Stress Corrosion Cracking of Additively Manufactured Alloy 625
Abstract
:1. Introduction
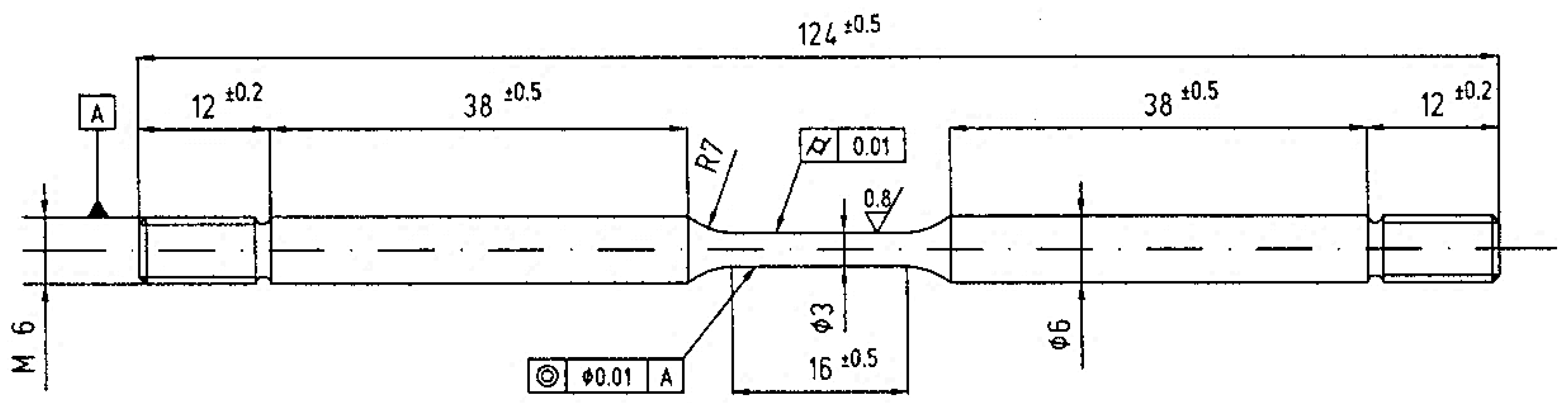
2. Materials and Methods
3. Results
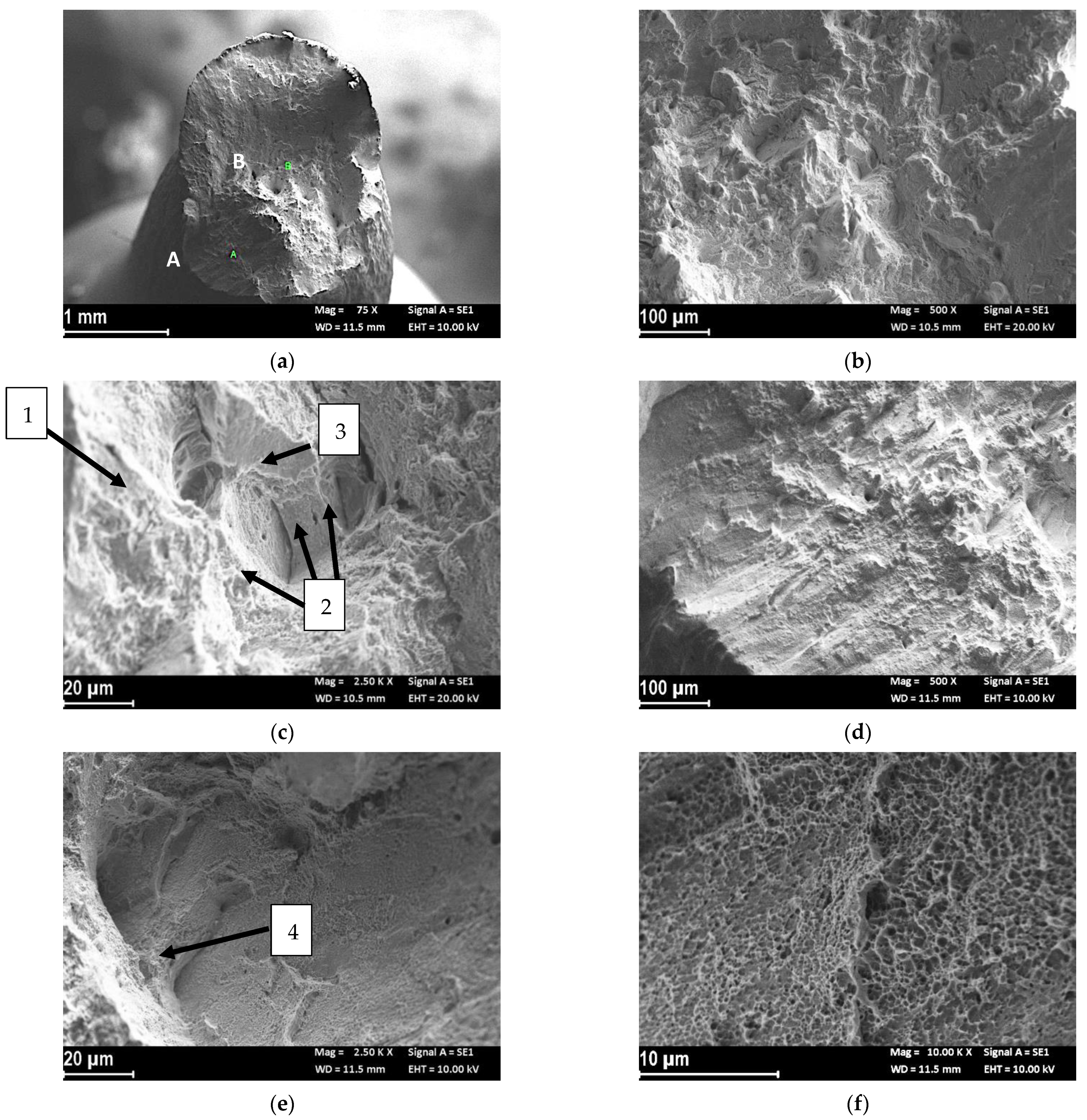
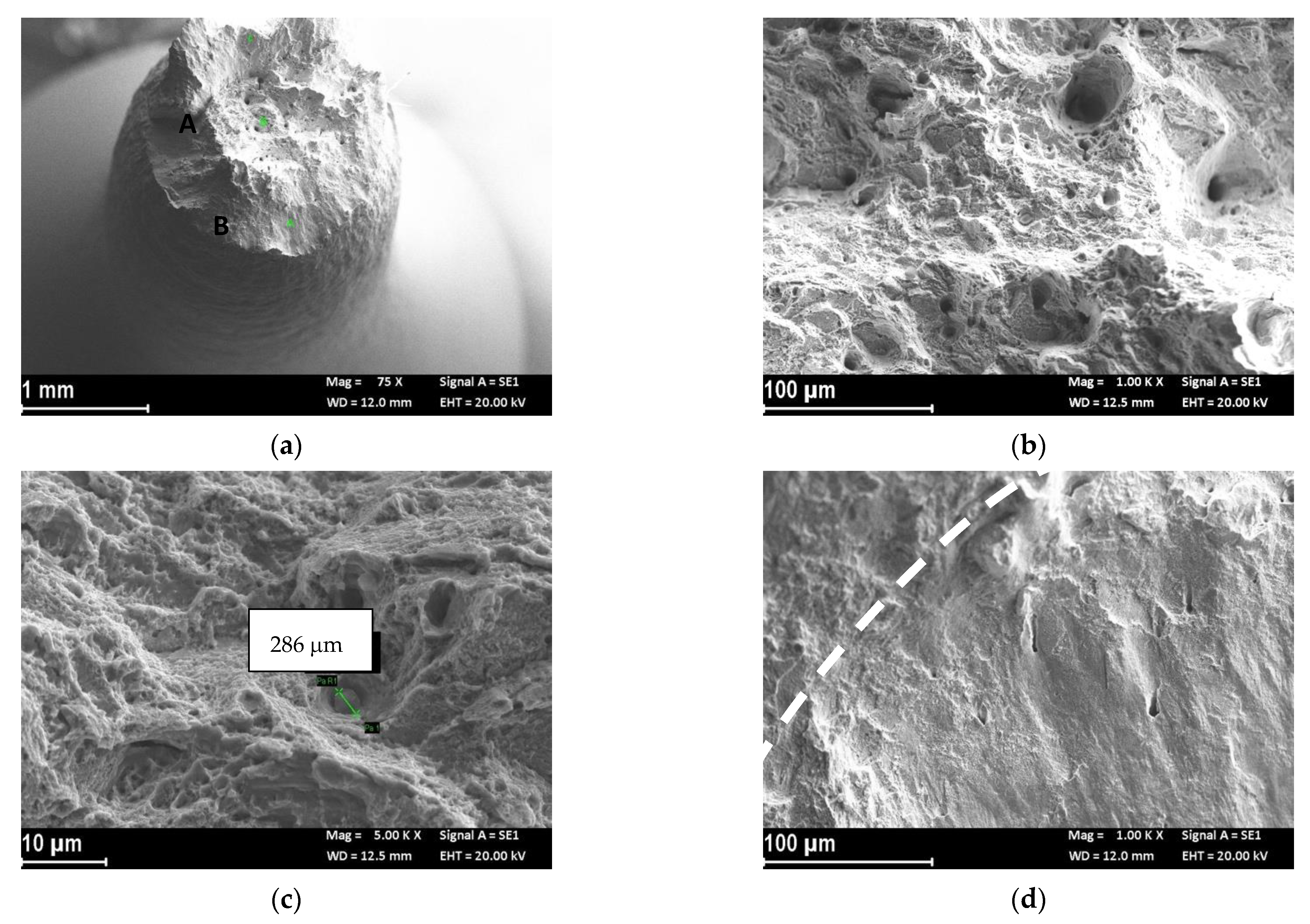
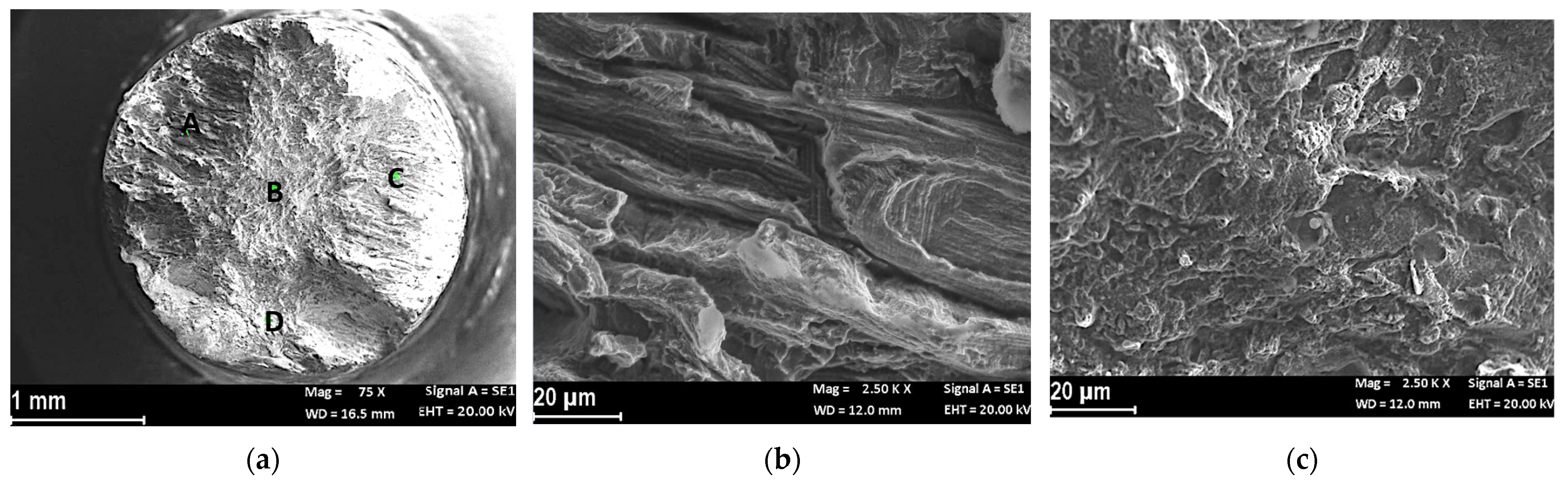
Fractographic Analysis
4. Discussion
5. Conclusions
- (1)
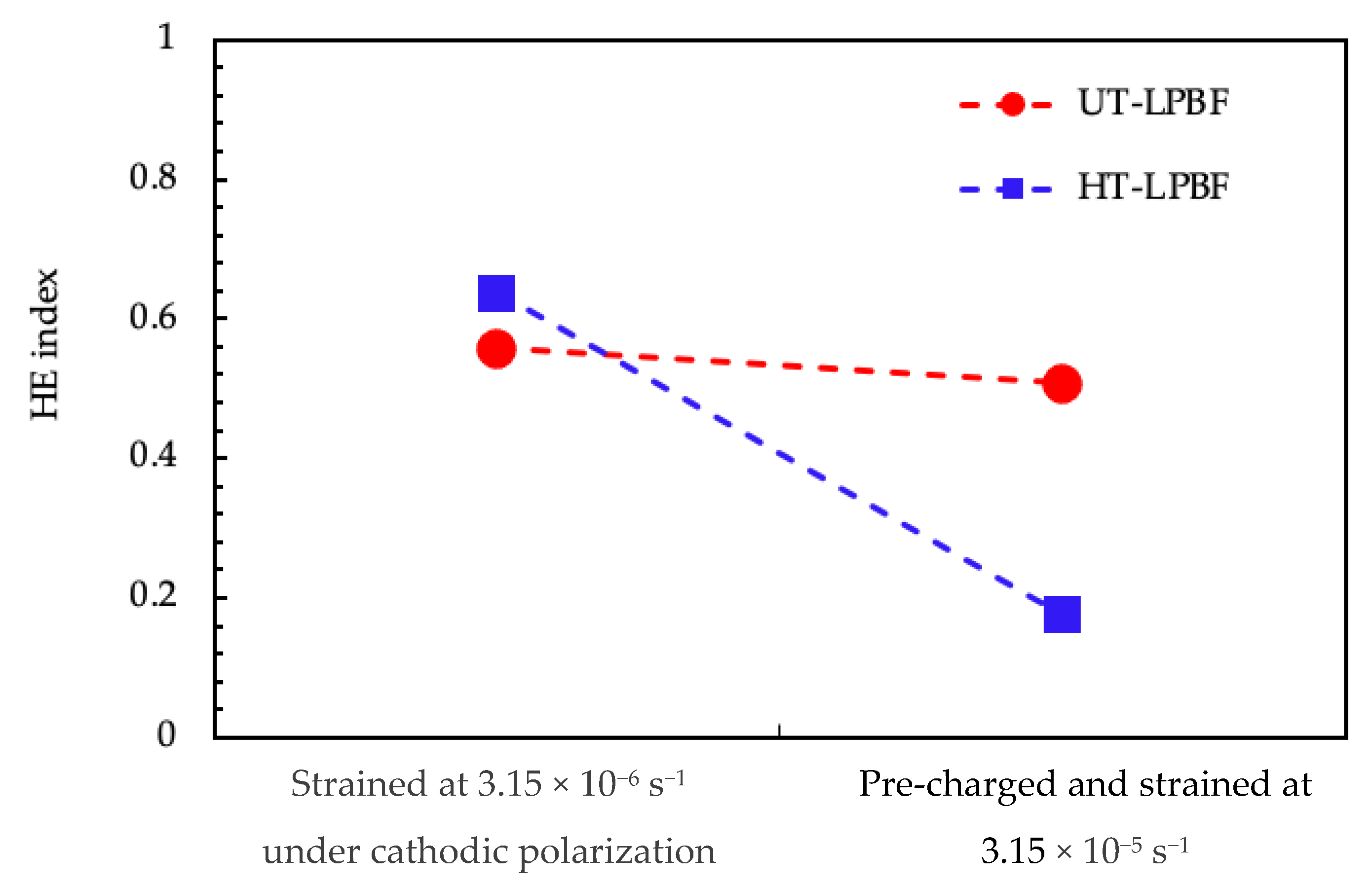
- Irrespective of the heat treatment, LPBF manufactured Alloy 625 was not susceptible to chlorides induced stress corrosion cracking;
- (2)
- The samples underwent hydrogen embrittlement when tested under slow strain rate tests in substitute ocean water when cathodically polarized at −1.1 V vs. Ag/AgCl. The HE effects were evident for the UT specimens both on a very low strain rate and at a higher strain rate on specimens pre-charged with hydrogen;
- (3)
- The HT specimens showed a similar behavior to the UT specimens in tests at the very low strain rate, but a good resistance in tests at the higher strain rate on hydrogen pre-charged specimens;
- (4)
- The different behavior of the heat-treated alloy was explained on the basis of the different microstructure: the very fine sub-grain structures, high concentration of dislocation and second phases increased the hydrogen diffusion and reversible trapping effect in the UT specimens;
- (5)
- It is postulated that the coalescence of precipitates during heat treatment increased their incoherency and made them irreversible traps for hydrogen that mitigated HE effects in the HT specimens.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frazier, W.E. Metal additive manufacturing: A review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Manfredi, D.; Calignano, F.; Krishnan, M.; Canali, R.; Ambrosio, E.P.; Atzeni, E. From powders to dense metal parts: Characterization of a commercial alsimg alloy processed through direct metal laser sintering. Materials (Basel) 2013, 6, 856–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Pavese, M.; Fino, P.; Ambrosio, E.P.; Calignano, F.; Manfredi, D. Corrosion resistance of direct metal laser sintering AlSiMg alloy. Surf. Interface Anal. 2016, 48, 818–826. [Google Scholar] [CrossRef]
- Mercelis, P.; Kruth, J. Residual stresses in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2006, 12, 254–265. [Google Scholar] [CrossRef]
- Zhang, D.; Niu, W.; Cao, X.; Liu, Z. Effect of standard heat treatment on the microstructure and mechanical properties of selective laser melting manufactured Inconel 718 superalloy. Mater. Sci. Eng. A 2015, 644, 32–40. [Google Scholar] [CrossRef]
- Trelewicz, J.R.; Halada, G.P.; Donaldson, O.K.; Manogharan, G. Microstructure and Corrosion Resistance of Laser Additively Manufactured 316L Stainless Steel. JOM 2016, 68, 850–859. [Google Scholar] [CrossRef]
- Cabrini, M.; Calignano, F.; Fino, P.; Lorenzi, S.; Lorusso, M.; Manfredi, D.; Testa, C.; Pastore, T.; Id, D.M.; Testa, C.; et al. Corrosion Behavior of Heat-Treated AlSi10Mg Manufactured by Laser Powder Bed Fusion. Materials (Basel) 2018, 11, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revilla, R.I.; Liang, J.; Godet, S.; De Graeve, I. Local Corrosion Behavior of Additive Manufactured AlSiMg Alloy Assessed by SEM and SKPFM. J. Electrochem. Soc. 2017, 164, C27–C35. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Testa, C.; Manfredi, D.; Lorusso, M.; Calignano, F.; Pavese, M.; Andreatta, F. Corrosion behavior of AlSi10Mg alloy produced by laser powder bed fusion under chloride exposure. Corros. Sci. 2019, 152, 101–108. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Testa, C.; Pastore, T.; Manfredi, D.; Lorusso, M.; Calignano, F.; Fino, P. Statistical approach for electrochemical evaluation of the effect of heat treatments on the corrosion resistance of AlSi10Mg alloy by laser powder bed fusion. Electrochim. Acta 2019, 305, 459–466. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Testa, C.; Manfredi, D.; Calignano, F.; Fino, P.; Cattano, G. Evaluation of corrosion resistance of aluminum silicon alloys manufactured by LPBF. LA Metall. Ital. 2017, 109, 120–124. [Google Scholar]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Testa, C.; Manfredi, D.; Cattano, G.; Calignano, F. Corrosion resistance in chloride solution of the AlSi10Mg alloy obtained by means of LPBF. Surf. Interface Anal. 2019, 51, 1159–1164. [Google Scholar] [CrossRef]
- Schmidt, D.P.; Jelis, E.; Clemente, M.P.; Ravindra, N.M. Corrosion of 3D printed steel. Mater. Sci. Technol. Conf. Exhib. 2015 MS T 2015 2015, 1, 93–100. [Google Scholar]
- Puskar, T.; Jevremovic, D.; Williams, R.J.; Eggbeer, D.; Vukelic, D.; Budak, I. A comparative analysis of the corrosive effect of artificial saliva of variable pH on DMLS and cast Co-Cr-Mo dental alloy. Materials (Basel) 2014, 6, 6486–6501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuna, S.H.; Özçiçek Pekmez, N.; Kürkçüoğlu, I. Corrosion resistance assessment of Co-Cr alloy frameworks fabricated by CAD/CAM milling, laser sintering, and casting methods. J. Prosthet. Dent. 2015, 114, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Ziętala, M.; Durejko, T.; Polański, M.; Kunce, I.; Płociński, T.; Zieliński, W.; Łazińska, M.; Stępniowski, W.; Czujko, T.; Kurzydłowski, K.J.; et al. The microstructure, mechanical properties and corrosion resistance of 316L stainless steel fabricated using laser engineered net shaping. Mater. Sci. Eng. A 2016, 677, 1–10. [Google Scholar] [CrossRef]
- De Damborenea, J.J.; Arenas, M.A.; Larosa, M.A.; Jardini, A.L.; de Carvalho Zavaglia, C.A.; Conde, A. Corrosion of Ti6Al4V pins produced by direct metal laser sintering. Appl. Surf. Sci. 2017, 393, 340–347. [Google Scholar] [CrossRef]
- Koike, M.; Martinez, K.; Guo, L.; Chahine, G.; Kovacevic, R.; Okabe, T. Evaluation of titanium alloy fabricated using electron beam melting system for dental applications. J. Mater. Process. Technol. 2011, 211, 1400–1408. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Testa, C.; Manfredi, D.; Ambrosio, E.P.; Calignano, F.; Lorusso, M.; Fino, P. Studio della resistenza alla corrosione della lega AlSi10Mg ottenuta per additive manufacturing in soluzione di cloruri (Analisys of corrosion resistance of the AlSi10Mg alloy obtained by additive manufacturing in chloride solution). Metall. Ital. 2016, 108, 137–146. [Google Scholar]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Testa, C.; Manfredi, D.; Calignano, F.; Lorusso, M.; Fino, P. Corrosion behavior of aluminum-silicon alloys obtained by Direct Metal Laser Sintering. In Proceedings of the EUROCORR 2017—The Annual Congress of the European Federation of Corrosion, 20th International Corrosion Congress and Process Safety Congress 2017, Prague, Czech Republic, 3–7 September 2017. [Google Scholar]
- Shankar, V.; Bhanu Sankara Rao, K.; Mannan, S. Microstructure and mechanical properties of Inconel 625 superalloy. J. Nucl. Mater. 2001, 288, 222–232. [Google Scholar] [CrossRef]
- Ezugwu, E.O. Key improvements in the machining of difficult-to-cut aerospace superalloys. Int. J. Mach. Tools Manuf. 2005, 45, 1353–1367. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Testa, C.; Brevi, F.; Biamino, S.; Fino, P.; Manfredi, D.; Marchese, G.; Calignano, F.; Pastore, T.; et al. Microstructure and Selective Corrosion of Alloy 625 Obtained by Means of Laser Powder Bed Fusion. Materials 2019, 12, 1742. [Google Scholar] [CrossRef] [Green Version]
- Cabrini, M.; Lorenzi, S.; Testa, C.; Pastore, T.; Brevi, F.; Biamino, S.; Fino, P.; Manfredi, D.; Marchese, G.; Calignano, F.; et al. Evaluation of Corrosion Resistance of Alloy 625 Obtained by Laser Powder Bed Fusion. J. Electrochem. Soc. 2019, 166, C3399–C3408. [Google Scholar] [CrossRef]
- Brown, M.H. The Relationship of Heat Treatment to the Corrosion Resistance of Stainless Alloys. CORROSION 1969, 25, 438–443. [Google Scholar] [CrossRef]
- Adnyana, D.N. Stress Corrosion Cracking in a Nickel-base alloy pre-heater expansion bellows. Majalah Metalurgi 2014, 29, 235–244. [Google Scholar]
- Was, G.S.; Ampornrat, P.; Gupta, G.; Teysseyre, S.; West, E.A.; Allen, T.R.; Sridharan, K.; Tan, L.; Chen, Y.; Ren, X.; et al. Corrosion and stress corrosion cracking in supercritical water. J. Nucl. Mater. 2007, 371, 176–201. [Google Scholar] [CrossRef]
- Mucci, J.; Harris, J.A. Influence of Gaseous Hydrogen on the Mechanical Properties of High Temperature Alloys; Final Report Contract FR-7746, NASA-CR-149962. 1976. Available online: https://ntrs.nasa.gov/citations/19760022312 (accessed on 7 August 2021).
- Harris, J.; van Wanderham, M.C. Properties of Materials in High Pressure Hydrogen At Cryogenic, Room, and Elevated Temperatures; Final Report Contract FR-5768, NASA-CR-26191. 1973. Available online: https://ntrs.nasa.gov/api/citations/19730023072/downloads/19730023072.pdf (accessed on 7 August 2021).
- Murakami, K.; Yabe, N.; Suzuki, H.; Takai, K.; Hagihara, Y.; Wada, Y. Substitution of high-pressure charge by electrolysis charge and hydrogen environment embrittlement susceptibilities for inconel 625 and SUS 316L. In Proceedings of the American Society of Mechanical Engineers, Pressure Vessels and Piping Division (Publication) PVP, Vancouver, BC, Canada, 23–17 July 2006; Volume 2006. [Google Scholar]
- Kane, R.D. Accelerated Hydrogen Charging of Nickel and Cobalt Base Alloys. CORROSION 1978, 34, 442–445. [Google Scholar] [CrossRef]
- Hicks, P.D.; Altstetter, C.J. Internal hydrogen effects on tensile properties of iron- and nickel-base superalloys. Metall. Trans. A 1990, 21, 365–372. [Google Scholar] [CrossRef]
- Chandler, H. ASM Handbook: Heat Treater’s Guide-Practices and Procedures for Nonferrous Alloys; ASM International: Geauga County, OH, USA, 1996; ISBN 0871705656. [Google Scholar]
- Stenerud, G.; Johnsen, R.; Olsen, J.S. Susceptibility to hydrogen Induced Stress Cracking of UNS N07718 and UNS N07725 under cathodic protection. In Proceedings of the Corrosion NACE, Dallas, TX, USA, 15–19 March 2015. [Google Scholar]
- Kagay, B.; Findley, K.; Coryell, S. Comparison of Slow Strain Rate, Incremental Step Load and Riding Displacement Hydrogen Embrittlement Testing of UNS N07718. In Proceedings of the Nace Corrosion, Phoenix, AZ, USA, 15–19 April 2018. [Google Scholar]
- Huang, W.; Sun, W.; Samson, A.; Muise, D.; Haarseth, C. Investigation of Hydrogen Embrittlement Susceptibility of Precipitation Hardened Nickel Alloys under Cathodic Protection Condition. In Proceedings of the Corrosion NACE 2014, San Antonio, TX, USA, 9–13 March 2014. [Google Scholar]
- Martelo, D.F.; Morana, R.; Akid, R. Understanding the mechanical behaviour of 718 and 625 + nickel based super-alloys under cathodic polarization. Theoret. Appl. Fracture Mech. 2021, 112, 102871. [Google Scholar] [CrossRef]
- Botinha, J.; Gehrmann, B.; Alves, H. Influence of the Hardening Phases on the Hydrogen Embrittlement Susceptibility of Ni-Alloys based on the UNS7718. In Proceedings of the NACE Corrosion, Houston, TX, USA, 14–18 June 2020. [Google Scholar]
- Marchese, G.; Lorusso, M.; Parizia, S.; Bassini, E.; Lee, J.W.; Calignano, F.; Manfredi, D.; Terner, M.; Hong, H.U.; Ugues, D.; et al. Influence of heat treatments on microstructure evolution and mechanical properties of Inconel 625 processed by laser powder bed fusion. Mater. Sci. Eng. A 2018, 729, 64–75. [Google Scholar] [CrossRef]
- Casale, I.B. Boiling Temperatures of MgCl2 Solutions—Their Application in Stress Corrosion Studies. Corrosion 1967, 23, 314–317. [Google Scholar] [CrossRef]
- Rebak, R.B. Stress corrosion cracking (SCC) of nickel-based alloys. In Stress Corrosion Cracking; Elsevier: Amsterdam, The Netherlands, 2011; pp. 273–306. [Google Scholar]
- Marchese, G.; Parizia, S.; Rashidi, M.; Saboori, A.; Manfredi, D.; Ugues, D.; Lombardi, M.; Hryha, E.; Biamino, S. The role of texturing and microstructure evolution on the tensile behavior of heat-treated Inconel 625 produced via laser powder bed fusion. Mater. Sci. Eng. A 2020, 769, 138500. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Thivillon, L.; Bertrand, P.; Smurov, I. Strategy of manufacturing components with designed internal structure by selective laser melting of metallic powder. Appl. Surf. Sci. 2007, 254, 980–983. [Google Scholar] [CrossRef]
- Kashaev, N.; Horstmann, M.; Ventzke, V.; Riekehr, S.; Huber, N. Comparative study of mechanical properties using standard and micro-specimens of base materials Inconel 625, Inconel 718 and Ti-6Al-4V. J. Mat. Res. Tech. 2013, 2, 43–47. [Google Scholar]
- Brown, C.U.; Jacob, G.; Stoudt, M.; Moylan, S.; Slotwinski, J.; Donmez, A. Interlaboratory Study for Nickel Alloy 625 Made by Laser Powder Bed Fusion to Quantify Mechanical Property Variability. J. Mater. Eng. Perform. 2016, 25, 3390–3397. [Google Scholar] [CrossRef]
- Li, C.; White, R.; Fang, X.Y.; Weaver, M.; Guo, Y.B. Microstructure evolution characteristics of Inconel 625 alloy from selective laser melting to heat treatment. Mater. Sci. Eng. A 2017, 705, 20–31. [Google Scholar] [CrossRef]
- Chêne, J.; Brass, A.M. Hydrogen transport by mobile dislocations in nickel base superalloy single crystals. Scr. Mater. 1999, 40, 537–542. [Google Scholar] [CrossRef]
- Boniszewski, T.; Smith, G.C. The influence of hydrogen on the plastic deformation ductility, and fracture of nickel in tension. Acta Metall. 1963, 11, 165–178. [Google Scholar] [CrossRef]
- Bruemmer, S.M.; Jones, R.H.; Thomas, M.T.; Baer, D.R. Influence of sulfur, phosphorus, and antimony segregation on the intergranular hydrogen embrittlement of nickel. Metall. Trans. A 1983, 14, 223–232. [Google Scholar] [CrossRef]
- Latanision, R.M.; Opperhauser, H. Further observations on the effect of grain boundary segregation in the hydrogen embrittlement of nickel. Metall. Trans. A 1975, 6, 233–234. [Google Scholar] [CrossRef]
- Berkowitz, B.J.; Kane, R.D. Effect of Impurity Segregation on the Hydrogen Embrittlement of a High Strength Nickel Base Alloy in H2S Environments. Corrosion 1980, 36, 24–29. [Google Scholar] [CrossRef]
- Fiore, N.F.; Kargol, J.A. Hydrogen-related embrittlement of Ni-base superalloys. In Proceedings of the Third International Conference on Effect of Hydrogen on Behavior of Materials, Moran, Wyoming, 26–31 August 1980; Bernstein, I.M., Thompson, A.W., Metallurgical Society of AIME, Physical Metallurgy Committee, Eds.; Metallurgical Society of AIME: Warrendale, PA, USA, 1981; pp. 851–862. [Google Scholar]
- Jothi, S.; Croft, T.N.; Wright, L.; Turnbull, A.; Brown, S.G.R. Multi-phase modelling of intergranular hydrogen segregation/trapping for hydrogen embrittlement. Int. J. Hydrogen Energy 2015, 40, 15105–15123. [Google Scholar] [CrossRef] [Green Version]
- Harris, T.M.; Latanision, M. Grain boundary diffusion of hydrogen in nickel. Metall. Trans. A 1991, 22, 351–355. [Google Scholar] [CrossRef]
- Brass, A.M.; Roux, D.; Chêne, J. Role of secondary γ′ precipitation and of hydrogen in the first stage of the plastic deformation of the υ matrix of a Ni base superalloy single crystal. Mater. Sci. Eng. A 2002, 323, 97–102. [Google Scholar] [CrossRef]



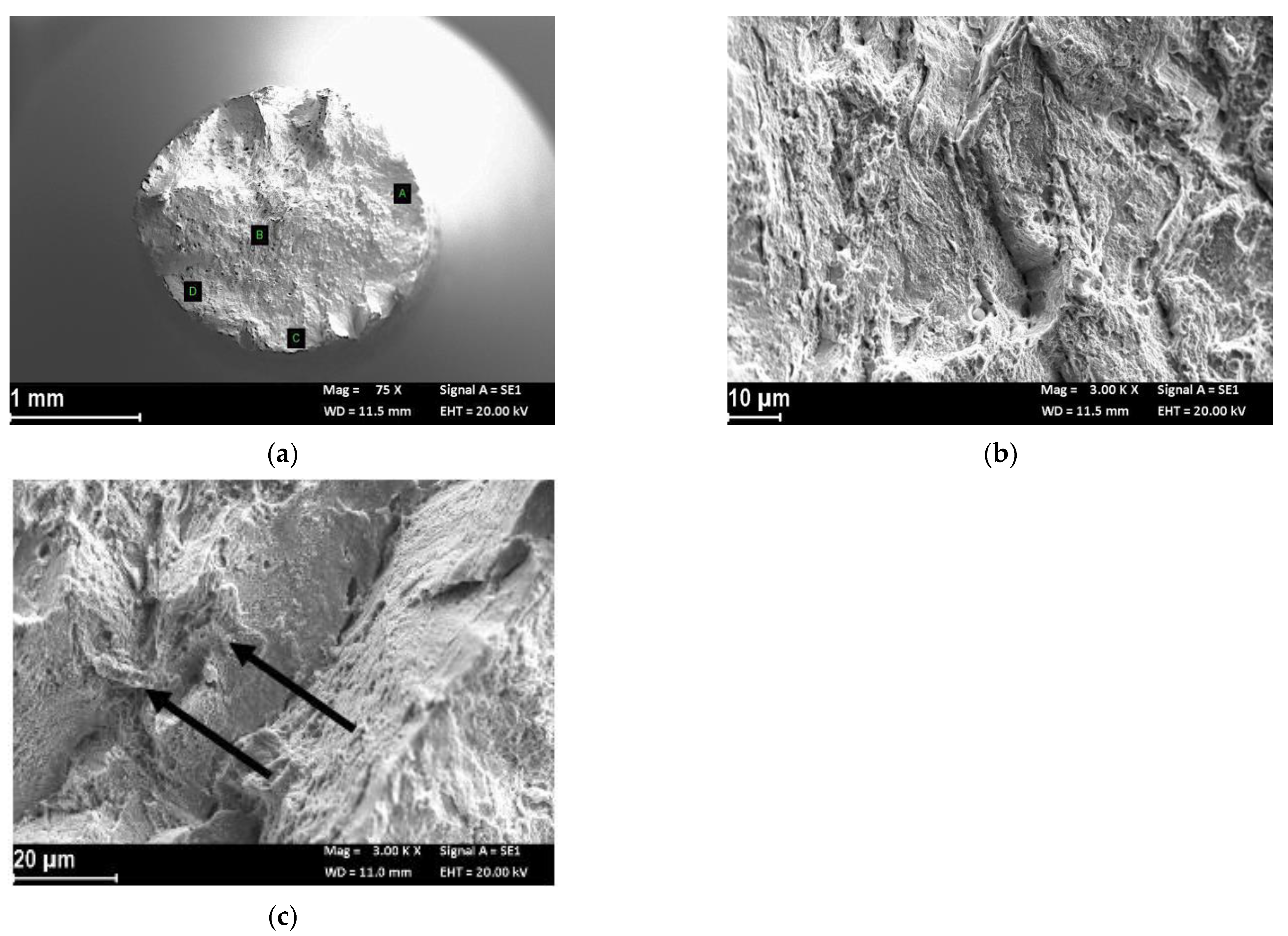
| Element (%wt) | C | Si | Mn | P | S | Cr | Mo | Ni | Nb | Ti | Al | Co | Ta | Fe | Nb + Ta |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPBF powder | 0.013 | 0.1 | 0.03 | <0.001 | 0.002 | 22.8 | 8.1 | Bal | 3.66 | 0.17 | <0.01 | 0.17 | 0.13 | 0.43 | 3.79 |
| LPBF metal | 0.01 | 0.08 | 0.03 | <0.001 | 0.002 | 22.4 | 8.2 | Bal | 3.73 | 0.18 | <0.01 | 0.17 | 0.13 | 0.45 | 3.86 |
| Specimen | Temperature [°C] | Building Direction | Initial Weight * [g] | Final Weight * [g] | ΔM [g] | Crack |
|---|---|---|---|---|---|---|
| U-bend LPBF UT | 155 | XY | 29.80806 | 29.80671 | 0.00134 | NO |
| Z | 27.21266 | 27.20922 | 0.00344 | NO | ||
| U-bend LPBF UT | 170 | XY | 27.16713 | 27.16435 | 0.00278 | NO |
| 26.90112 | 26.89606 | 0.00507 | NO | |||
| Z | 31.46677 | 31.46087 | 0.00591 | NO | ||
| 31.46682 | 31.46087 | 0.00596 | NO | |||
| U-bend LPBF HT 980 °C | 170 | XY | 27.85226 | 27.84929 | 0.00297 | NO |
| 28.27807 | 28.27352 | 0.00455 | NO | |||
| Z | 28.59065 | 28.58455 | 0.00610 | NO | ||
| 28.76252 | 28.58455 | 0.17797 | NO |
| Specimens | Test | Strain Rate (s−1) | σmax (MPa) | σmax env/ σmax air | εmax (%) | RA (%) | HE index (%) |
|---|---|---|---|---|---|---|---|
| LPBF-UT | Air | 3.15 × 10−6 | 1018 | - | 32 | 61 | - |
| SOW E = −1.1 V vs. Ag/AgCl | 3.15 × 10−6 | 995 | 0.98 | 25 | 27 | 56 | |
| 16 h pre-charging in SOW E = −1.1 V vs. Ag/AgCl than test in the same solution | 3.15 × 10−5 | 985 | 0.97 | 26 | 30 | 51 | |
| LPBF-HT | Air | 3.15 × 10−6 | 953 | - | 41 | 61 | - |
| SOW E = −1.1 V vs. Ag/AgCl | 3.15 × 10−6 | 894 | 0.94 | 19 | 22 | 64 | |
| 16 h pre-charging in SOW E = −1.1 V vs. Ag/AgCl than test in the same solution | 3.15 × 10−5 | 874 | 0.92 | 38 | 50 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrini, M.; Lorenzi, S.; Testa, C.; Carugo, F.; Pastore, T.; Manfredi, D.; Biamino, S.; Marchese, G.; Parizia, S.; Scenini, F. Stress Corrosion Cracking of Additively Manufactured Alloy 625. Materials 2021, 14, 6115. https://doi.org/10.3390/ma14206115
Cabrini M, Lorenzi S, Testa C, Carugo F, Pastore T, Manfredi D, Biamino S, Marchese G, Parizia S, Scenini F. Stress Corrosion Cracking of Additively Manufactured Alloy 625. Materials. 2021; 14(20):6115. https://doi.org/10.3390/ma14206115
Chicago/Turabian StyleCabrini, Marina, Sergio Lorenzi, Cristian Testa, Francesco Carugo, Tommaso Pastore, Diego Manfredi, Sara Biamino, Giulio Marchese, Simone Parizia, and Fabio Scenini. 2021. "Stress Corrosion Cracking of Additively Manufactured Alloy 625" Materials 14, no. 20: 6115. https://doi.org/10.3390/ma14206115
APA StyleCabrini, M., Lorenzi, S., Testa, C., Carugo, F., Pastore, T., Manfredi, D., Biamino, S., Marchese, G., Parizia, S., & Scenini, F. (2021). Stress Corrosion Cracking of Additively Manufactured Alloy 625. Materials, 14(20), 6115. https://doi.org/10.3390/ma14206115











