Biomechanical Analysis of Non-Metallic Biomaterial in the Manufacture of a New Knee Prosthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Materials
2.2. Test Methods
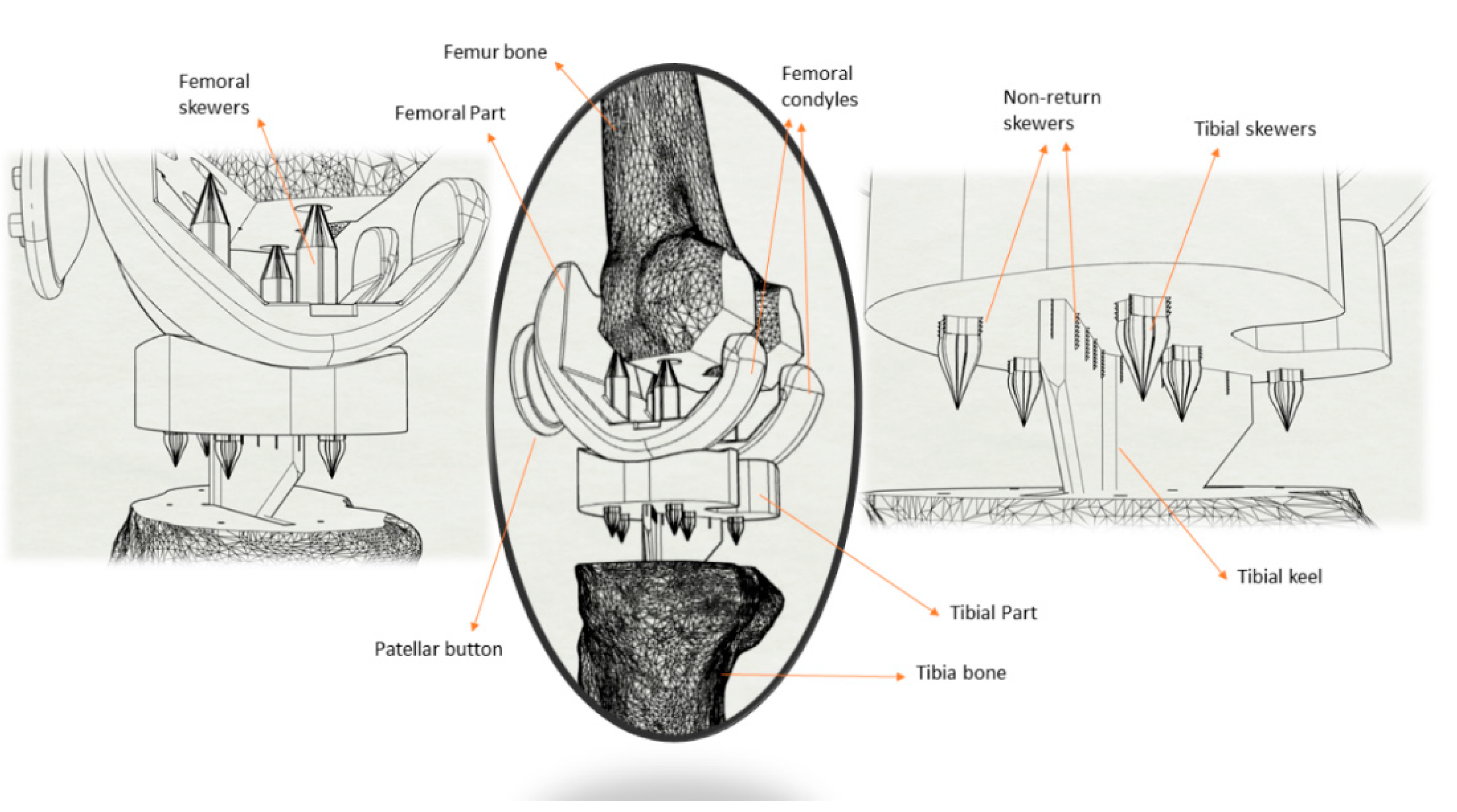
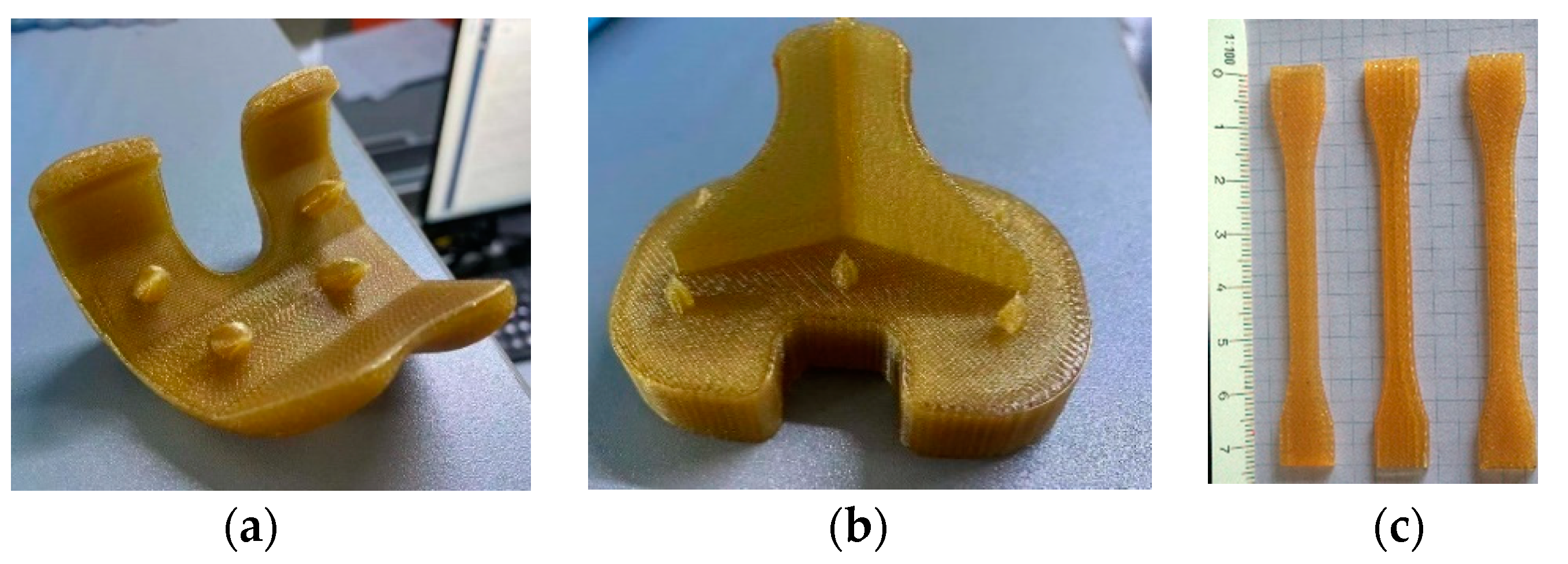
2.2.1. Parts and Specimens Additive Manufacturing
2.2.2. Tensile Test
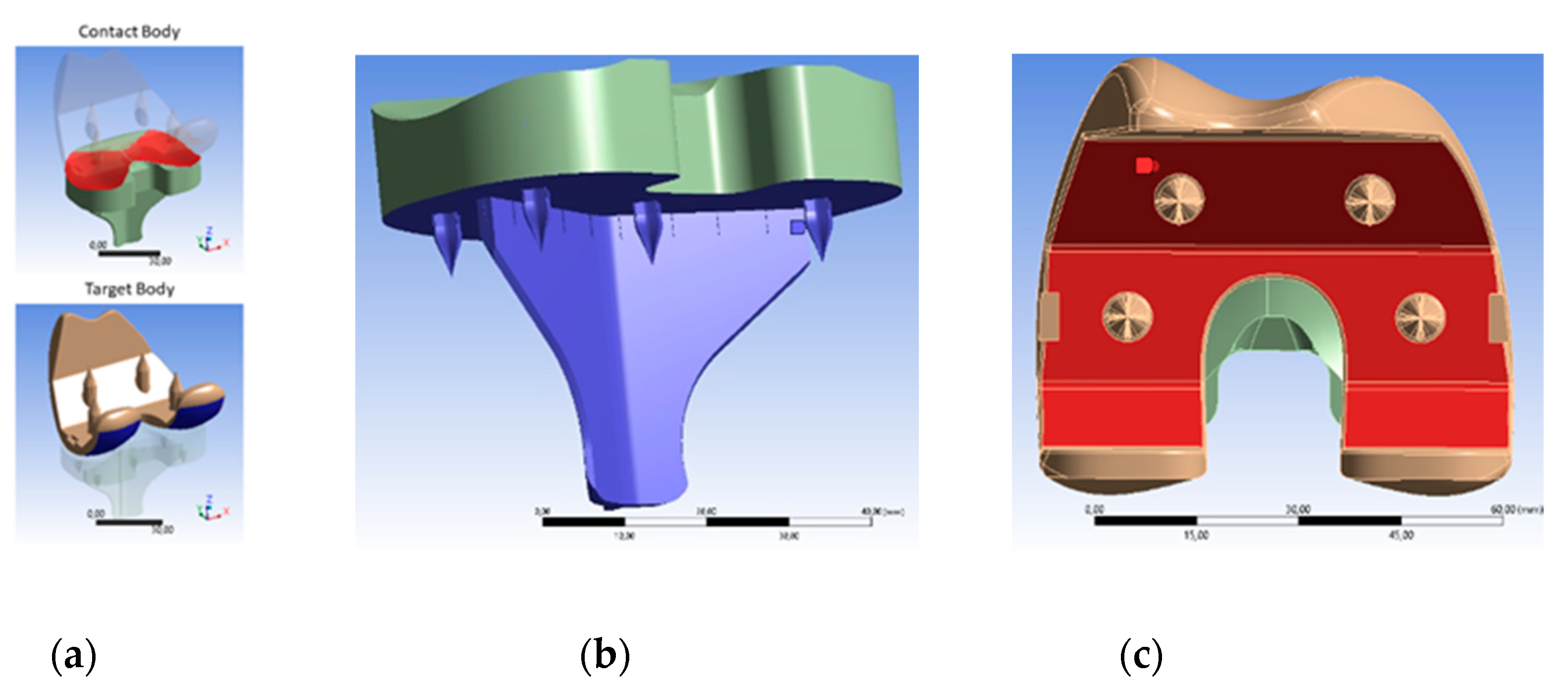
2.2.3. FEM Simulation
2.2.4. Cell Viability Assay (Ratio Live/Dead)
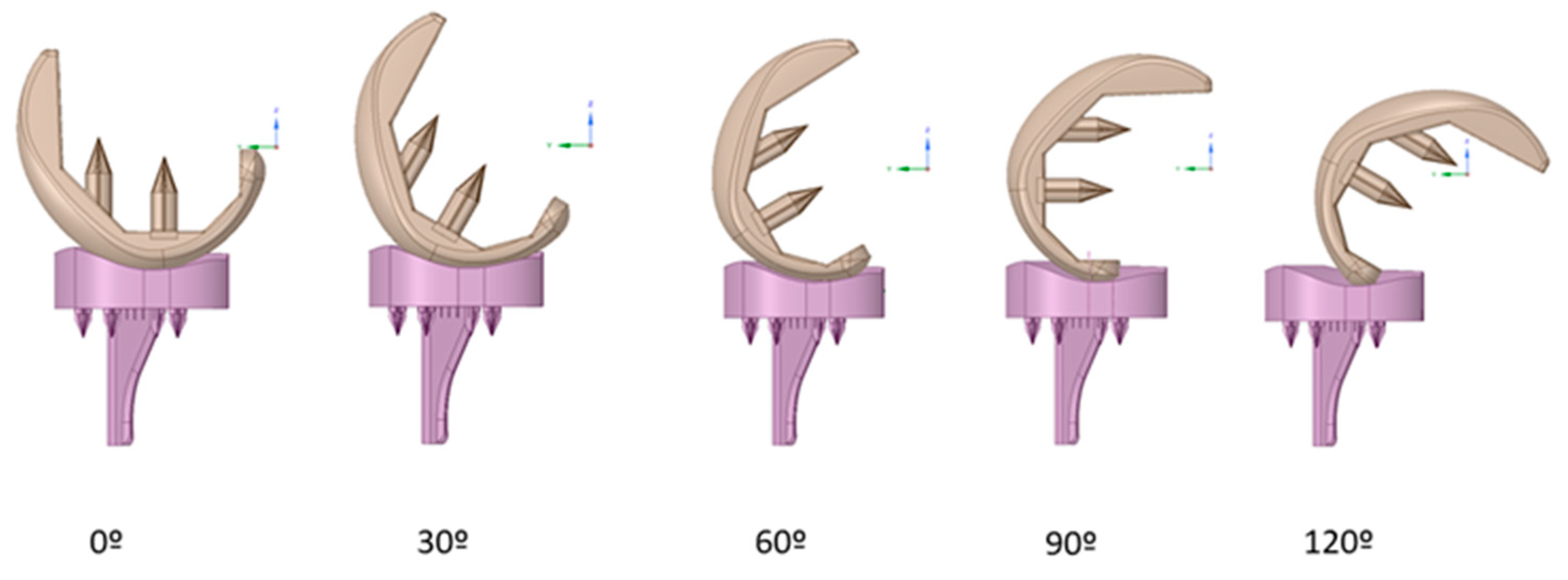
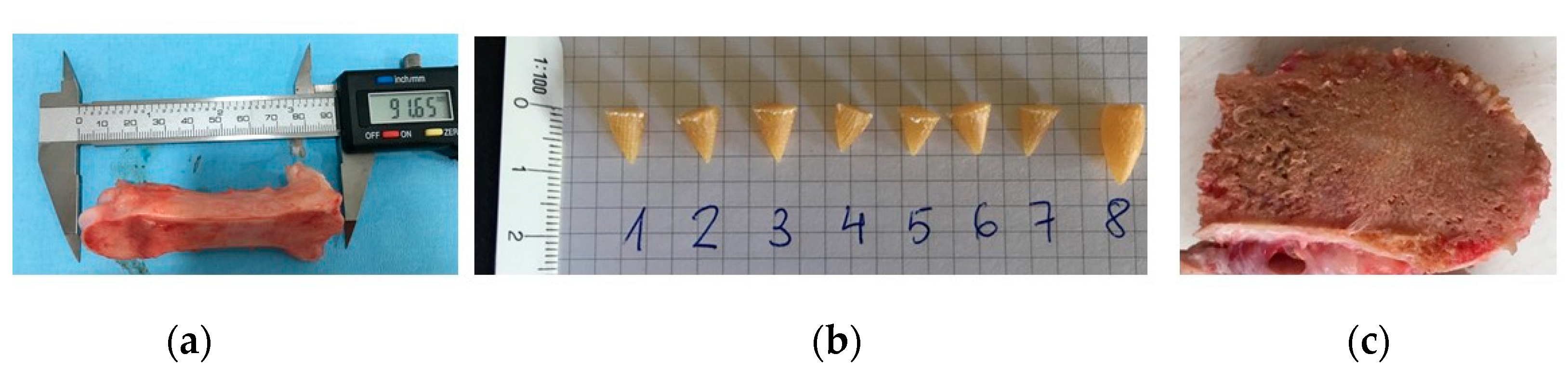
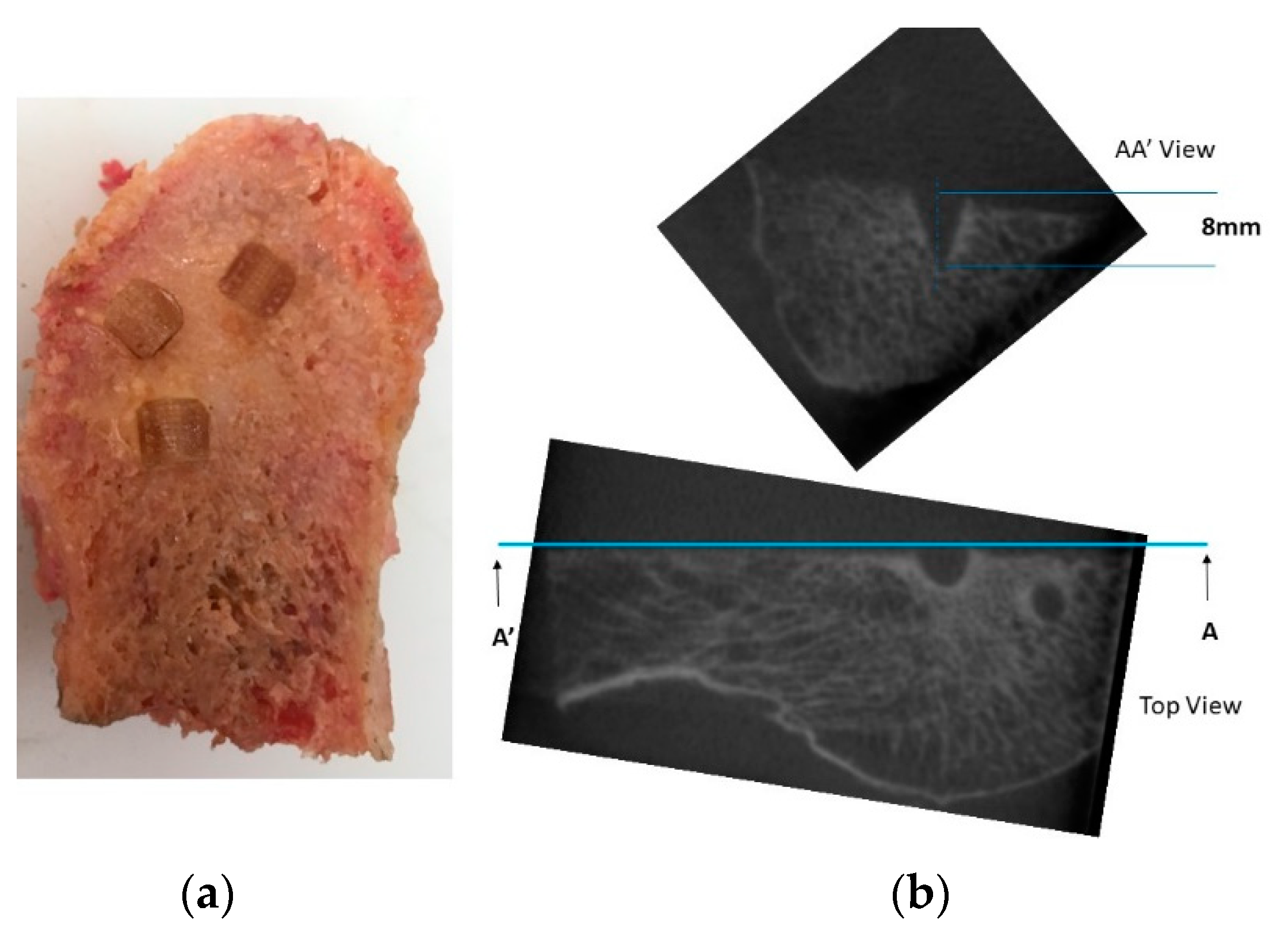
2.2.5. Penetration and Fixation Test
3. Results and Discussion
3.1. Specimen U1010 Tensile Test
3.2. FEM Simulation
3.3. Live/Dead Test
3.4. Penetration and Fixation Test
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [Green Version]
- OECD. Health at a Glance 2019: OECD Indicators. OECD Publ. 2019. [Google Scholar] [CrossRef]
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D.J.; Met, D. Patient Satisfaction after Total Knee Arthroplasty Who is Satisfied and Who is Not ? Clin. Orthop. Relat. Res. 2010, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravipour, I.; Pejhan, S.; Luo, Y.; Wyss, U.P. Customized surface-guided knee implant: Contact analysis and experimental test. J. Eng. Med. 2018, 232, 90–100. [Google Scholar] [CrossRef]
- Fitzpatrick, C.K.; Clary, C.W.; Rullkoetter, P.J. The role of patient, surgical, and implant design variation in total knee replacement performance. J. Biomech. 2012, 45, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, M.A.; Clary, C.W.; Fitzpatrick, C.K.; Deacy, J.S.; Maletsky, L.P.; Rullkoetter, P.J. Dynamic finite element knee simulation for evaluation of knee replacement mechanics. J. Biomech. 2012, 45, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Boorla, R.; Prabeena, T. Fabrication of patient specific knee implant by fused deposition modeling. Mater. Today Proc. 2019, 18, 3638–3642. [Google Scholar] [CrossRef]
- Thompson, M.K.; Moroni, G.; Vaneker, T.; Fadel, G.; Campbell, R.I.; Gibson, I.; Bernard, A.; Schulz, J.; Graf, P.; Ahuja, B.; et al. Design for Additive Manufacturing: Trends, opportunities, considerations, and constraints. CIRP Ann. Manuf. Technol. 2016, 65, 737–760. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- ISO (the International Organization for Standardization). ISO/ASTM 52900:2015 Additive Manufacturing—General Principles—Terminology; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Zakeri, S.; Vippola, M.; Levänen, E. A comprehensive review of the photopolymerization of ceramic resins used in stereolithography. Addit. Manuf. 2020, 35, 101177. [Google Scholar] [CrossRef]
- Paul, S. Finite element analysis in fused deposition modeling research: A literature review. Measurement 2021, 178, 109320. [Google Scholar] [CrossRef]
- Byberg, K.I.; Gebisa, A.W.; Lemu, H.G. Mechanical properties of ULTEM 9085 material processed by fused deposition modeling. Polym. Test. 2018, 72, 335–347. [Google Scholar] [CrossRef]
- White Paper—Sabic UltemTM Filament Performance Assessment. Available online: https://www.sabic.com/en/products/specialties/filaments/ultem-filament (accessed on 6 September 2021).
- Hooper, J.; Schwarzkopf, R.; Fernandez, E.; Buckland, A.; Werner, J.; Einhorn, T.; Walker, P.S. Feasibility of single-use 3D-printed instruments for total knee arthroplasty. Bone Joint J. 2019, 101-B, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Suffo, M.; Vilches-Pérez, J.I.; Salido-Peracaula, M. Comparative Analysis of the Adhesion of Metallic Inserts on Dental Implants-Prosthetic Assembly Generated by Polymeric Materials Used for Additive Manufacturing. In Advances in Design Engineering; Lecture Notes in Mechanical Engineering; Springer: Berlin, Germany, 2020; Volume 1, pp. 245–253. ISBN 978-3-03041-199-2. [Google Scholar]
- Suffo, M. Determination of Adhesive to Be Applied in the Fabrication of Prototypes Using FDM Techniques of 3D Printing in Component Parts Using ULTEMTM 1010. In Design Tools and Methods in Industrial Engineering; Springer: Berlin, Germany, 2020; pp. 959–969. ISBN 978-3-03031-154-4. [Google Scholar]
- Xin, W.; Yu, L.; Yuan, F.; Haode, C.; Li, D. Macrophage polarization in aseptic bone resorption around dental implants induced by Ti particles in a murine model. J. Periodontal Res. 2019, 54, 329–338. [Google Scholar] [CrossRef]
- Popescu, D.; Baciu, F.; Vlăsceanu, D.; Cotruţ, C.M.; Marinescu, R. Effects of multiple sterilizations and natural aging on the mechanical behavior of 3D-printed ABS. Mech. Mater. 2020, 148, 103423. [Google Scholar] [CrossRef]
- Deng, X.; Zeng, Z.; Peng, B.; Yan, S.; Ke, W. Mechanical Properties Optimization of Poly-Ether-Ether-Ketone via Fused Deposition Modeling. Materials 2018, 11, 216. [Google Scholar] [CrossRef] [Green Version]
- Black, J. Requirements for successful total knee replacement. Material considerations. Orthop. Clin. North Am. 1989, 20, 1–13. [Google Scholar]
- Sharkey, P.F.; Hozack, W.; Rothman, R.; Shastri, S.; Sidney, J. Why Are Total Knee Arthroplasties Failing Today ? Clin. Orthop. Relat. Res. 2002, 7–13. [Google Scholar] [CrossRef]
- Schroer, W.C.; Berend, K.R.; Lombardi, A.V.; Barnes, C.L.; Bolognesi, M.P.; Berend, M.E.; Ritter, M.A.; Nunley, R.M. Why Are Total Knees Failing Today ? Etiology of Total Knee Revision in 2010 and 2011. J. Arthroplast. 2013, 28, 116–119. [Google Scholar] [CrossRef]
- Brien, S.T.O.; Luo, Y.; Brandt, J. In-vitro and in-silico investigations on the in fl uence of contact pressure on cross-linked polyethylene wear in total knee replacements. Wear 2015, 333, 687–693. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Adeoye, A.O.M. 3D printing of bone scaffolds with hybrid biomaterials. Compos. Part B Eng. 2019, 158, 428–436. [Google Scholar] [CrossRef]
- Zhao, M.; An, M.; Wang, Q.; Liu, X.; Lai, W.; Zhao, X.; Wei, S.; Ji, J. Quantitative proteomic analysis of human osteoblast-like MG-63 cells in response to bioinert implant material titanium and polyetheretherketone. J. Proteom. 2012, 75, 3560–3573. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Abolfazl Zahedi, S.; Vahidnia, F.; Ikumapayi, O.M.; Farooq, M.U. Three-dimensional finite element analysis of a porcelain crowned tooth. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 461–464. [Google Scholar] [CrossRef]
- Polyetherimide (PEI): A Comprehensive Review. Available online: https://omnexus.specialchem.com/selection-guide/polyetherimide-pei-high-heat-plastic?src=omproperty&utm_source=selection-resources&utm_medium=polymer-properties&utm_campaign=hardness-m (accessed on 6 September 2021).
- Ma, H.M.; Lu, Y.C.; Kwok, T.G.; Ho, F.Y.; Huang, C.Y.; Huang, C.H. The effect of the design of the femoral component on the conformity of the patellofemoral joint in total knee replacement. J. Bone Jt. Surg. Ser. B 2007, 89, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Triathlon: Total Knee System. Available online: https://www.stryker.com/us/en/joint-replacement/products/triathlon-total-knee-system.html (accessed on 6 September 2021).
- Suffo, M.; de la Mata, M.; Molina, S.I. A sugar-beet waste based thermoplastic agro-composite as substitute for raw materials. J. Clean. Prod. 2020, 257, 120382. [Google Scholar] [CrossRef]
- International Organization for Standardization. Implants for Surgery—Wear of Total Knee-Joint Prostheses, Part3: Loading and Displacement Parameters for Wear-Testing Machines with Displacement Control and Corresponding Environmental Conditions for Test; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- O’Brien, S.; Luo, Y.; Wu, C.; Petrak, M.; Bohm, E.; Brandt, J.M. Computational development of a polyethylene wear model for the articular and backside surfaces in modular total knee replacements. Tribol. Int. 2013, 59, 284–291. [Google Scholar] [CrossRef]
- O’Brien, S.T.; Bohm, E.R.; Petrak, M.J.; Wyss, U.P.; Brandt, J.M. An energy dissipation and cross shear time dependent computational wear model for the analysis of polyethylene wear in total knee replacements. J. Biomech. 2014, 47, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, T.; Wimmer, M.A. Cross-shear in metal-on-polyethylene articulation of orthopaedic implants and its relationship to wear. Wear 2013, 301, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, L.; Galvin, A.L.; Brown, T.D.; Jin, Z.; Fisher, J. Quantification of the Effect of Cross-shear on the Wear of Conventional and Highly Cross-linked UHMWPE. J. Biomech. 2008, 41, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Peces, M.V.; Pérez-Moreno, A.; De-Los-santos, D.M.; Mesa-Díaz, M.D.M.; Pinaglia-Tobaruela, G.; Vilches-Pérez, J.I.; Fernández-Montesinos, R.; Salido, M.; de la Rosa-Fox, N.; Piñero, M. Chitosan-gptms-silica hybrid mesoporous aerogels for bone tissue engineering. Polymers 2020, 12, 2723. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, A.; Reyes-Peces, M.d.l.V.; de los Santos, D.M.; Pinaglia-Tobaruela, G.; de la Orden, E.; Vilches-Pérez, J.I.; Salido, M.; Piñero, M.; de la Rosa-Fox, N. Hydroxyl groups induce bioactivity in silica/chitosan aerogels designed for bone tissue engineering. In vitro model for the assessment of osteoblasts behavior. Polymers 2020, 12, 2802. [Google Scholar] [CrossRef] [PubMed]
- Carruth, P.; He, J.; Benson, B.W.; Schneiderman, E.D. Analysis of the Size and Position of the Mental Foramen Using the CS 9000 Cone-beam Computed Tomographic Unit. J. Endod. 2015, 41, 1032–1036. [Google Scholar] [CrossRef]
- Gach, P.; Tuchtan-Torrents, L.; Delteil, C.; Adalian, P.; Piercecchi, M.D.; Ebert, L.C.; Gorincour, G. Virtual reconstruction of paranasal sinuses from CT data: A feasibility study for forensic application. Diagn. Interv. Imaging 2019, 100, 163–168. [Google Scholar] [CrossRef]
- Van Den Heever, D.J.; Scheffer, C.; Erasmus, P.; Dillon, E. Contact stresses in a patient-specific unicompartmental knee replacement. Clin. Biomech. 2011, 26, 159–166. [Google Scholar] [CrossRef]
- Stukenborg-Colsman, C.; Ostermeier, S.; Hurschler, C.; Wirth, C.J. Tibiofemoral contact stress after total knee arthroplasty. Acta Orthop. Scand. 2002, 73, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Forum Ansys. Available online: https://forum.ansys.com/discussion/27554/frictional-stress (accessed on 6 September 2021).
- Kšiňan, J.; Vodička, R. An 2-D SGBEM formulation of contact models coupling the interface damage and Coulomb friction in fibre–matrix composites. Eng. Fract. Mech. 2016, 168, 76–92. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Xu, F.-F.; Yu, Z.-C.; Lu, K.-Y.; Zheng, Z.-B.; Zhao, S.-D. Coulomb, Tresca and Coulomb-Tresca friction models used in analytical analysis for rolling process of external spline. J. Mater. Process. Technol. 2021, 292, 117059. [Google Scholar] [CrossRef]
- D’Lima, D.D.; Chen, P.C.; Colwell, C.W. Polyethylene contact stresses, articular congruity, and knee alignment. Clin. Orthop. Relat. Res. 2001, 392, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, T.; Lui, Y.F.; Sze, K.Y.; Lu, W.W. The measured mechanical properties of osteoporotic trabecular bone decline with the increment of deformation volume during micro-indentation. J. Mech. Behav. Biomed. Mater. 2020, 103, 103546. [Google Scholar] [CrossRef]
- Raffa, M.L.; Nguyen, V.-H.; Hernigou, P.; Flouzat-Lachaniette, C.-H.; Haiat, G. Stress shielding at the bone-implant interface: Influence of surface roughness and of the bone-implant contact ratio. J. Orthop. Res. 2021, 39, 1174–1183. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suffo, M.; Revenga, C. Biomechanical Analysis of Non-Metallic Biomaterial in the Manufacture of a New Knee Prosthesis. Materials 2021, 14, 5951. https://doi.org/10.3390/ma14205951
Suffo M, Revenga C. Biomechanical Analysis of Non-Metallic Biomaterial in the Manufacture of a New Knee Prosthesis. Materials. 2021; 14(20):5951. https://doi.org/10.3390/ma14205951
Chicago/Turabian StyleSuffo, Miguel, and Carlos Revenga. 2021. "Biomechanical Analysis of Non-Metallic Biomaterial in the Manufacture of a New Knee Prosthesis" Materials 14, no. 20: 5951. https://doi.org/10.3390/ma14205951
APA StyleSuffo, M., & Revenga, C. (2021). Biomechanical Analysis of Non-Metallic Biomaterial in the Manufacture of a New Knee Prosthesis. Materials, 14(20), 5951. https://doi.org/10.3390/ma14205951







