Corrosion Properties of Cr27Fe24Co18Ni26Nb5 Alloy in 1 N Sulfuric Acid and 1 N Hydrochloric Acid Solutions
Abstract
:1. Introduction
2. Materials and Methods
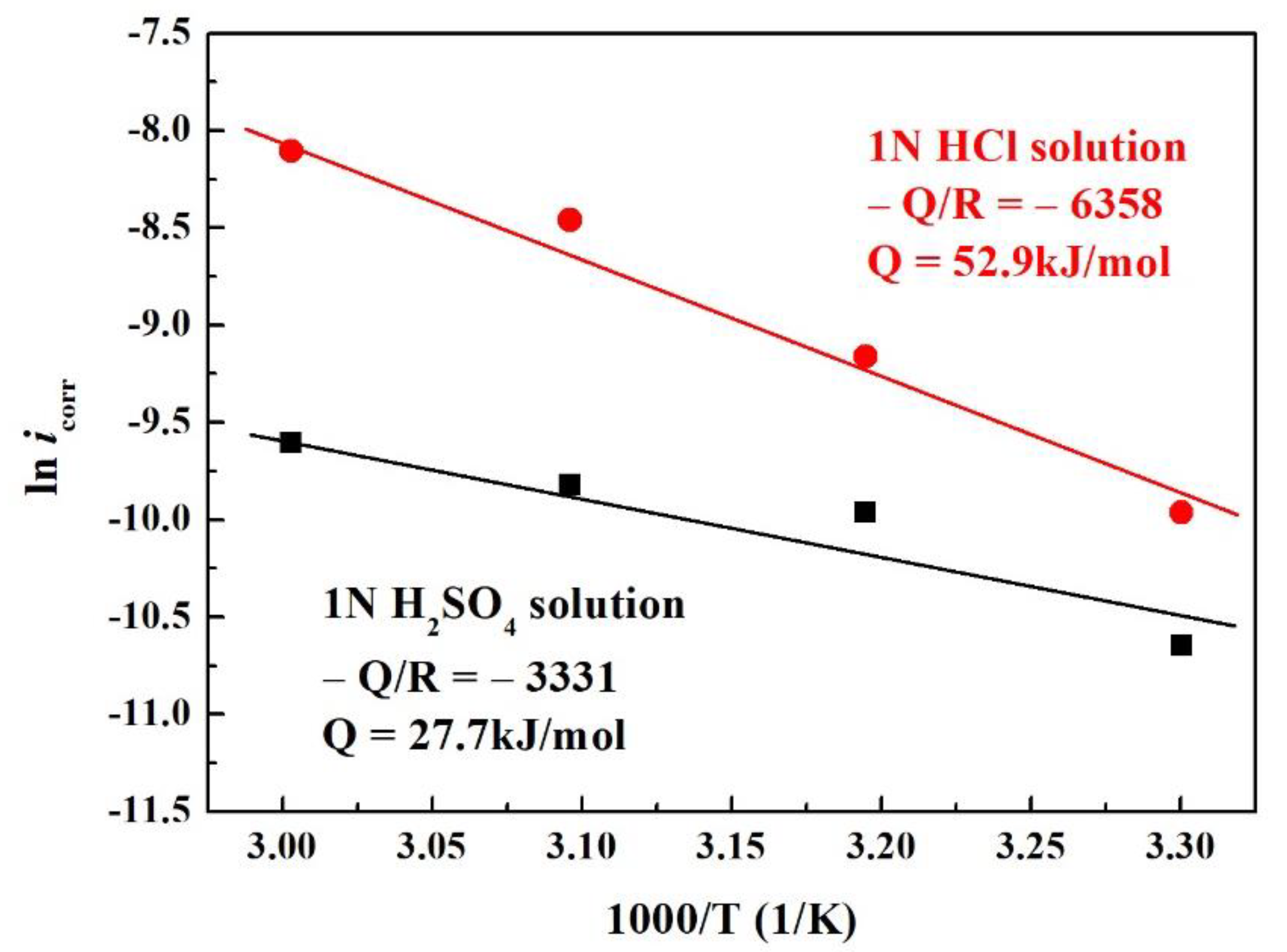
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.-J.; Gan, J.W.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. High-Entropy Alloys, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 13–30. [Google Scholar]
- Yeh, J.W. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Pouraliakbar, H.; Shim, S.H.; Kim, Y.K.; Rizi, M.S.; Noh, H.; Hong, S.I. Microstructure evolution and mechanical properties of (CoCrNi)90(AlTiZr)5(CuFeMo)5 multicomponent alloy: A pathway through multicomponent alloys toward new superalloys. J. Alloys Compd. 2021, 860, 158412. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Duval, T.; Hung, U.D.; Yeh, J.W.; Shih, H.C. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Fujieda, T.; Shiratori, H.; Kuwabara, K.; Kato, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. First demonstration of promising selective electron beam melting method for utilizing high-entropy alloys as engineering materials. Mater. Lett. 2015, 159, 12–15. [Google Scholar] [CrossRef]
- Tsau, C.H.; Wang, W.L. Microstructures, Hardness and Corrosion Behaviors of FeCoNiNb0.5Mo0.5 and FeCoNiNb High-Entropy Alloys. Materials 2018, 11, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muangtong, P.; Namus, R.M.; Goodall, R. Improved Tribocorrosion Resistance by Addition of Sn to CrFeCoNi High Entropy Alloy. Metals 2021, 11, 13. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Cheng, C.-Q.; Shang, J.-L.; Wang, R.; Li, P.; Zhao, J. Oxidation behavior of high-entropy alloys AlxCoCrFeNi (x = 0.15, 0.4) in supercritical water and comparison with HR3C steel. Trans. Nonferrous Met. Soc. China 2015, 25, 1341–1351. [Google Scholar] [CrossRef]
- Huang, K.; Chen, L.; Lin, X.; Huang, H.; Tang, S.; Du, F. Wear and Corrosion Resistance of Al0.5CoCrCuFeNi High-Entropy Alloy Coating Deposited on AZ91D Magnesium Alloy by Laser Cladding. Entropy 2018, 20, 915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Q.; Wang, H.; Chen, M.; Chen, Z.; Li, R.; Jin, P.; Zhang, Y. Mechanical Properties and Corrosion Resistance of NbTiAlSiZrNx High-Entropy Films Prepared by RF Magnetron Sputtering. Entropy 2019, 21, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.Q.; Zhang, Y.X.; Wang, X.R.; Wang, Z.Q.; He, P. Microstructure and corrosion properties of AlCrxNiCu0.5Mo (x = 0, 0.5, 1.0, 1.5, 2.0) high entropy alloy coatings on Q235 steel by electrospark—Computer numerical control deposition. Mater. Lett. 2021, 292, 129642. [Google Scholar] [CrossRef]
- Kiahosseini, S.R.; Baygi, S.J.M.; Khalaj, G.; Khoshakhlagh, A.; Samadipour, R. A Study on Structural, Corrosion, and Sensitization Behavior of Ultrafine and Coarse Grain 316 Stainless Steel Processed by Multiaxial Forging and Heat Treatment. J. Mater. Eng. Perform. 2018, 27, 271–281. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- Tsau, C.H.; Tsai, M.C. The effects of Mo and Nb on the microstructures and properties of CrFeCoNi(Nb,Mo) alloys. Entropy 2018, 20, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000; the table on inside back cover. [Google Scholar]
- Baker, H. ASM Handbook, Volume 3: Alloy Phase Diagrams, 9th ed.; ASM International: Materials Park, OH, USA, 1992; pp. 2–144. [Google Scholar]
- Tsau, C.H.; Yeh, C.Y.; Tsai, M.C. The Effect of Nb-Content on the Microstructures and Corrosion Properties of CrFeCoNiNbx High-Entropy Alloys. Materials 2019, 12, 3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
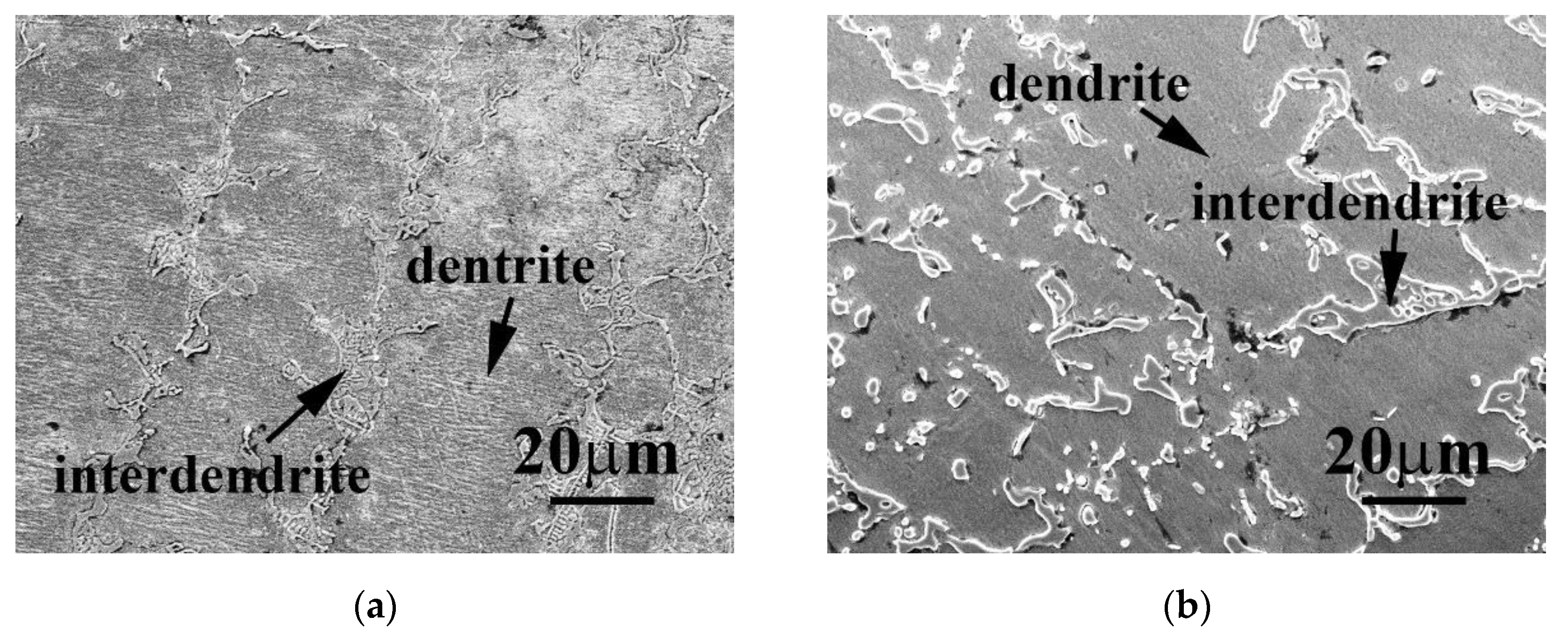

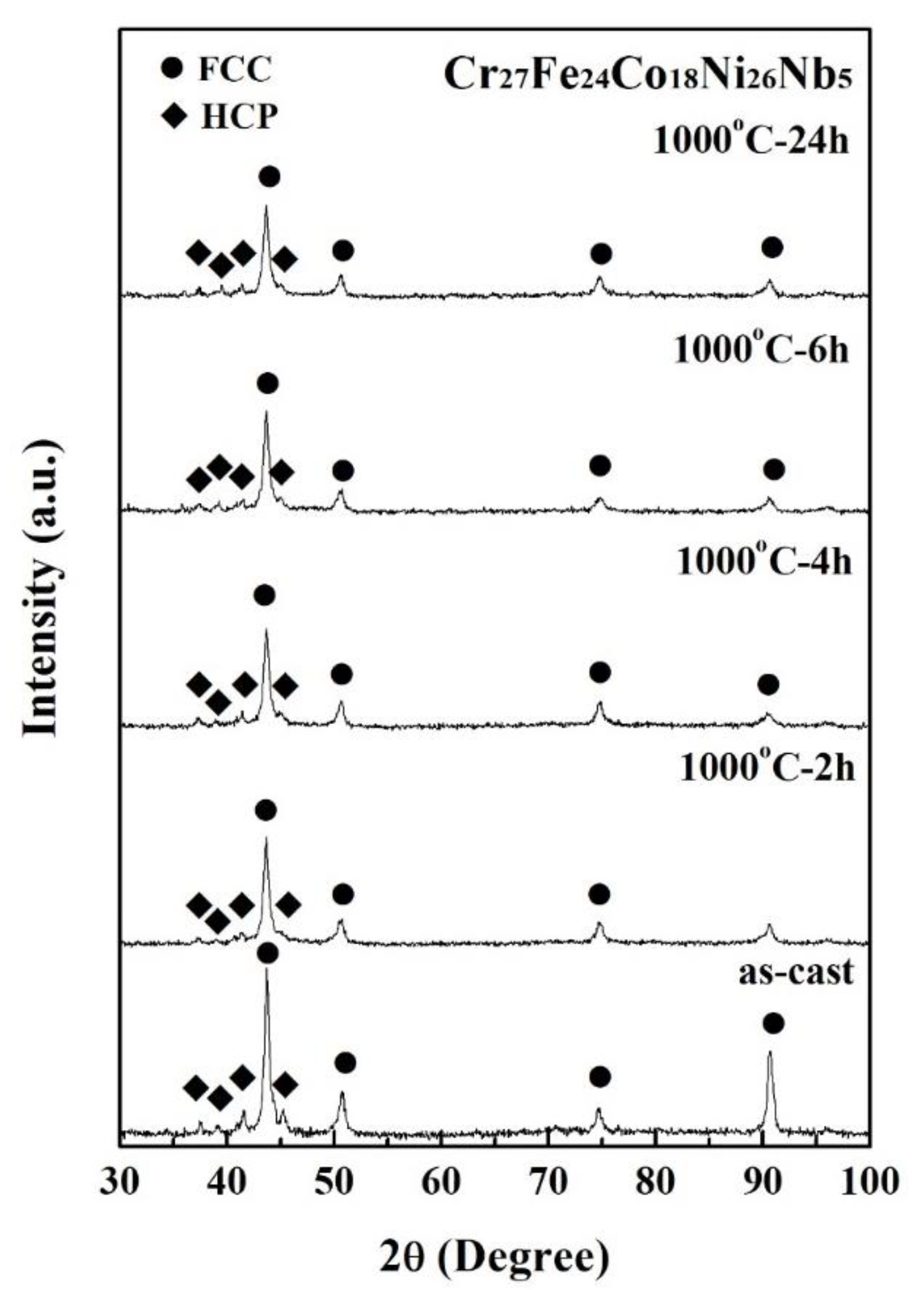
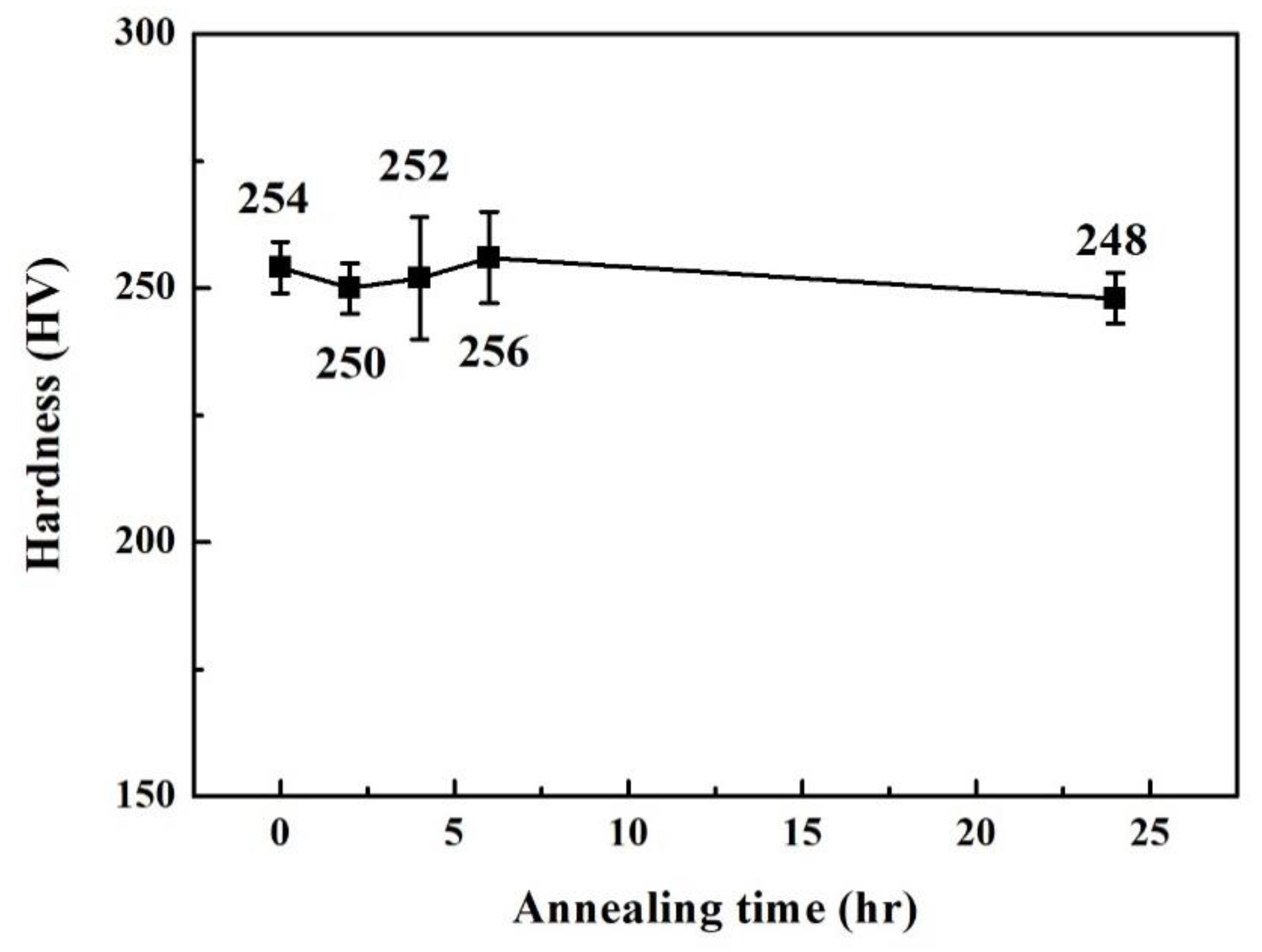
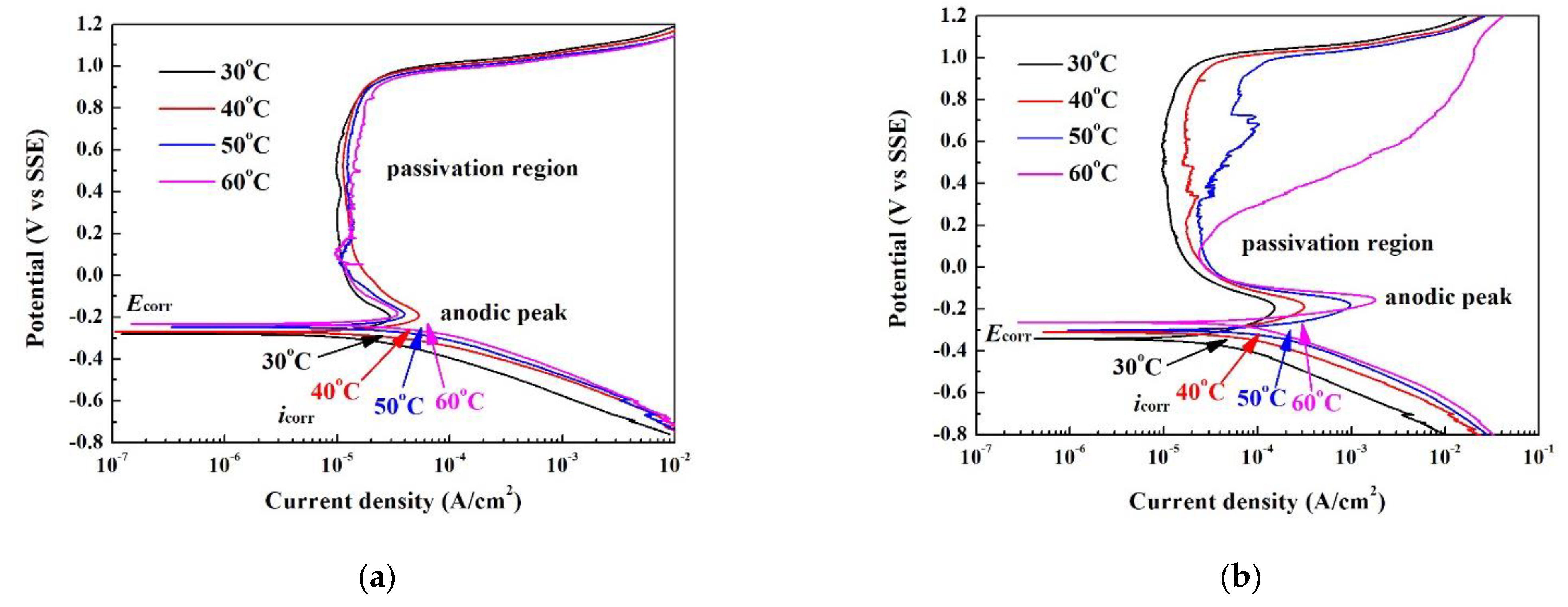
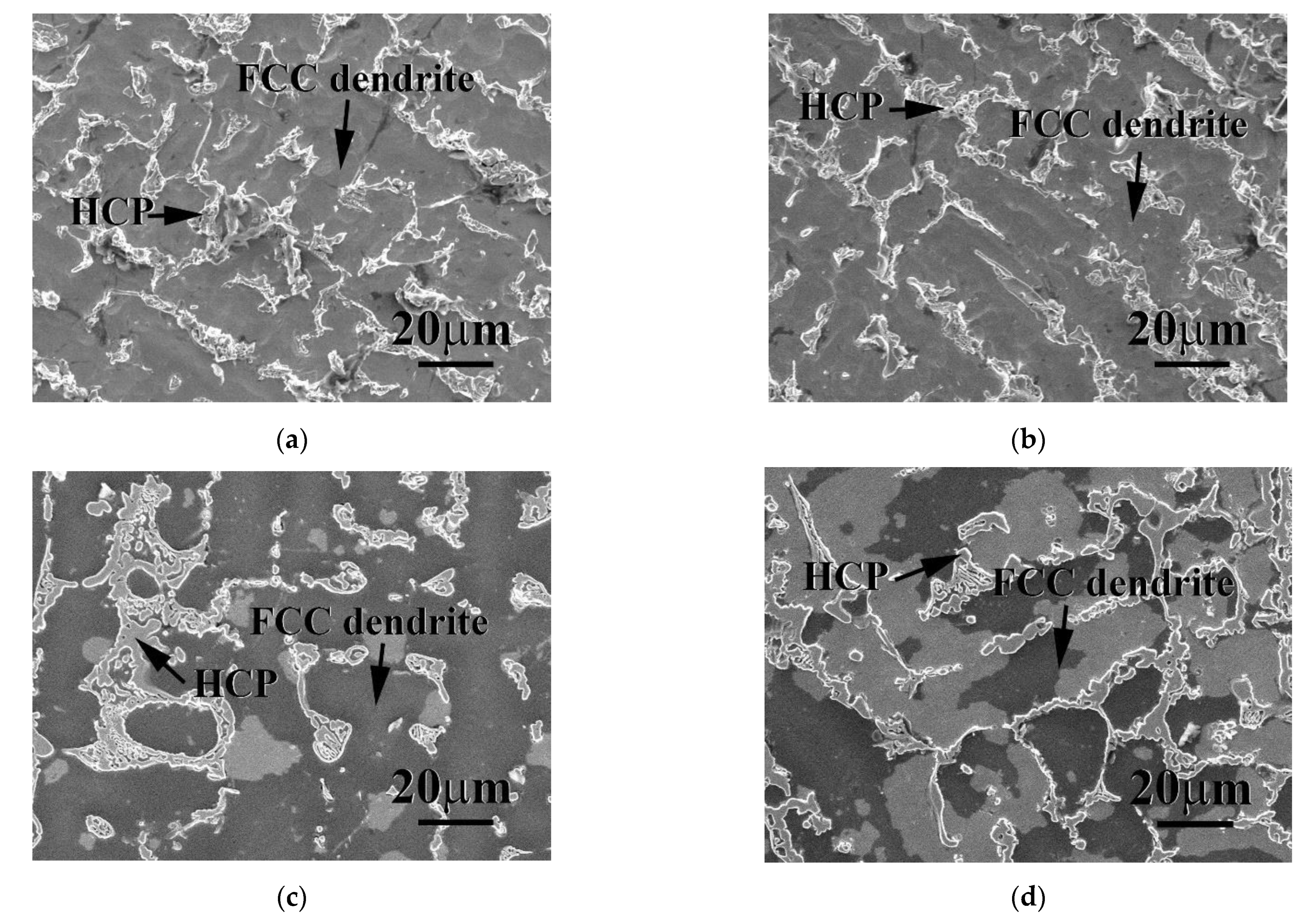
| Phase | Cr | Fe | Co | Ni | Nb |
|---|---|---|---|---|---|
| Overall | 26.0 ± 0.8 | 24.1 ± 0.9 | 18.1 ± 0.8 | 25.8 ± 0.7 | 6.0 ± 0.5 |
| FCC | 27.5 ± 0.5 | 25.7 ± 0.6 | 17.7 ± 1.0 | 26.5 ± 0.8 | 2.6 ± 0.5 |
| HCP | 18.1 ± 1.6 | 15.4 ± 2.1 | 26.4 ± 2.1 | 19.7 ± 2.8 | 30.4 ± 4.3 |
| Solution | Items | 30 °C | 40 °C | 50 °C | 60 °C |
|---|---|---|---|---|---|
| 1 N | βc (V·cm2/A) | 0.178 | 0.177 | 0.187 | 0.200 |
| H2SO4 | Ecorr (V vs. SSE) | −0.280 | −0.272 | −0.246 | −0.233 |
| solution | icorr (μA/cm2) | 23.7 | 47.0 | 54.2 | 67.3 |
| Epp (V vs. SSE) | −0.200 | −0.192 | −0.186 | −0.181 | |
| ipp (μA/cm2) | 29.3 | 52.9 | 39.4 | 34.3 | |
| ipass (μA/cm2) | 10.1 | 12.1 | 11.6 | 10.0 | |
| Eb (V vs. SSE) | 0.981 | 0.972 | 0.960 | 0.955 | |
| 1 N | βc (V·cm2/A) | 1.17 | 1.34 | 1.31 | 1.37 |
| HCl | Ecorr (V vs. SSE) | −0.343 | −0.312 | −0.302 | −0.265 |
| solution | icorr (μA/cm2) | 47.1 | 105 | 212 | 302 |
| Epp (V vs. SSE) | −0.196 | −0.188 | −0.180 | −0.156 | |
| ipp (mA/cm2) | 0.152 | 0.315 | 0.971 | 1.82 | |
| ipass (μA/cm2) | 9.78 | 10.8 | 23.5 | 23.7 | |
| Eb (V vs. SSE) | 1.009 | 1.001 | 0.994 | 0.233 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsau, C.-H.; Chen, P.-M. Corrosion Properties of Cr27Fe24Co18Ni26Nb5 Alloy in 1 N Sulfuric Acid and 1 N Hydrochloric Acid Solutions. Materials 2021, 14, 5924. https://doi.org/10.3390/ma14205924
Tsau C-H, Chen P-M. Corrosion Properties of Cr27Fe24Co18Ni26Nb5 Alloy in 1 N Sulfuric Acid and 1 N Hydrochloric Acid Solutions. Materials. 2021; 14(20):5924. https://doi.org/10.3390/ma14205924
Chicago/Turabian StyleTsau, Chun-Huei, and Po-Min Chen. 2021. "Corrosion Properties of Cr27Fe24Co18Ni26Nb5 Alloy in 1 N Sulfuric Acid and 1 N Hydrochloric Acid Solutions" Materials 14, no. 20: 5924. https://doi.org/10.3390/ma14205924





