Comparative Study on the Thermal Performance of Cr-CrxOy and YSZ-CoNiCrAlY Coatings Exposed at 900 °C
Abstract
:1. Introduction
2. Materials and Experimental Procedure
2.1. Materials and Coatings Deposition
2.2. Heat Treatment and Oxidation
2.3. Characterisation
3. Results and Discussions
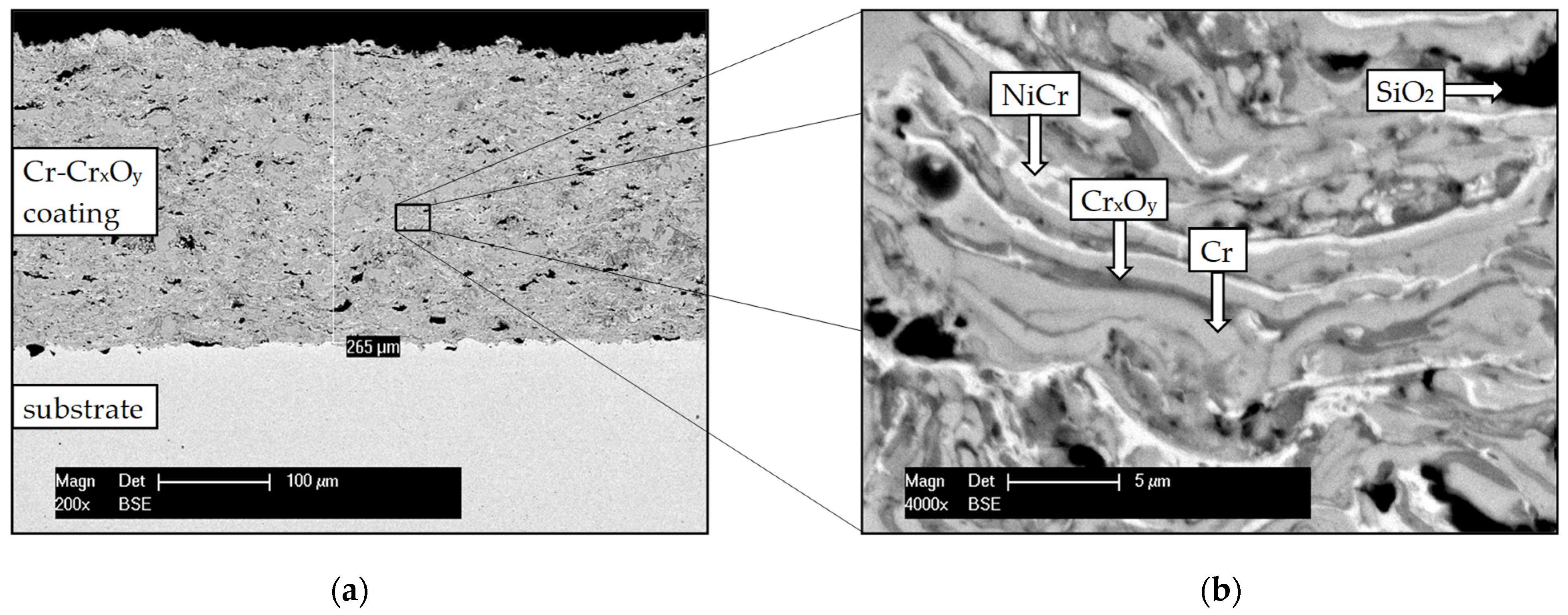
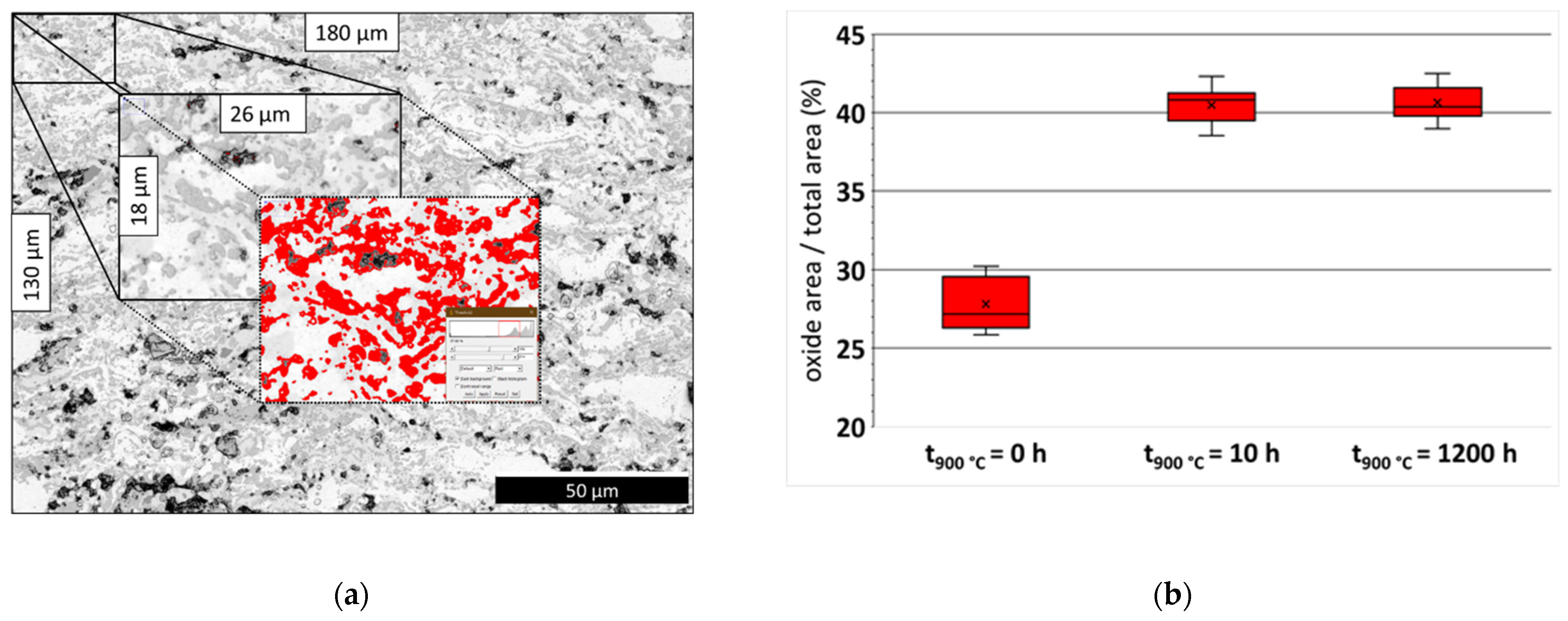
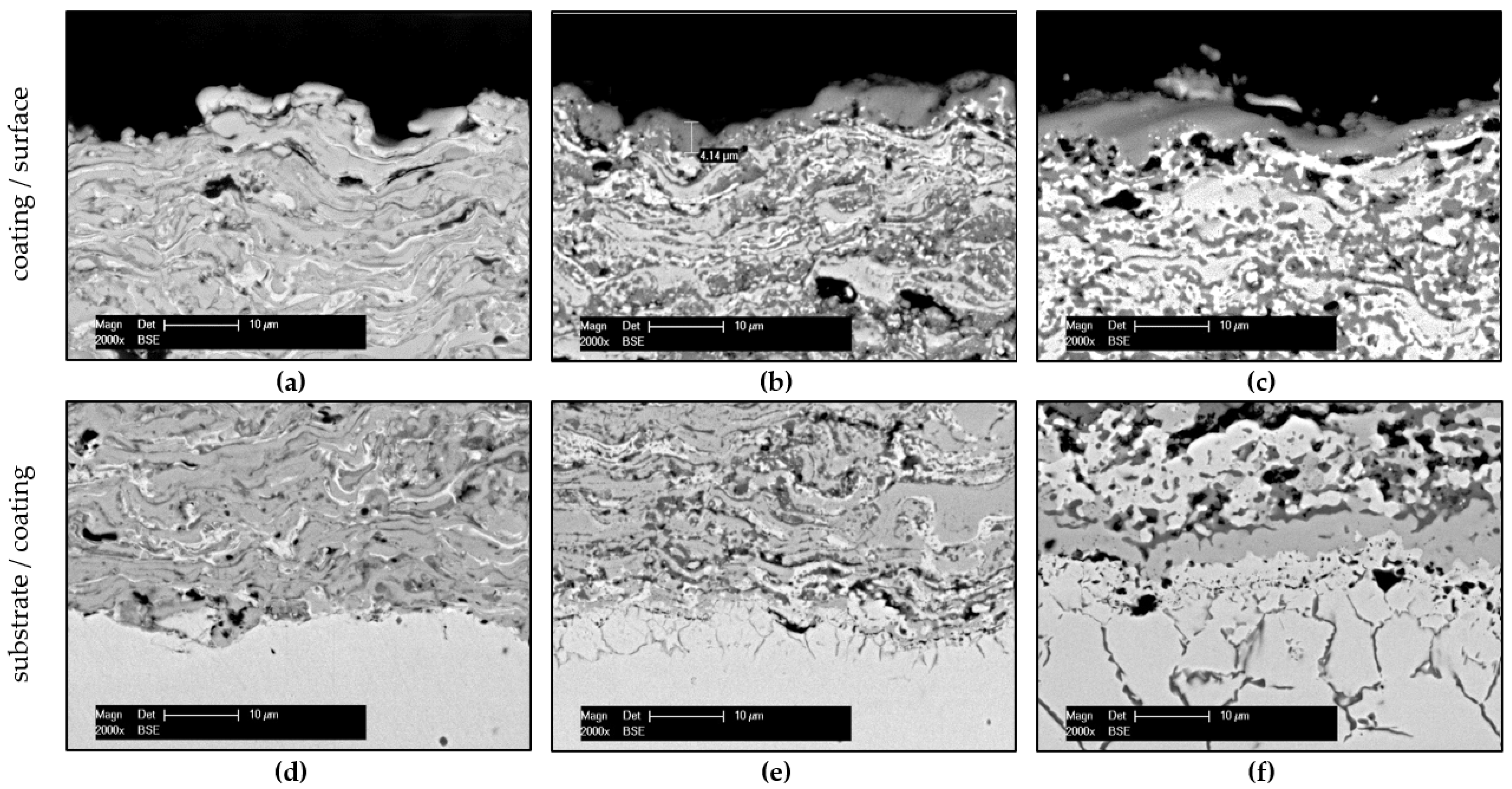
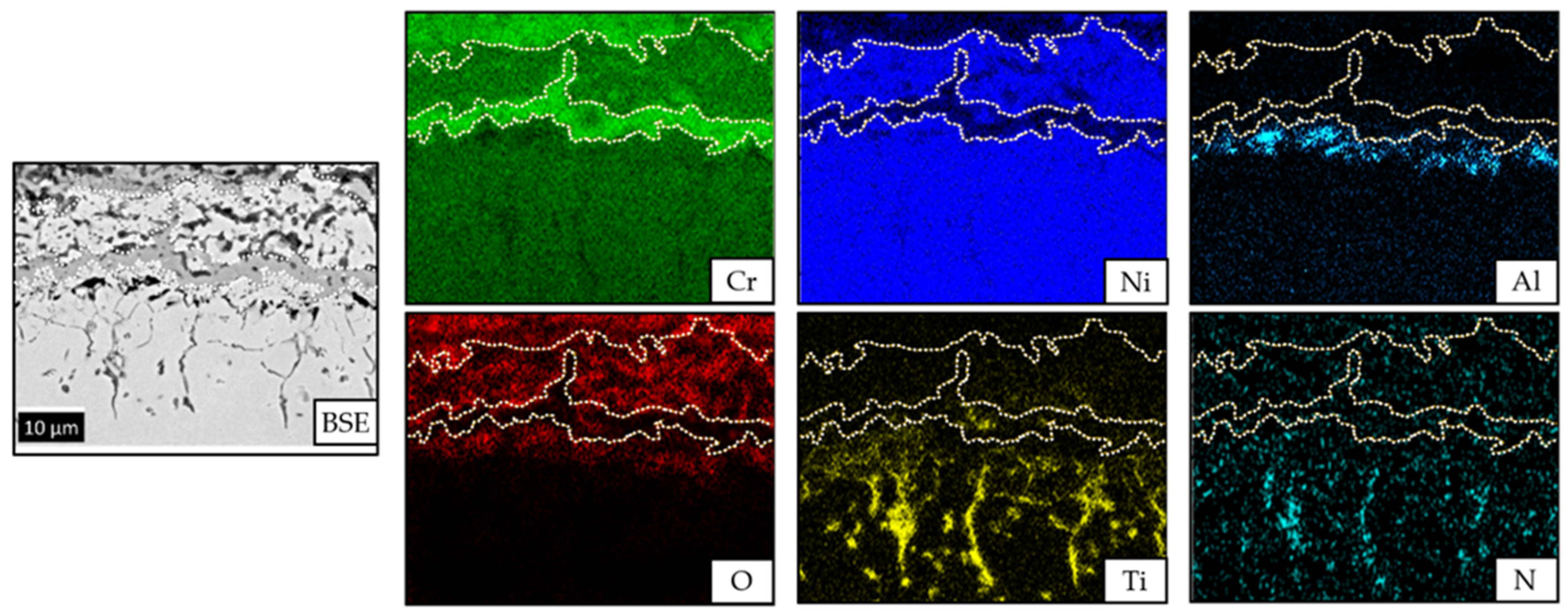
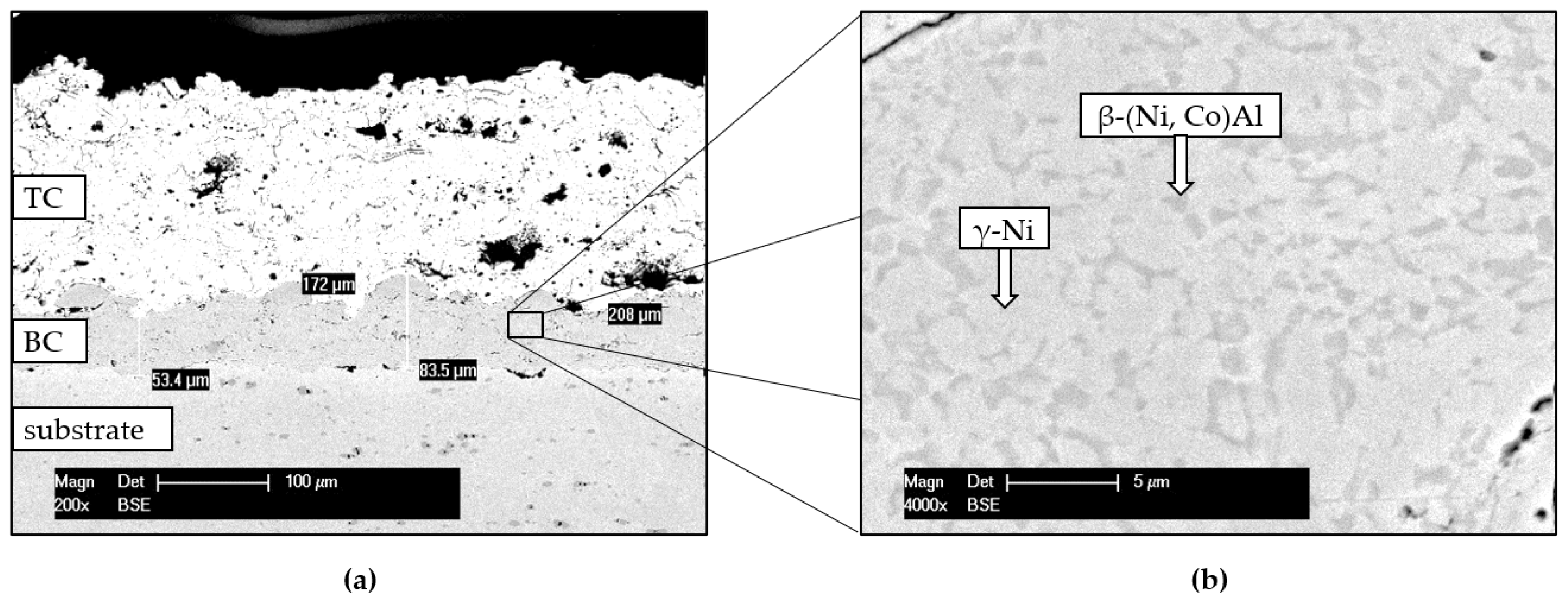
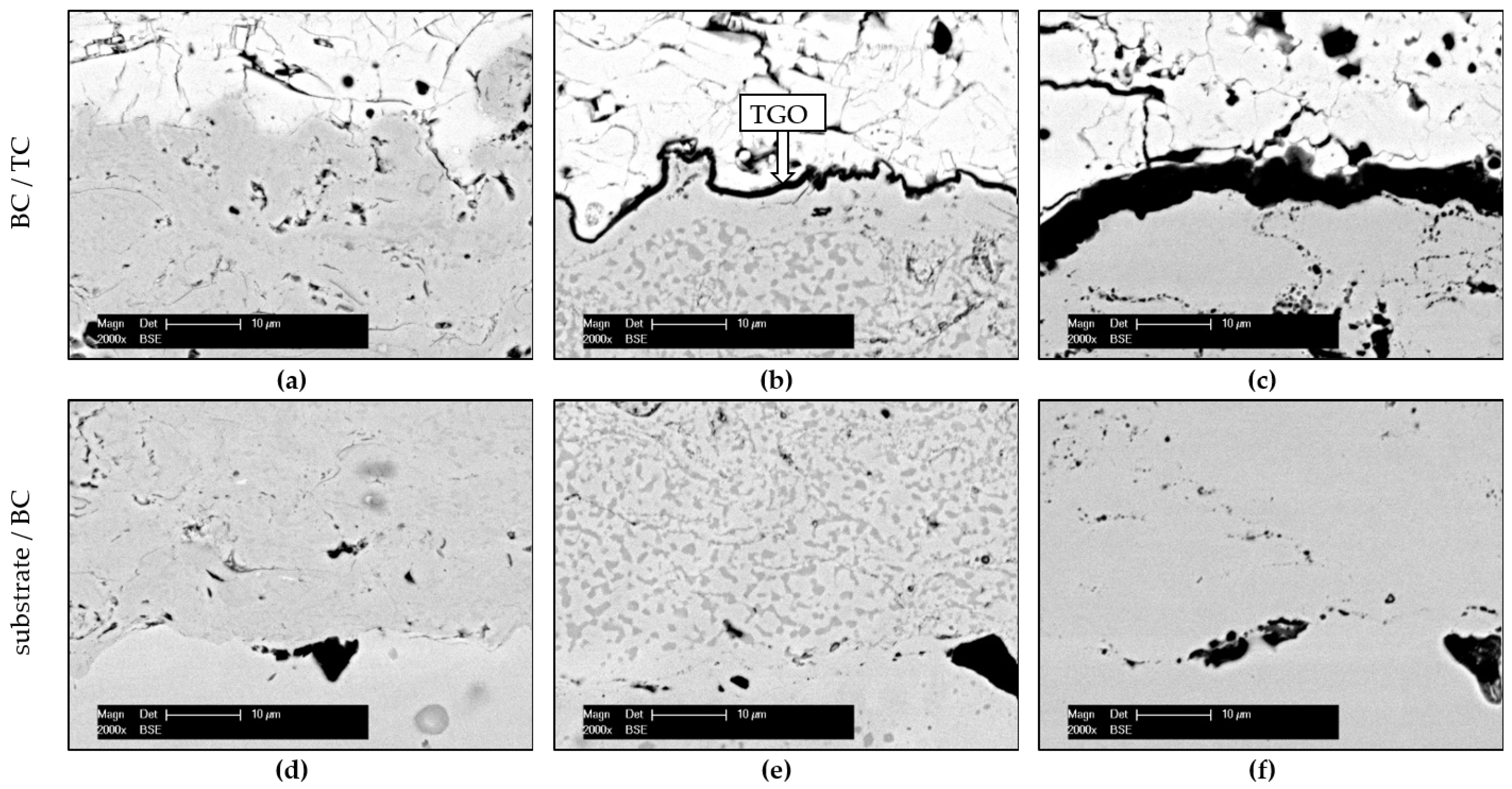
3.1. Structural Characterisation
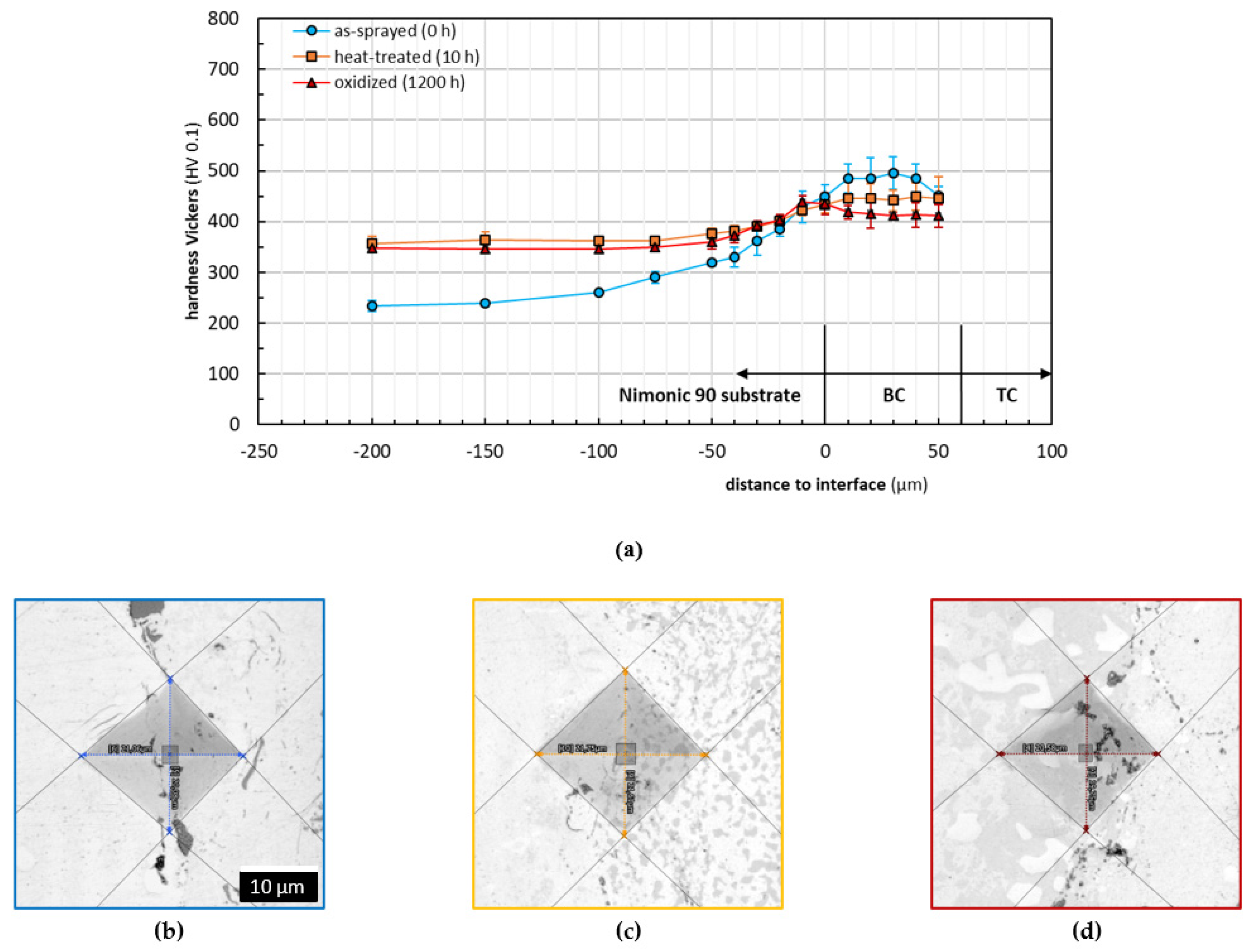
3.2. Microhardness and Adhesion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal Barrier Coatings for Gas-Turbine Engine Applications. Science 2002, 296, 280. [Google Scholar] [CrossRef]
- Carter, T.J. Common Failures in Gas Turbine Blades. Eng. Fail. Anal. 2005, 12, 237–247. [Google Scholar] [CrossRef]
- Rolls, R. The Jet Engine, 5th ed.; Wiley-VCH: Weinheim, Germany, 2015; ISBN 978-1-119-06599-9. [Google Scholar]
- Goward, G.W. Progress in Coatings for Gas Turbine Airfoils. Surf. Coat. Technol. 1998, 108–109, 73–79. [Google Scholar] [CrossRef]
- Nicholls, J.R.; Simms, N.J.; Chan, W.Y.; Evans, H.E. Smart Overlay Coatings—Concept and Practice. Surf. Coat. Technol. 2002, 149, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Heine, B. Nickelbasis-Superlegierungen für Flugzeugantriebe Aus Metallkundlicher Sicht; WOMag: Waldshut-Tiengen, Germany, 2014. [Google Scholar]
- Perepezko, J.H. The Hotter the Engine, the Better. Science 2009, 326, 1068. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, A.; Saremi, M.; Kobayashi, A. A Comparative Study on Hot Corrosion Resistance of Three Types of Thermal Barrier Coatings: YSZ, YSZ+Al2O3 and YSZ/Al2O3. Mater. Sci. Eng. A 2008, 478, 264–269. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.J.; Suo, H.L.; Meng, Y.C. Hot-Corrosion Behavior of Modified YSZ Thermal Barrier Coating. Appl. Mech. Mater. 2014, 492, 361–364. [Google Scholar] [CrossRef]
- Drexler, J.M.; Shinoda, K.; Ortiz, A.L.; Li, D.; Vasiliev, A.L.; Gledhill, A.D.; Sampath, S.; Padture, N.P. Air-Plasma-Sprayed Thermal Barrier Coatings That Are Resistant to High-Temperature Attack by Glassy Deposits. Acta Mater. 2010, 58, 6835–6844. [Google Scholar] [CrossRef]
- Khajezadeh, M.H.; Mohammadi, M.; Ghatee, M. Hot Corrosion Performance and Electrochemical Study of CoNiCrAlY/YSZ/YSZ-La2O3 Multilayer Thermal Barrier Coatings in the Presence of Molten Salt. Mater. Chem. Phys. 2018, 220, 23–34. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Hu, X.B.; Zheng, S.J.; Zhu, Y.L.; Wei, H.; Ma, X.L. Microstructural Evolution of the Interface between NiCrAlY Coating and Superalloy during Isothermal Oxidation. Mater. Des. 2015, 80, 63–69. [Google Scholar] [CrossRef]
- Cao, F.; Tryon, B.; Torbet, C.J.; Pollock, T.M. Microstructural Evolution and Failure Characteristics of a NiCoCrAlY Bond Coat in “Hot Spot” Cyclic Oxidation. Acta Mater. 2009, 57, 3885–3894. [Google Scholar] [CrossRef]
- Keyvani, A.; Saremi, M.; Sohi, M.H. An Investigation on Oxidation, Hot Corrosion and Mechanical Properties of Plas-ma-Sprayed Conventional and Nanostructured YSZ Coatings. Surf. Coat. Technol. 2011, 206, 208–216. [Google Scholar] [CrossRef]
- Nicholls, J.R. Designing Oxidation-Resistant Coatings. JOM 2000, 52, 28–35. [Google Scholar] [CrossRef]
- Wood, G.C.; Hodgkiess, T.; Whittle, D.P. A Comparison of the Scaling Behaviour of Pure Iron-Chromium and Nickel-Chromium Alloys in Oxygen. Corros. Sci. 1966, 6, 129–147. [Google Scholar] [CrossRef]
- Bürgel, R.; Grünling, H.W.; Schneider, K. Erfahrungen Mit Hochtemperatur-Korrosionsschutzschichten in Stationären Gasturbinen. Mater. Corros. 1987, 38, 549–555. [Google Scholar] [CrossRef]
- Pang, X.; Gao, K.; Volinsky, A.A. Microstructure and Mechanical Properties of Chromium Oxide Coatings. J. Mater. Res. 2007, 22, 3531–3537. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Lee, C.; Hwang, S.Y. The Effect of Nano-Sized Cr2O3 Addition on the Characteristics of NiCr–Cr2O3–Ag–BaF2/CaF2 Coating. Surf. Coat. Technol. 2006, 201, 38–44. [Google Scholar] [CrossRef]
- Verlotski, V. Process for Producing a Protective Chromium Layer. International Application No. PCT/EP2012/004197, 30 May 2013. [Google Scholar]
- Cramer, S.D.; Covino, B.S. Corrosion: Materials; ASM International: Novelty, OH, USA, 2005; Volume 13B, ISBN 978-0-87170-707-9. [Google Scholar]
- König, C. Nanomagnetismus von Epitaktischen Fe(110)- und CrO2(100)-Strukturen im Hinblick auf Potentielle Inelekt-Ronische Anwendungen; Rheinisch-Westfälische Technische Hochschule Aachen: Aachen, Germany, 2006. [Google Scholar]
- Glemser, O.; Hauschild, U.; Trüpel, F. Über Chromoxyde Zwischen Cr2O3 Und CrO3. Z. Anorg. Allg. Chem. 1954, 277, 113–126. [Google Scholar] [CrossRef]
- Ishibashi, S.; Namikawa, T.; Satou, M. Epitaxial Growth of Ferromagnetic CrO2 Films in Air. Mater. Res. Bull. 1979, 14, 51–57. [Google Scholar] [CrossRef]
- Singh, G.P.; Ram, S.; Eckert, J.; Fecht, H.-J. Synthesis and Morphological Stability in CrO2 single Crystals of a Half-Metallic Ferromagnetic Compound. J. Phys. Conf. Ser. 2009, 144, 012110. [Google Scholar] [CrossRef]
- Ivanov, P.G.; Watts, S.M.; Lind, D.M. Epitaxial Growth of CrO2 Thin Films by Chemical-Vapor Deposition from a Cr8O21 Precursor. J. Appl. Phys. 2001, 89, 1035–1040. [Google Scholar] [CrossRef]
- Hagel, W.C. Anion Diffusion in α-Cr2O3. J. Am. Ceram. Soc. 1965, 48, 70–75. [Google Scholar] [CrossRef]
- Cao, P.; Wells, D.; Short, M.P. Anisotropic Ion Diffusion in α-Cr2O3: An Atomistic Simulation Study. Phys. Chem. Chem. Phys. 2017, 19, 13658–13663. [Google Scholar] [CrossRef] [Green Version]
- Buscail, H.; Jacob, Y.P.; Stroosnijder, M.F.; Caudron, E.; Cueff, R.; Rabaste, F.; Perrier, S. X-Ray Diffraction to Study the Oxidation Mechanism of Chromium at Elevated Temperatures. Mater. Sci. Forum 2004, 461–464, 93–100. [Google Scholar] [CrossRef]
- Schmalzried, H. Chemical Kinetics of Solids; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1995; ISBN 978-3-527-61553-7. [Google Scholar]
- Kidson, G.V. Some Aspects of the Growth of Diffusion Layers in Binary Systems. J. Nucl. Mater. 1961, 3, 21–29. [Google Scholar] [CrossRef]
- Okamoto, H.; Schleesinger, M.E.; Mueller, E.M. Alloy Phase Diagrams, Revised ed.; ASM International: Novelty, OH, USA, 2016; Volume 3. [Google Scholar]
- Ren, P.; Zhu, S.; Wang, F. Spontaneous Reaction Formation of Cr23C6 Diffusion Barrier Layer between Nanocrystalline MCrAlY Coating and Ni-Base Superalloy at High Temperature. Corros. Sci. 2015, 99, 219–226. [Google Scholar] [CrossRef]
- Toscano, J.; Gil, A.; Hüttel, T.; Wessel, E.; Naumenko, D.; Singheiser, L.; Quadakkers, W.J. Temperature Dependence of Phase Relationships in Different Types of MCrAlY-Coatings. Surf. Coat. Technol. 2007, 202, 603–607. [Google Scholar] [CrossRef]
- Grabke, H.J. Oxidation of NiAl and FeAl. Intermetallics 1999, 7, 1153–1158. [Google Scholar] [CrossRef]
- Sharma, K.; Chatha, S.; Sidhu, H.; Singh, H. Heat Treatment of Thermal Spray Coatings: A Review. In Proceedings of the National Conference on Advancements and Futuristic Trends in Mechanical and Materials Engineering, Jalandhar, India, 7–8 October 2011. [Google Scholar]
- Babu, P.S.; Sen, D.; Jyothirmayi, A.; Krishna, L.R.; Rao, D.S. Influence of Microstructure on the Wear and Corrosion Behavior of Detonation Sprayed Cr2O3-Al2O3 and Plasma Sprayed Cr2O3 Coatings. Ceram. Int. 2018, 44, 2351–2357. [Google Scholar] [CrossRef]
- Lesage, J.; Staia, M.H.; Chicot, D.; Godoy, C.; De Miranda, P.E.V. Effect of Thermal Treatments on Adhesive Properties of a NiCr Thermal Sprayed Coating. Thin Solid Films 2000, 377–378, 681–686. [Google Scholar] [CrossRef]
- Jang, H.-J.; Park, D.-H.; Jung, Y.-G.; Jang, J.-C.; Choi, S.-C.; Paik, U. Mechanical Characterization and Thermal Behavior of HVOF-Sprayed Bond Coat in Thermal Barrier Coatings (TBCs). Surf. Coat. Technol. 2006, 200, 4355–4362. [Google Scholar] [CrossRef]
- Ghasemi, R.; Vakilifard, H. Plasma-Sprayed Nanostructured YSZ Thermal Barrier Coatings: Thermal Insulation Capa-bility and Adhesion Strength. Ceram. Int. 2017, 43, 8556–8563. [Google Scholar] [CrossRef]
- Mahade, S.; Björklund, S.; Govindarajan, S.; Olsson, M.; Joshi, S. Novel Wear Resistant Carbide-Laden Coatings Depos-ited by Powder-Suspension Hybrid Plasma Spray: Characterization and Testing. Surf. Coat. Technol. 2020, 399, 126147. [Google Scholar] [CrossRef]
- Saeidi, S.; Voisey, K.T.; McCartney, D.G. Mechanical Properties and Microstructure of VPS and HVOF CoNiCrAlY Coatings. J. Therm. Spray Technol. 2011, 20, 1231–1243. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiryc, M.; Kazamer, N.; Kurumlu, D.; Marginean, G. Comparative Study on the Thermal Performance of Cr-CrxOy and YSZ-CoNiCrAlY Coatings Exposed at 900 °C. Materials 2021, 14, 6040. https://doi.org/10.3390/ma14206040
Kiryc M, Kazamer N, Kurumlu D, Marginean G. Comparative Study on the Thermal Performance of Cr-CrxOy and YSZ-CoNiCrAlY Coatings Exposed at 900 °C. Materials. 2021; 14(20):6040. https://doi.org/10.3390/ma14206040
Chicago/Turabian StyleKiryc, Markus, Norbert Kazamer, Deniz Kurumlu, and Gabriela Marginean. 2021. "Comparative Study on the Thermal Performance of Cr-CrxOy and YSZ-CoNiCrAlY Coatings Exposed at 900 °C" Materials 14, no. 20: 6040. https://doi.org/10.3390/ma14206040
APA StyleKiryc, M., Kazamer, N., Kurumlu, D., & Marginean, G. (2021). Comparative Study on the Thermal Performance of Cr-CrxOy and YSZ-CoNiCrAlY Coatings Exposed at 900 °C. Materials, 14(20), 6040. https://doi.org/10.3390/ma14206040








