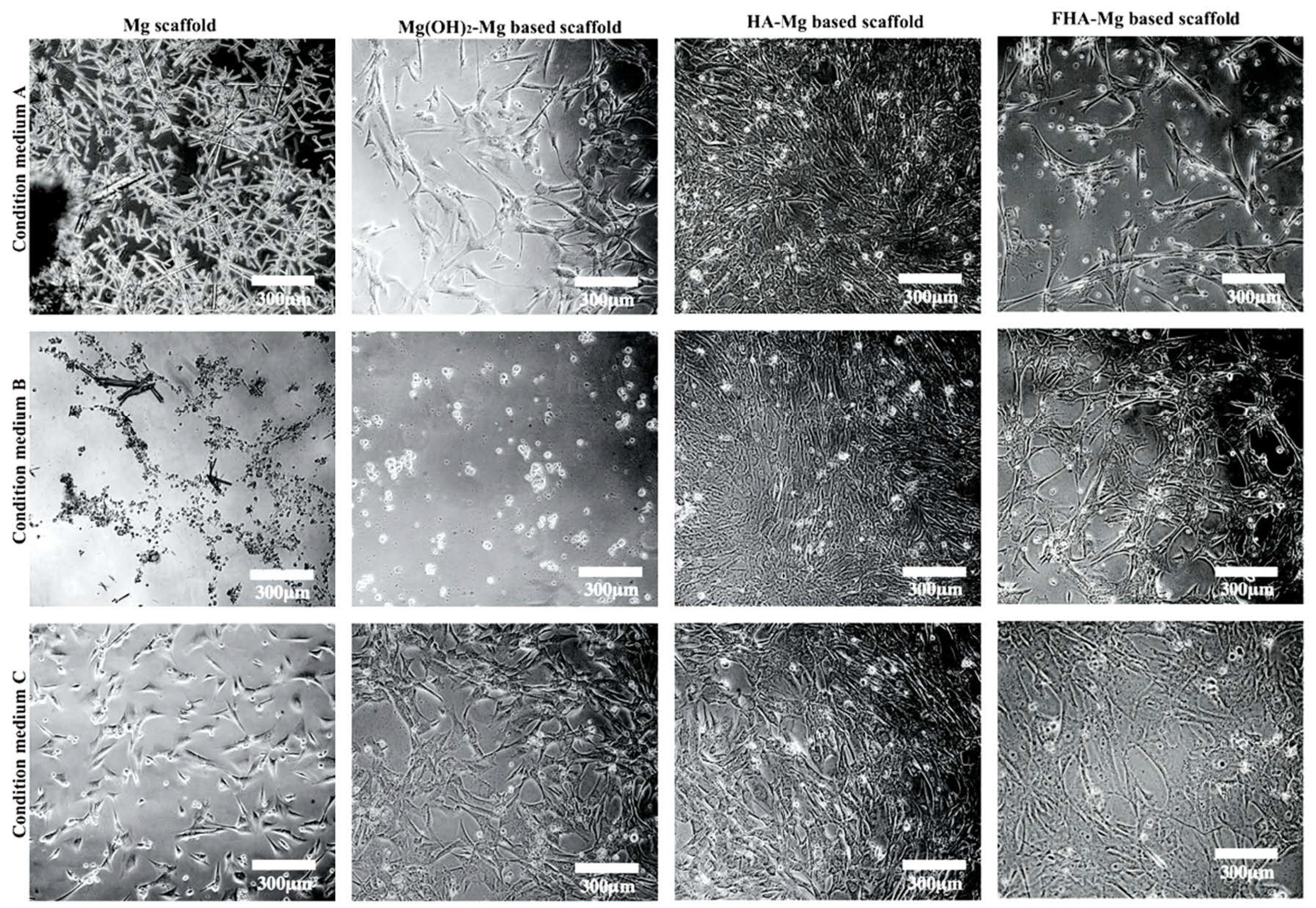
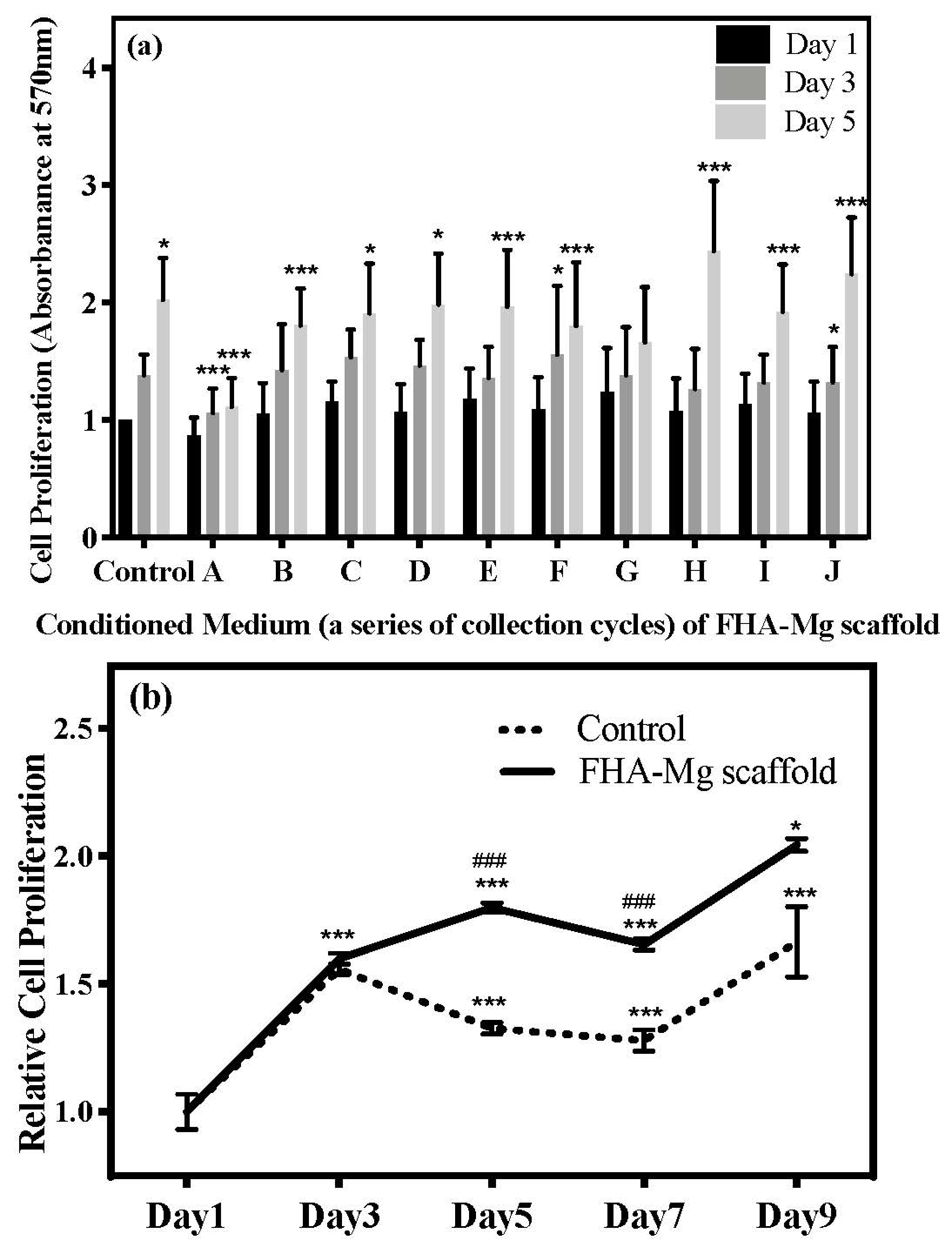
3.3. Effect of Released Mg2+ ions on Cell Viability
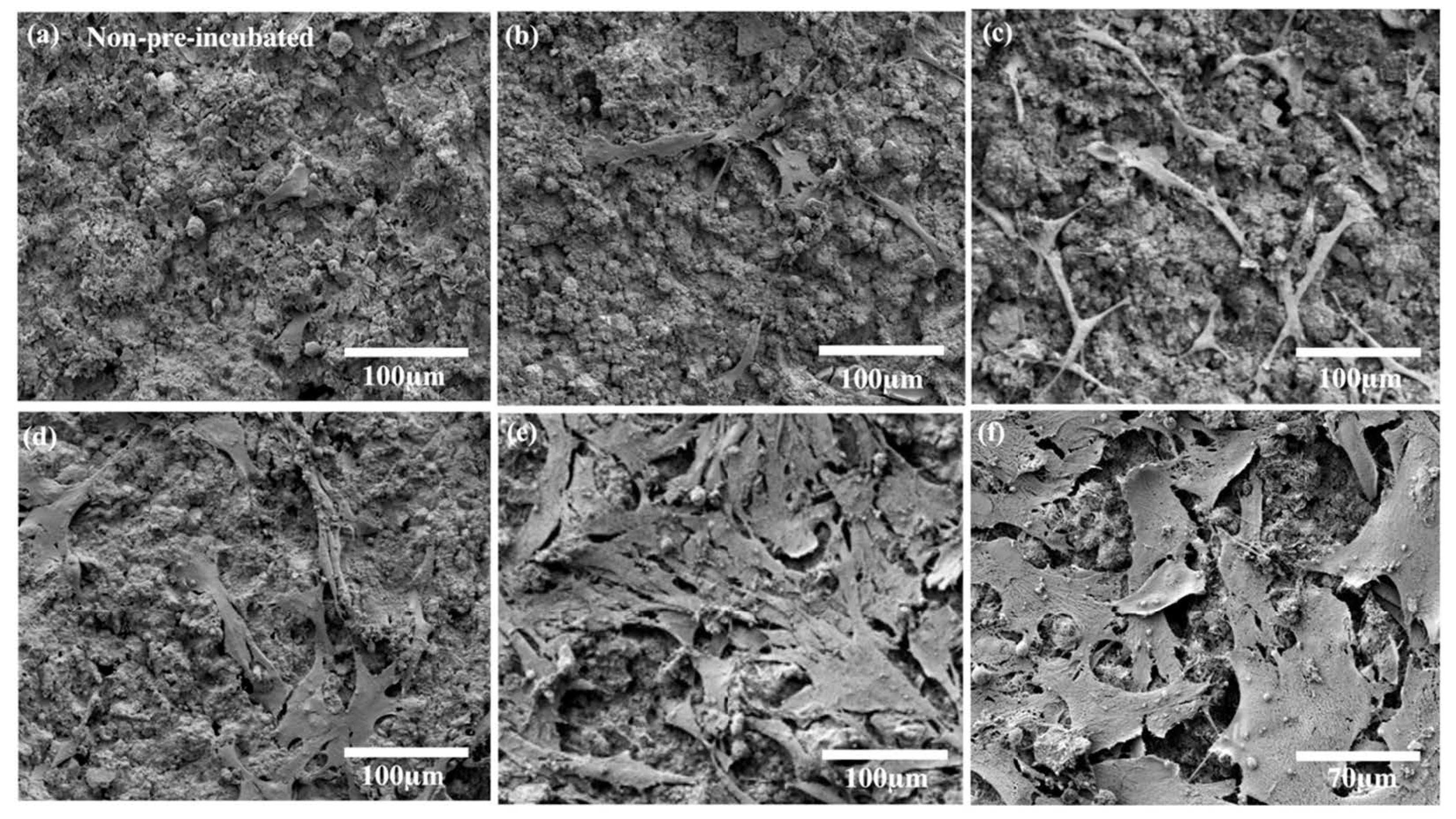
3.4. hMSC Morphology and Proliferation on FHA-Mg Scaffolds
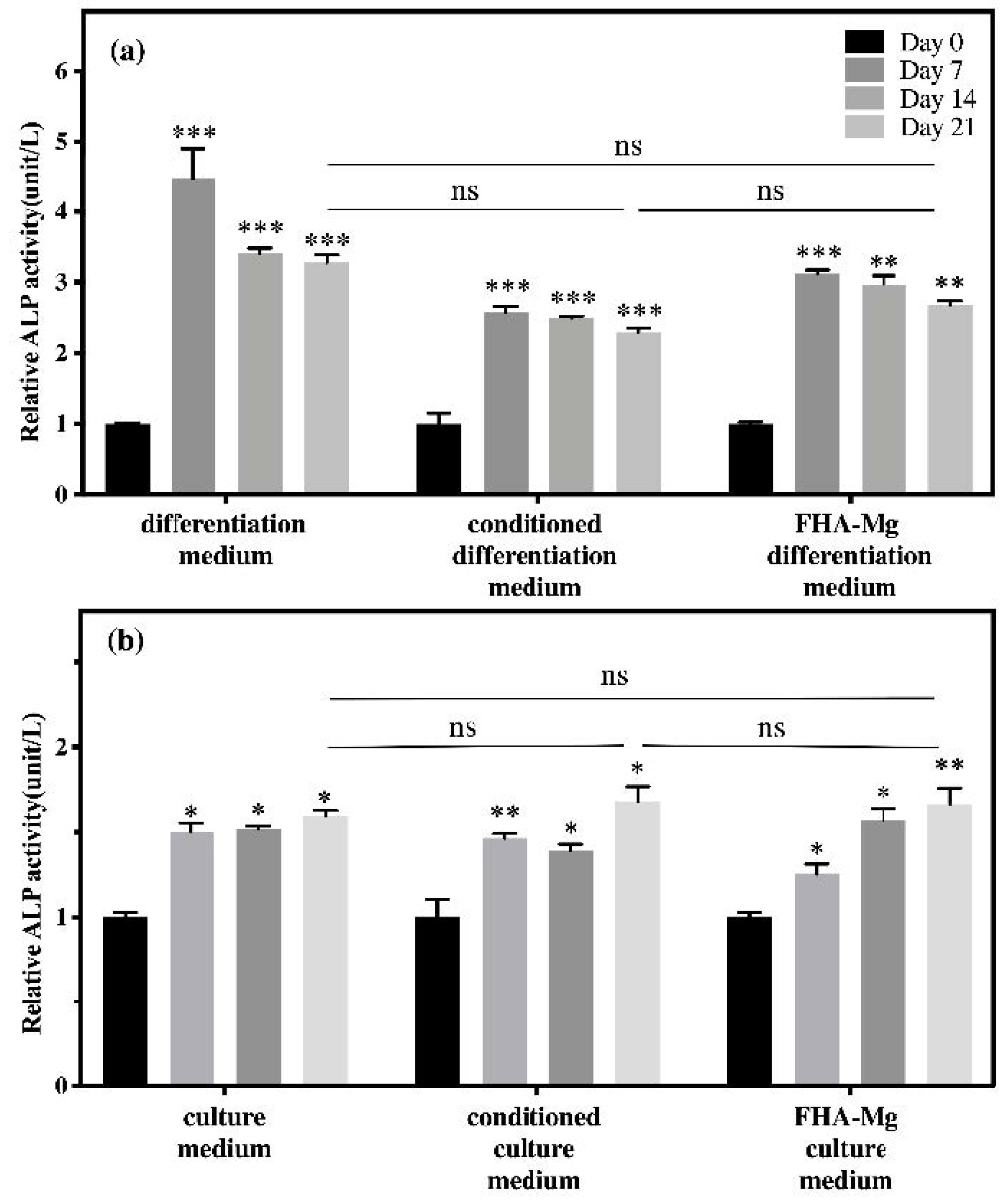
3.5. Osteogenic Differentiation of hMSCs on FHA-Mg Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Wang, D.; Blocki, A.; Tuan, R.S. Chapter 49—Mesenchymal stem cells in musculoskeletal tissue engineering. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 883–915. [Google Scholar]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef]
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Angrisani, N.; Reifenrath, J.; Zimmermann, F.; Eifler, R.; Meyer-Lindenberg, A.; Vano-Herrera, K.; Vogt, C. Biocompatibility and degradation of LAE442-based magnesium alloys after implantation of up to 3.5 years in a rabbit model. Acta Biomater. 2016, 44, 355–365. [Google Scholar] [CrossRef]
- Waizy, H.; Diekmann, J.; Weizbauer, A.; Reifenrath, J.; Bartsch, I.; Neubert, V.; Schavan, R.; Windhagen, H. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months. J. Biomater. Appl. 2014, 28, 667–675. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef] [Green Version]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.-X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Yang, C.-W.; Lee, T.-M. Evaluation of microstructural features and in vitro biocompatibility of hydrothermally coated fluorohydroxyapatite on AZ80 Mg alloy. Ind. Eng. Chem. Res. 2016, 55, 5207–5215. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Pei, J.; Tian, Y.; Zhang, J.; Jiang, C.; Huang, J.; Pang, Z.; Cao, Y.; Wang, X.; et al. Degradation and osteogenic induction of a SrHPO4-coated Mg–Nd–Zn–Zr alloy intramedullary nail in a rat femoral shaft fracture model. Biomaterials 2020, 247, 119962. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Savabi, O.; Tayebi, L.; Vashaee, D. Biodegradable Magnesium Bone Implants Coated with a Novel Bioceramic Nanocomposite. Materials 2020, 13, 1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Wang, J.; Gao, C.; Wang, Z.; Zhou, X.; Tang, M.; Yu, K.; Deng, Y. Enhanced osteoinductivity and corrosion resistance of dopamine/gelatin/rhBMP-2–coated β-TCP/Mg-Zn orthopedic implants: An in vitro and in vivo study. PLoS ONE 2020, 15, e0228247. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; He, X.; Chen, Z.; He, L.; Lu, M.; Ge, J.; Weng, J.; Mu, Y.; Duan, K. Effect of magnesium particle fraction on osteoinduction of hydroxyapatite sphere-based scaffolds. J. Mater. Chem. B 2019, 7, 5648–5660. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Mohd Daud, N.; Sing, N.B.; Yusop, A.H.; Abdul Majid, F.A.; Hermawan, H. Degradation and in vitro cell–material interaction studies on hydroxyapatite-coated biodegradable porous iron for hard tissue scaffolds. J. Orthop. Transl. 2014, 2, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Willumeit-Römer, R. The Interface Between Degradable Mg and Tissue. JOM 2019, 71, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef]
- Sojka, J.E.; Weaver, C.M. Magnesium supplementation and osteoporosis. Nutr. Rev. 1995, 53, 71–74. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Grigolato, R.; Pizzi, N.; Brotto, M.C.; Corrocher, G.; Desando, G.; Grigolo, B. Magnesium-enriched hydroxyapatite as bone filler in an ameloblastoma mandibular defect. Int. J. Clin. Exp. Med. 2015, 8, 281–288. [Google Scholar] [PubMed]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Wang, Y.M.; Jing, Y.B.; Zhuang, J.P.; Yan, J.L.; Shao, Z.K.; Jin, M.S.; Wu, C.J.; Zhou, Y. In vivo study of microarc oxidation coated biodegradable magnesium plate to heal bone fracture defect of 3mm width. Colloids Surf. B Biointerfaces 2017, 158, 147–156. [Google Scholar] [CrossRef]
- Yun, Y.; Dong, Z.; Yang, D.; Schulz, M.J.; Shanov, V.N.; Yarmolenko, S.; Xu, Z.; Kumta, P.; Sfeir, C. Biodegradable Mg corrosion and osteoblast cell culture studies. Mater. Sci. Eng. C 2009, 29, 1814–1821. [Google Scholar] [CrossRef]
- Roy, M.E.; Nishimoto, S.K. Matrix Gla protein binding to hydroxyapatite is dependent on the ionic environment: Calcium enhances binding affinity but phosphate and magnesium decrease affinity. Bone 2002, 31, 296–302. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Xie, X.; Zhang, P.; Lai, Y.; Li, Y.; Qin, L. Surface coating reduces degradation rate of magnesium alloy developed for orthopaedic applications. J. Orthop. Transl. 2013, 1, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Tsao, Y.-T.; Shih, Y.-Y.; Liu, Y.-A.; Liu, Y.-S.; Lee, O.K. Knockdown of SLC41A1 magnesium transporter promotes mineralization and attenuates magnesium inhibition during osteogenesis of mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.; Jin, S.; Gu, S.; Sun, R.; Liang, Q. High concentration magnesium inhibits extracellular matrix calcification and protects articular cartilage via Erk/autophagy pathway. J. Cell Physiol. 2019, 234, 23190–23201. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Y.; Li, T.; Ding, S.; Zhang, W.; Gu, Y.; Liu, C. Facilitated receptor-recognition and enhanced bioactivity of bone morphogenetic protein-2 on magnesium-substituted hydroxyapatite surface. Sci. Rep. 2016, 6, 24323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omidi, M.; Agha, N.A.; Müller, A.; Feyerabend, F.; Helmholz, H.; Willumeit-Römer, R.; Schlüter, H.; Luthringer-Feyerabend, B. Investigation of the impact of magnesium versus titanium implants on protein composition in osteoblast by label free quantification. Metallomics 2020, 12, 916–934. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Liu, X. Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regen Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, X. Effect of fluorine addition on the corrosion resistance of hydroxyapatite ceramics. Ceram. Int. 2004, 30, 1961–1965. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Daroonparvar, M.; Ebrahimi-Kahrizsangi, R.; Medraj, M. In-vitro corrosion inhibition mechanism of fluorine-doped hydroxyapatite and brushite coated Mg–Ca alloys for biomedical applications. Ceram. Int. 2014, 40, 7971–7982. [Google Scholar] [CrossRef]
- Zhao, C.; Hou, P.; Ni, J.; Han, P.; Chai, Y.; Zhang, X. Ag-incorporated FHA coating on pure Mg: degradation and in vitro antibacterial properties. Acs Appl. Mater. Interfaces 2016, 8, 5093–5103. [Google Scholar] [CrossRef]
- Ebrahimi-Kahrizsangi, R.; Nasiri-Tabrizi, B.; Chami, A. Characterization of single-crystal fluorapatite nanoparticles synthesized via mechanochemical method. Particuology 2011, 9, 537–544. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Daroonparvar, M.; Yajid, M.; Kasiri-Asgarani, M.; Abdul-Kadir, M.; Medraj, M. In-vitro degradation behavior of Mg alloy coated by fluorine doped hydroxyapatite and calcium deficient hydroxyapatite. Trans. Nonferrous Met. Soc. China 2014, 24, 2516–2528. [Google Scholar] [CrossRef]
- Ansari, Z.; Kalantar, M.; Kharaziha, M.; Ambrosio, L.; Raucci, M.G. Polycaprolactone/fluoride substituted-hydroxyapatite (PCL/FHA) nanocomposite coatings prepared by in-situ sol-gel process for dental implant applications. Prog. Org. Coat. 2020, 147, 105873. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Ma, L.; Xie, L.; Yang, H.; Li, Y.; Wang, S.; Qiao, H.; Lin, H.; Lan, J.; et al. Chemical stability, antibacterial and osteogenic activities study of strontium-silver co-substituted fluorohydroxyapatite nanopillars: A potential multifunctional biological coating. Ceram. Int. 2020, 46, 27758–27773. [Google Scholar] [CrossRef]
- Shen, S.; Cai, S.; Bao, X.; Xu, P.; Li, Y.; Jiang, S.; Xu, G. Biomimetic fluoridated hydroxyapatite coating with micron/nano-topography on magnesium alloy for orthopaedic application. Chem. Eng. J. 2018, 339, 7–13. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, H.; Liu, H.; Ji, H.; Fei, W.; Luo, E. Fluorine-contained hydroxyapatite suppresses bone resorption through inhibiting osteoclasts differentiation and function in vitro and in vivo. Cell Prolif. 2019, 52, e12613. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Savabi, O.; Boroni, M. A review of degradation properties of Mg based biodegradable implants. Res. Rev. Mater. Sci. Chem 2012, 1, 15–58. [Google Scholar]
- Wang, Y.; Li, X.; Chen, M.; Zhao, Y.; You, C.; Li, Y.; Chen, G. In Vitro and in Vivo Degradation Behavior and Biocompatibility Evaluation of Microarc Oxidation-Fluoridated Hydroxyapatite-Coated Mg–Zn–Zr–Sr Alloy for Bone Application. ACS Biomater. Sci. Eng. 2019, 5, 2858–2876. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, J.T.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Wallin, R.F.; Arscott, E. A practical guide to ISO 10993-5: Cytotoxicity. Med. Device Diagn. Ind. 1998, 20, 96–98. [Google Scholar]
- Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. Association for the Advancement of Medical Instrumentation; American National Standard: Arlington, VA, USA, 2012; ANSI/AAMI/ISO 10993-12:2012.
- Ge, J.; Guo, L.; Wang, S.; Zhang, Y.; Cai, T.; Zhao, R.C.H.; Wu, Y. The Size of Mesenchymal Stem Cells is a Significant Cause of Vascular Obstructions and Stroke. Stem Cell Rev. Rep. 2014, 10, 295–303. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Kawamura, N.; Nakao, Y.; Ishikawa, R.; Tsuchida, D.; Iijima, M. Degradation and Biocompatibility of AZ31 Magnesium Alloy Implants in vitro and in vivo: A Micro-Computed Tomography Study in Rats. Materials 2020, 13, 473. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Chen, D.; Ding, Z.; Qiu, M.; Zhang, Z.; Shen, J.; Zhang, X.; Zhang, S.; He, Y.; Shi, Z. Synergistic effect of a biodegradable Mg–Zn alloy on osteogenic activity and anti-biofilm ability: an in vitro and in vivo study. RSC Adv. 2016, 6, 45219–45230. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vivo assessments of bioabsorbable AZ91 magnesium implants coated with nanostructured fluoridated hydroxyapatite by MAO/EPD technique for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Akbari Ghavimi, S.; Allen, B.N.; Stromsdorfer, J.L.; Kramer, J.S.; Li, X.; Ulery, B.D. Calcium and phosphate ions as simple signaling molecules with versatile osteoinductivity. Biomed. Mater. 2018, 13, 055005. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Weng, J.; Yang, B.C.; Qu, S.X.; Zhang, X.D. Characterization of titanium surfaces with calcium and phosphate and osteoblast adhesion. Biomaterials 2004, 25, 3421–3428. [Google Scholar] [CrossRef]
- Qi, T.; Weng, J.; Yu, F.; Zhang, W.; Li, G.; Qin, H.; Tan, Z.; Zeng, H. Insights into the Role of Magnesium Ions in Affecting Osteogenic Differentiation of Mesenchymal Stem Cells. Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef]
- Luthringer, B.J.; Willumeit-Romer, R. Effects of magnesium degradation products on mesenchymal stem cell fate and osteoblastogenesis. Gene 2016, 575, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, C.; Li, J.; Zhu, Y.; Zhang, X. High extracellular magnesium inhibits mineralized matrix deposition and modulates intracellular calcium signaling in human bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014, 450, 1390–1395. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, L.; Qi, H.; Zhao, Q.; Liu, Y.; Zhang, Y. Dual Function of Magnesium in Bone Biomineralization. Adv. Healthc. Mater. 2019, 8, e1901030. [Google Scholar] [CrossRef]


| Days | 0–3 | 4–6 | 7–9 | 10–12 | 13–15 | 16–18 | 19–21 | 22–24 | 25–27 | 28–30 |
|---|---|---|---|---|---|---|---|---|---|---|
| Uncoated Mg conditioned medium | A | B | C | D | E | F | G | H | I | J |
| Mg(OH)2-Mg conditioned medium | A | B | C | D | E | F | G | H | I | J |
| HA-Mg conditioned medium | A | B | C | D | E | F | G | H | I | J |
| FHA-Mg conditioned medium | A | B | C | D | E | F | G | H | I | J |
| Gene | Size (bp) | Sequences (5′ to 3′) | |
|---|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | forward | 71 | GAA GGT GAA GGT CGG AGT CAA C |
| reverse | CAG AGT TAA AAG CAG CCC TGG T | ||
| Alkaline phosphatase (ALP) | forward | 476 | ACG TGG CTA AGA ATG TCA TC |
| reverse | CTG GTA GGC GAT GTC CTT A | ||
| Osteocalcin (OCN) | forward | 315 | CAT GAG AGC CCT CAC A |
| reverse | AGA GCG ACA CCC TAG AC | ||
| Uncoated Mg Scaffold | Mg(OH)2-Mg Scaffold | HA-Mg Scaffold | FHA-Mg Scaffold | |
|---|---|---|---|---|
| Pore size (μm) | N/A | 48.5 ± 1.6 | 45.6 ± 1.0 | 61.3 ± 0.6 |
| Porosity (vol.%) | N/A | 18.4 ± 1.6 | 13.1 ± 0.9 | 8.5 ± 0.5 |
| Roughness (μm) | 0.09 ± 0.03 | 9.46 ± 0.92 | 8.30 ± 1.74 | 4.12 ± 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-H.; Lee, S.-P.; Yang, C.-W.; Lo, C.-M. Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. Materials 2021, 14, 441. https://doi.org/10.3390/ma14020441
Wang S-H, Lee S-P, Yang C-W, Lo C-M. Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. Materials. 2021; 14(2):441. https://doi.org/10.3390/ma14020441
Chicago/Turabian StyleWang, Si-Han, Shiao-Pieng Lee, Chung-Wei Yang, and Chun-Min Lo. 2021. "Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation" Materials 14, no. 2: 441. https://doi.org/10.3390/ma14020441





