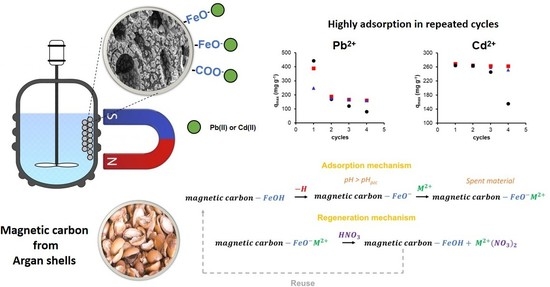
Synthesis of Magnetic Adsorbents Based Carbon Highly Efficient and Stable for Use in the Removal of Pb(II) and Cd(II) in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Activated Carbon Impregnated with Magnetite and Cobalt Ferrite: Magnetic Adsorbents
2.2. Physicochemical Characterization of the Magnetic Adsorbents
2.3. Adsorption Studies of Metal Cations in Aqueous Solutions
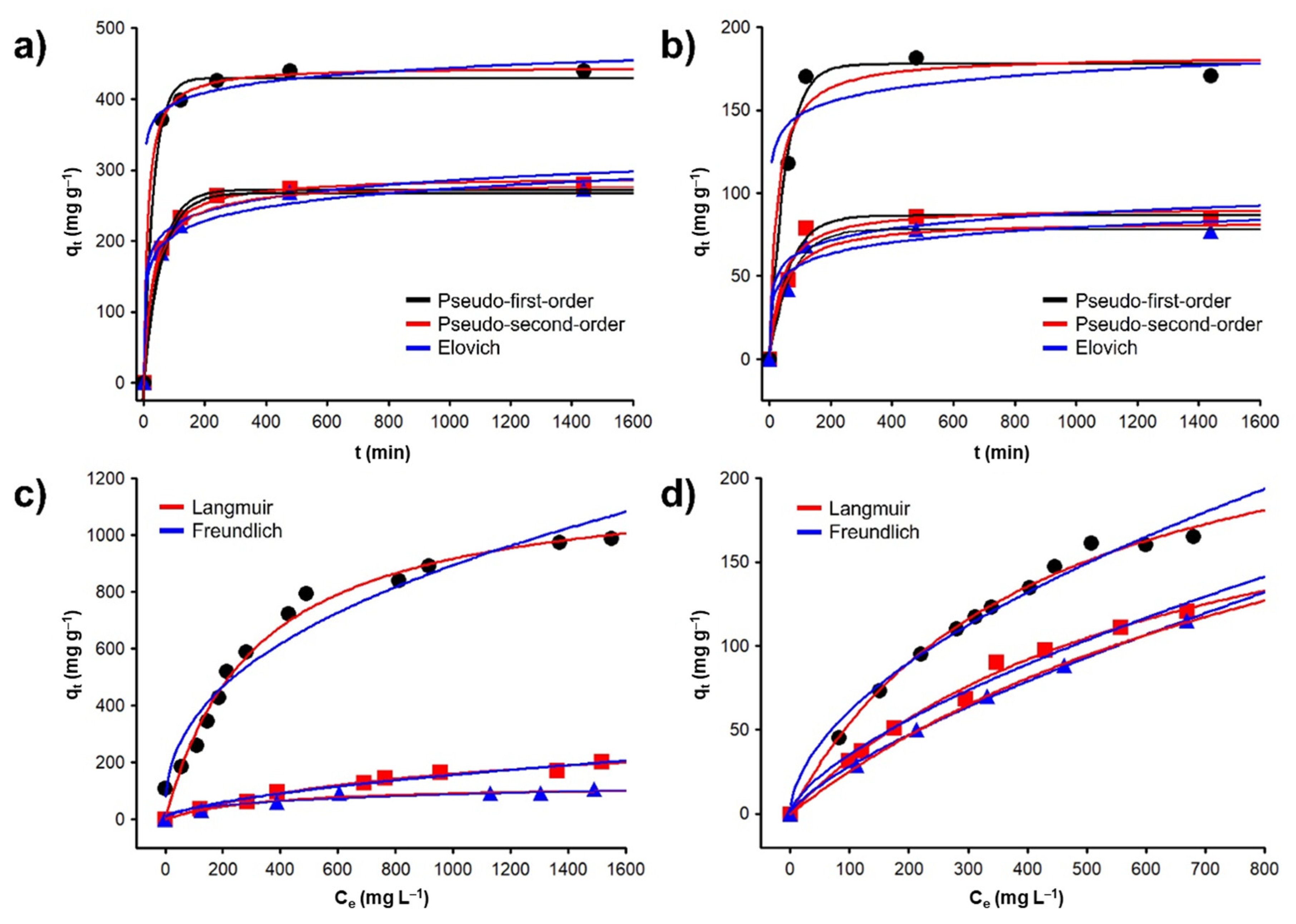
2.3.1. Adsorption Kinetics
2.3.2. Adsorption Equilibrium
2.3.3. Reuse of the Materials (Adsorption—Desorption Studies)
2.4. Statistical Analysis
3. Results and Discussion
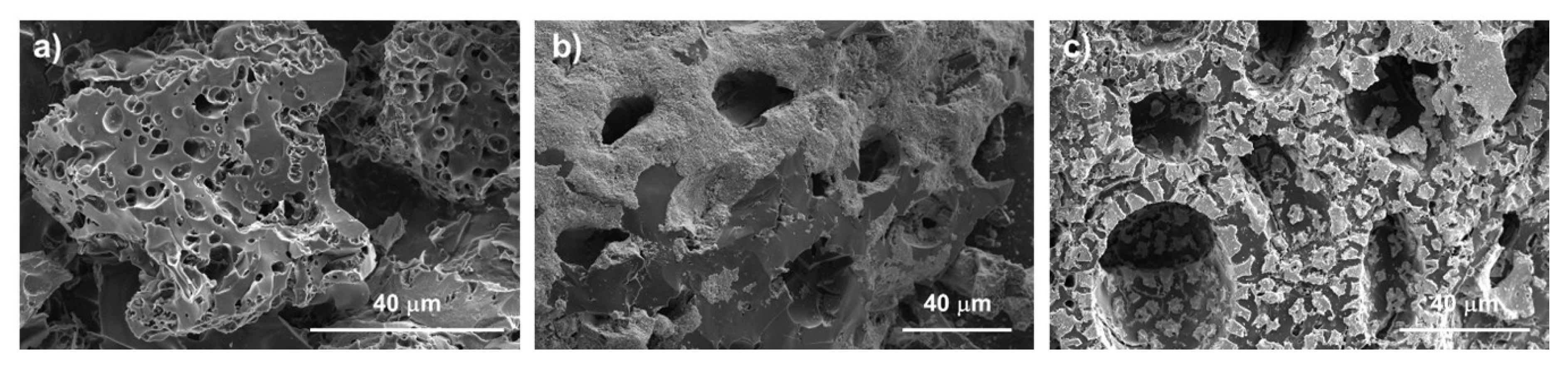
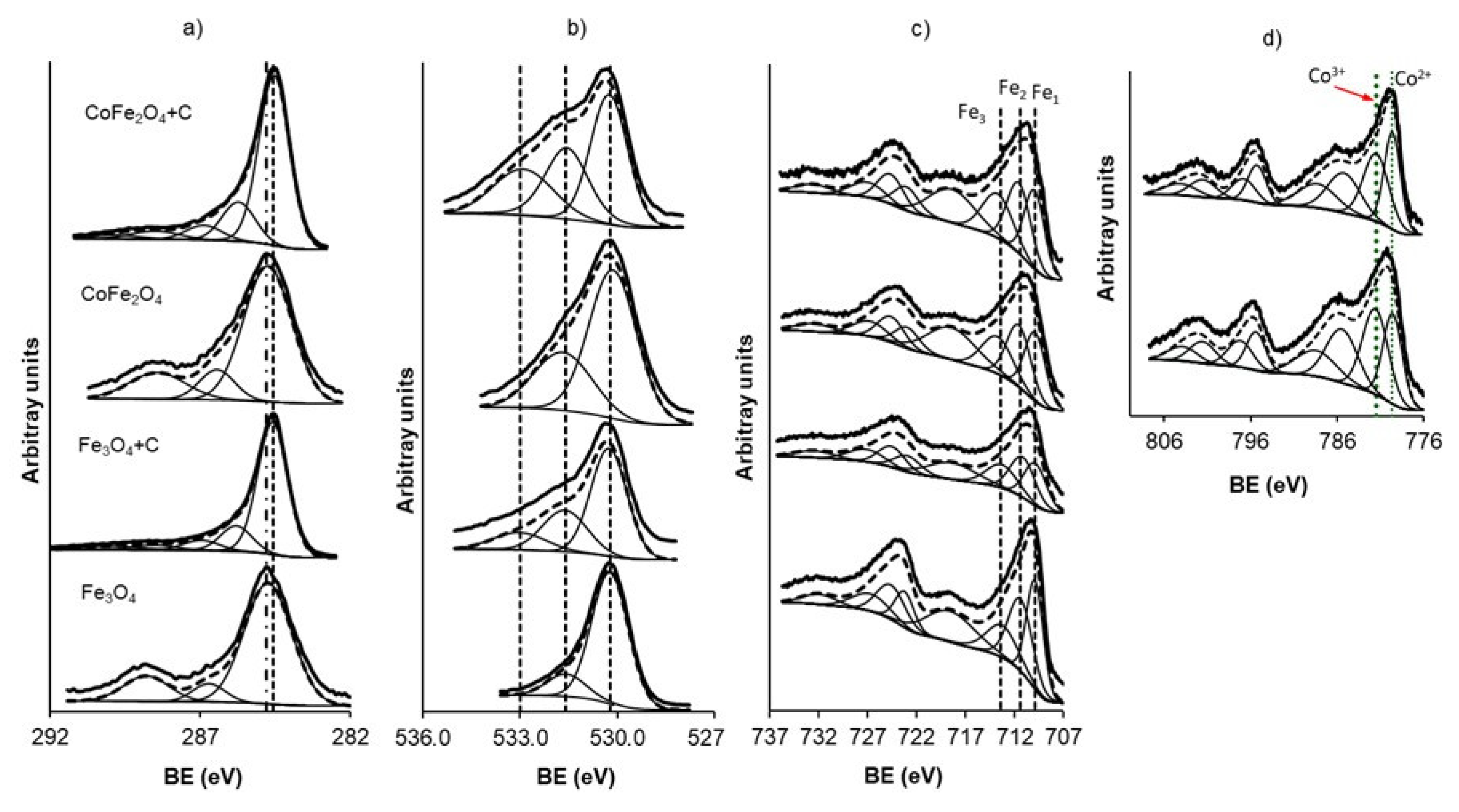
3.1. Physicochemical Characterization of the Magnetic Adsorbents
3.2. Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kołodyńska, D.; Wnetrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Liu, C.; Wu, T.; Hsu, P.C.; Xie, J.; Zhao, J.; Liu, K.; Sun, J.; Xu, J.; Tang, J.; Ye, Z.; et al. Direct/alternating current electrochemical method for removing and recovering heavy metal from water using graphene oxide electrode. ACS Nano 2019, 13, 6431–6437. [Google Scholar] [CrossRef]
- Bora, A.J.; Dutta, R.K. Removal of metals (Pb, Cd, Cu, Cr, Ni, and Co)from drinking water by oxidation-coagulation-absorption at optimized pH. J. Water Process. Eng. 2019, 31, 100839. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, A.; Kadeche, A.; Adjdir, M.; Taleb, Z.; Ikhou, D.; Taleb, S.; Deratani, A. Lead and cadmium removal by adsorption process using hydroxyapatite porous materials. Water Pract. Technol. 2020, 15, 130–141. [Google Scholar] [CrossRef]
- Kongsuwan, A.; Patnukao, P.; Pavasant, P. Binary component sorption of Cu(II) and Pb(II) with activated carbon from Eucalyptus camaldulensis Dehn bark. J. Ind. Eng. Chem. 2009, 15, 465–470. [Google Scholar] [CrossRef]
- El-ashtoukhy, E.Z.; Amin, N.K.; Abdelwahab, O. Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent. Desalination 2008, 223, 162–173. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.N.; Mohanty, C.R.; Meikap, B.C. Removal of lead (II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Anitha, K.; Namsani, S.; Singh, J.K. Removal of Heavy Metal Ions Using a Functionalized Single-Walled Carbon Nanotube: A Molecular Dynamics Study. J. Phys. Chem. A 2015, 119, 8349–8358. [Google Scholar] [CrossRef]
- Li, Y.; Ding, J.; Luan, Z.; Di, Z.; Zhu, Y.; Xu, C. Competitive adsorption of Pb 2, Cu 2 and Cd 2 ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Stafiej, A.; Pyrzynska, K. Solid phase extraction of metal ions using carbon nanotubes. Microchem. J. 2008, 89, 29–33. [Google Scholar] [CrossRef]
- Deng, X.; Lü, L.; Li, H.; Luo, F. The adsorption properties of Pb (II) and Cd (II) on functionalized graphene prepared by electrolysis method. J. Hazard. Mater. 2010, 183, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Yuan, T.; Sun, R. A lignosulfonate-modified graphene hydrogel with ultrahigh adsorption capacity for Pb(ii) removal. J. Mater. Chem. A 2016, 11888–11896. [Google Scholar] [CrossRef]
- Samonin, V.V.; Nikonova, V.Y.; Podvyaznikov, M.L. Carbon Adsorbents on the Basis of the Hydrolytic Lignin Modi fi ed with Fullerenes in Producing. Russ. J. Appl. Chem. 2014, 87, 190–193. [Google Scholar] [CrossRef]
- Hur, J.; Shin, J.; Yoo, J.; Seo, Y. Competitive Adsorption of Metals onto Magnetic Graphene Oxide: Comparison with Other Carbonaceous Adsorbents. Sci. World J. 2015, 2015, 836287. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and application of magnetic biochar in water treatment: A critical review. Sci. Total Environ. 2020, 711, 134847. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Wan, Y.; Wang, S.; Gao, B. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yu, K.; Wan, C.; Zhu, J.; Li, X.; Tong, S.; Zhao, Y. Comparison of the nickel addition patterns on the catalytic performances of LaCoO3 for low-temperature CO oxidation. Catal. Today 2017, 281, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Preparation of magnetic porous carbon from waste hydrochar by simultaneous activation and magnetization for tetracycline removal. Bioresour. Technol. 2014, 154, 209–214. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Wang, H.; Li, X.; Cheng, J.J.; Wu, W. Improving methane yield from organic fraction of municipal solid waste (OFMSW) with magnetic rice-straw biochar. Bioresour. Technol. 2017, 245, 1058–1066. [Google Scholar] [CrossRef]
- Liu, S.; Li, M.; Liu, Y.; Liu, N.; Tan, X.; Jiang, L.; Wen, J.; Hu, X.; Yin, Z. Removal of 17β-estradiol from aqueous solution by graphene oxide supported activated magnetic biochar: Adsorption behavior and mechanism. J. Taiwan Inst. Chem. Eng. 2019, 102, 330–339. [Google Scholar] [CrossRef]
- Benjedim, S.; Romero-Cano, L.A.; Pérez-Cadenas, A.F.; Bautista-Toledo, M.I.; Lotfi, E.M.; Carrasco-Marín, F. Removal of emerging pollutants present in water using an E-coli biofilm supported onto activated carbons prepared from argan wastes: Adsorption studies in batch and fixed bed. Sci. Total Environ. 2020, 720. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sáez, N.; Villela-Martinez, D.E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Pastrana-Martínez, L.M. Heteroatom-doped graphene aerogels and carbon-magnetite catalysts for the heterogeneous electro-Fenton degradation of acetaminophen in aqueous solution. J. Catal. 2019, 378, 68–79. [Google Scholar] [CrossRef]
- Kavitha, D. Adsorptive removal of phenol by thermally modified activated carbon: Equilibrium, kinetics and thermodynamics. J. Environ. Biotechnol. Res. 2016, 3, 24–34. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Zeldowitsch, J. Uber den mechanismus der katalytischen oxidation von CO an MnO2. Acta Physicochim. 1934, 1, 364–449. [Google Scholar]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Kapillarchemie. Kapillarchemie Akad. Verl. Ger. Leipzig 1909, 15, 948. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitor electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of Binding Energy Positions with C1s for XPS Results. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Abdelwahab, A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Carbon xerogels hydrothermally doped with bimetal oxides for oxygen reduction reaction. Materials 2019, 12, 2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biniak, S.; Szymansky, G.; Siedlewski, J.; Swiatkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon N. Y. 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Figueiredo, J.; Pereira, M.F.; Freitas, M.M.; Órfão, J.J. Modification of the surface chemistry of activated carbons. Carbon N. Y. 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Zielke, U.; Hüttinger, K.J.; Hoffman, W.P. Surface-oxidized carbon fibers: I. Surface structure and chemistry. Carbon N. Y. 1996, 34, 983–998. [Google Scholar] [CrossRef]
- Zárate-Guzmán, A.I.; González-Gutiérrez, L.V.; Ocampo-Pérez, R.; Carrasco-Marín, F.; Romero-Cano, L.A. Iron precursor salt effect on the generation of [rad]OH radicals and sulfamethoxazole degradation through a heterogeneous Fenton process using Carbon-Fe catalysts. J. Water Process. Eng. 2020, 36, 101273. [Google Scholar] [CrossRef]
- Rey, A.; Hungria, A.B.; Duran-Valle, C.J.; Faraldos, M.; Bahamonde, A.; Casas, J.A.; Rodriguez, J.J. On the optimization of activated carbon-supported iron catalysts in catalytic wet peroxide oxidation process. Appl. Catal. B Environ. 2016, 181, 249–259. [Google Scholar] [CrossRef]
- Magno De Lima Alves, T.; Amorim, B.F.; Morales Torres, M.A.; Bezerra, C.G.; Nóbrega De Medeiros, S.; Gastelois, P.L.; Fernandez Outon, L.E.; Augusto De Almeida Macedo, W. Wasp-waisted behavior in magnetic hysteresis curves of CoFe2O4 nanopowder at a low temperature: Experimental evidence and theoretical approach. RSC Adv. 2017, 7, 22187–22196. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Huang, S.; Laird, D.A.; Wang, X.; Meng, Z. Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single- and binary-metal systems. Chemosphere 2019, 218, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhao, Y.; Sun, K.; Gao, B.; Wang, Z.; Jin, J.; Zhang, Z.; Wang, S.; Yan, Y.; Liu, X.; et al. Cadmium adsorption on plant- and manure-derived biochar and biochar-amended sandy soils: Impact of bulk and surface properties. Chemosphere 2014, 111, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Trakal, L.; Veselská, V.; Šafařík, I.; Vítková, M.; Číhalová, S.; Komárek, M. Lead and cadmium sorption mechanisms on magnetically modified biochars. Bioresour. Technol. 2016, 203, 318–324. [Google Scholar] [CrossRef]
- Trakal, L.; Bingöl, D.; Pohořelý, M.; Hruška, M.; Komárek, M. Geochemical and spectroscopic investigations of Cd and Pb sorption mechanisms on contrasting biochars: Engineering implications. Bioresour. Technol. 2014, 171, 442–451. [Google Scholar] [CrossRef]
- Zuo, W.Q.; Chen, C.; Cui, H.J.; Fu, M.L. Enhanced removal of Cd(ii) from aqueous solution using CaCO3 nanoparticle modified sewage sludge biochar. RSC Adv. 2017, 7, 16238–16243. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; He, J.; Li, Y.; Cai, X.; Zhang, K.; Liu, T.; Hu, Y.; Lin, D.; Kong, L.; Liu, J. Removal of cadmium and lead ions from water by sulfonated magnetic nanoparticle adsorbents. J. Colloid Interface Sci. 2017, 494, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, Y.; Fu, J.; Yuan, L.; Li, Z.; Liu, C.; Zhao, D.; Wang, X. A novel magnetic biochar/MgFe-layered double hydroxides composite removing Pb2+ from aqueous solution: Isotherms, kinetics and thermodynamics. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 567, 278–287. [Google Scholar] [CrossRef]
- Li, R.; Deng, H.; Zhang, X.; Wang, J.J.; Awasthi, M.K.; Wang, Q.; Xiao, R.; Zhou, B.; Du, J.; Zhang, Z. High-efficiency removal of Pb(II) and humate by a CeO2–MoS2 hybrid magnetic biochar. Bioresour. Technol. 2019, 273, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.d.; Ho, S.H.; Wang, D.; Wei, Z.s.; Chang, J.S.; Ren, N. qi Lead removal by a magnetic biochar derived from persulfate-ZVI treated sludge together with one-pot pyrolysis. Bioresour. Technol. 2018, 247, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, J.; Liu, Y.; Guo, J.; Ren, J.; Zhou, F. Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd(II) removal in water. Fuel 2018, 233, 469–479. [Google Scholar] [CrossRef]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Zhou, J.; Guo, J.; Ren, J.; Zhou, F. Efficient removal of lead from aqueous solution by urea-functionalized magnetic biochar: Preparation, characterization and mechanism study. J. Taiwan Inst. Chem. Eng. 2018, 91, 457–467. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, P.; Sarswat, A.; Steele, P.H.; Pittman, C.U. Lead sorptive removal using magnetic and nonmagnetic fast pyrolysis energy cane biochars. J. Colloid Interface Sci. 2015, 448, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Kong, L.; Qu, Z.; Li, L.; Shen, G. Magnetic biochar decorated with ZnS nanocrytals for Pb (II) removal. ACS Sustain. Chem. Eng. 2015, 3, 125–132. [Google Scholar] [CrossRef]

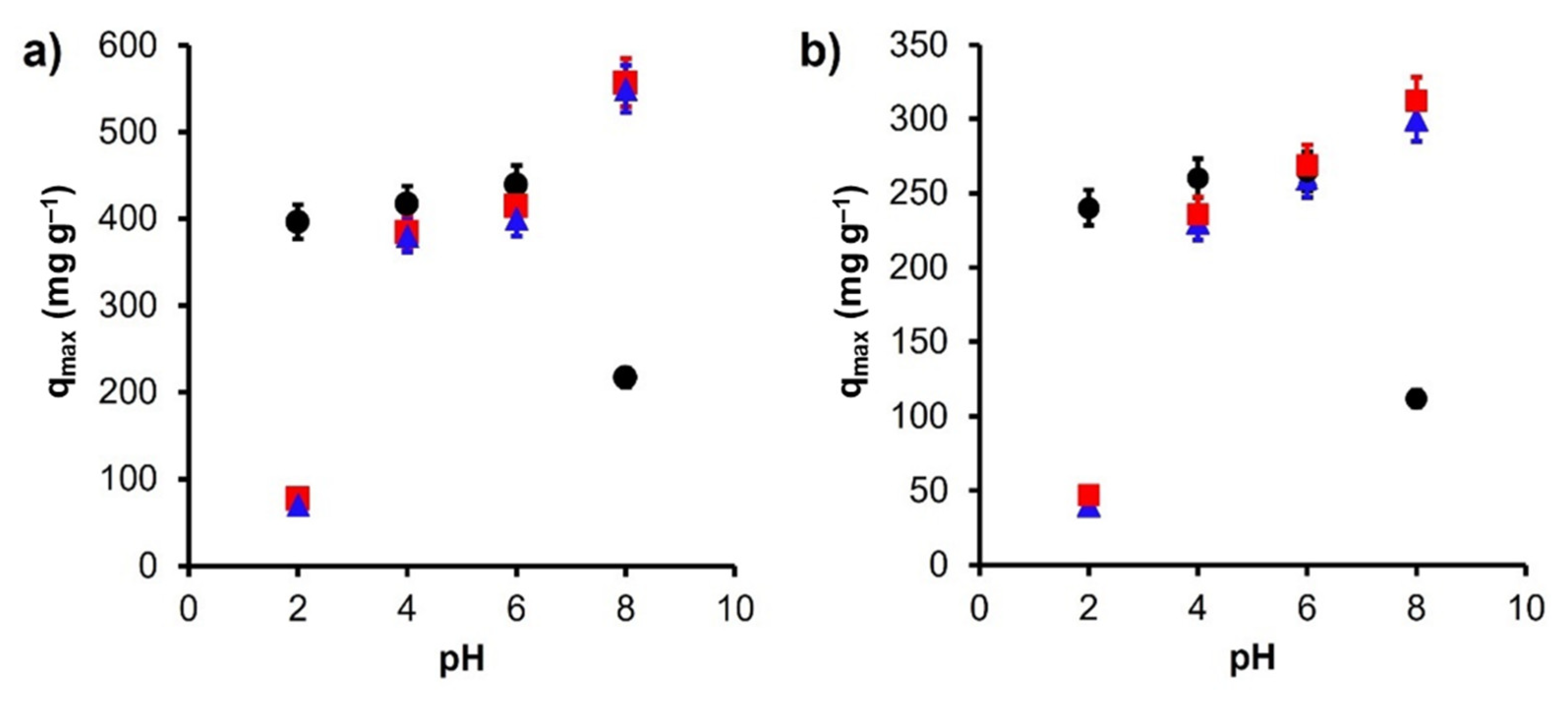
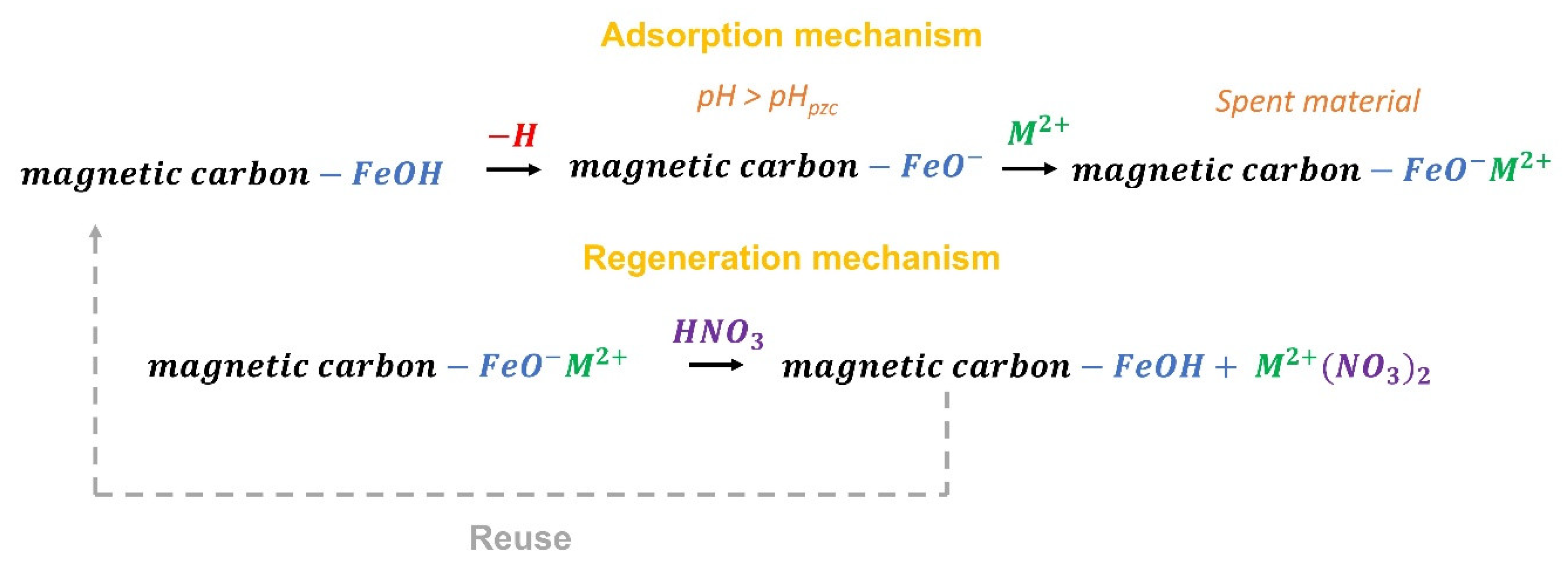
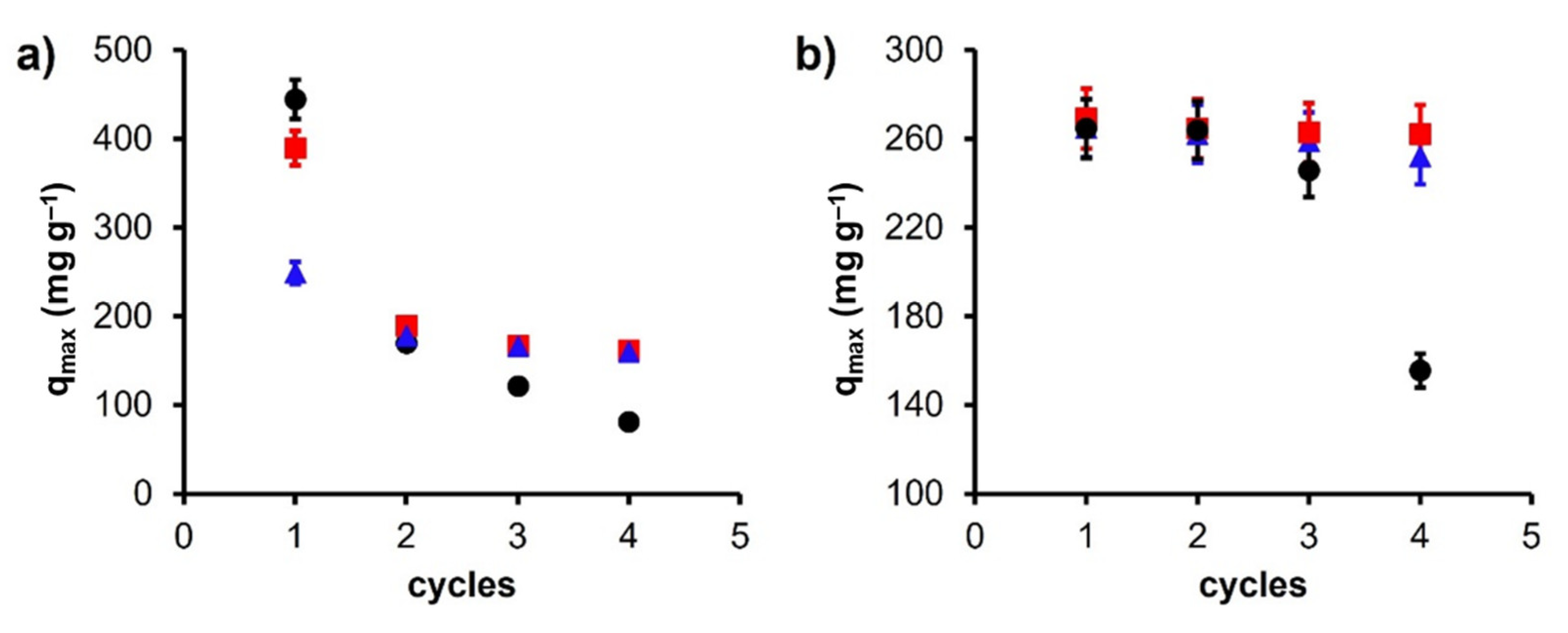
| Model | Equation | Parameters | Reference |
|---|---|---|---|
| Pseudo-first-order | qe (mg g−1) and qt (mg g−1): the amounts of adsorbed adsorbate at equilibrium and at time t. k1 (min−1): rate constant of pseudo-first-order adsorption. | [26] | |
| Pseudo-second-order | k2 (g mg−1 min−1): equilibrium rate constant of pseudo-second-order adsorption. | [27] | |
| Elovich | α (mg g−1 min−1): sorption rate. β (g mg−1): extent of surface coverage and activation energy for chemisorption. | [28] | |
| Intraparticular diffusion | ki (mg g−1 min−1/2): intraparticle diffusion rate constant. | [28] | |
| Langmuir | qmax (mg g−1): adsorption capacity of the material. K (L mg g−1): Langmuir constant. | [29] | |
| Freundlich | Kf: Freundlich constant. 1/n: heterogeneity factor. | [30] |
| Material | SBET | L0 | W0 | V0.95 | Vmeso | pHpzc |
|---|---|---|---|---|---|---|
| (m2 g−1) | (nm) | (cm3 g−1) | (cm3 g−1) | (cm3 g−1) | ||
| C | 1635 | 1.00 | 0.612 | 0.715 | 0.103 | 8.0 |
| Fe3O4+C | 394 | 1.04 | 0.158 | 0.209 | 0.051 | 3.5 |
| CoFe2O4+C | 359 | 1.22 | 0.144 | 0.239 | 0.095 | 3.4 |
| Sample | C1s | FWHM * | Peak | O1s | Peak | O | Sample | Fe2p | Fe | Co2p | Peak | Co | %Fe(II) | %Fe(III) | %Fe(III) | %Fe(III) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eV | eV | % | eV | % | % (Mass) | % (Atomic) | eV | % (Mass) | % (Atomic) | eV | % | % (Mass) | % (Atomic) | Octa | Tetra | Octa | Tetra | |||
| Fe3O4 | 284.8 | 1.86 | 77 | 530.2 | 85 | 31.8 | 49.8 | Fe3O4 | 709.9 | 38.0 | 56.18 | 25.2 | 0.0 | 38.0 | 62.0 | 61.5 | 38.5 | |||
| 286.7 | 8 | 531.6 | 15 | 711.4 | 38.1 | |||||||||||||||
| 288.8 | 15 | 713.3 | 23.8 | |||||||||||||||||
| 718.6 | ||||||||||||||||||||
| 723.2 | ||||||||||||||||||||
| 724.6 | ||||||||||||||||||||
| 726.7 | ||||||||||||||||||||
| 731.9 | ||||||||||||||||||||
| Fe3O4+C | 284.6 | 1.18 | 72 | 530.3 | 59 | 16.4 | 15.9 | Fe3O4+C | 709.9 | 34.1 | 23.42 | 6.5 | 0.0 | 34.1 | 65.9 | 57.7 | 42.3 | |||
| 285.8 | 14 | 531.6 | 27 | 711.2 | 38.1 | |||||||||||||||
| 287.0 | 7 | 533.0 | 13 | 713.3 | 27.9 | |||||||||||||||
| 288.5 | 4 | 718.4 | ||||||||||||||||||
| 290.0 | 3 | 722.9 | ||||||||||||||||||
| 724.5 | ||||||||||||||||||||
| 727.1 | ||||||||||||||||||||
| 732.4 | ||||||||||||||||||||
| CoFe2O4 | 284.8 | 1.89 | 72 | 530.1 | 68 | 30.7 | 46.6 | CoFe2O4 | 709.9 | 35.3 | 33.26 | 14.5 | 779.6 | 44.7 | 21.03 | 8.7 | 35.3 | 64.7 | 56.3 | 43.7 |
| 286.4 | 11 | 531.7 | 32 | 711.6 | 36.4 | 781.6 | 55.3 | |||||||||||||
| 288.4 | 16 | 713.7 | 28.3 | 785.4 | ||||||||||||||||
| 718.4 | 788.4 | |||||||||||||||||||
| 723.0 | 795.4 | |||||||||||||||||||
| 724.6 | 797.2 | |||||||||||||||||||
| 726.8 | 801.6 | |||||||||||||||||||
| 732.5 | 803.9 | |||||||||||||||||||
| CoFe2O4+C | 284.6 | 1.21 | 69 | 530.3 | 44 | 16.2 | 14.8 | CoFe2O4+C | 709.9 | 34.6 | 10.76 | 2.8 | 779.6 | 47.8 | 7.34 | 1.8 | 34.6 | 65.4 | 55.1 | 44.9 |
| 285.7 | 16 | 531.6 | 31 | 711.5 | 36.1 | 781.5 | 52.2 | |||||||||||||
| 286.9 | 7 | 532.9 | 25 | 713.7 | 29.3 | 785.2 | ||||||||||||||
| 288.5 | 5 | 718.4 | 788.3 | |||||||||||||||||
| 289.9 | 3 | 723.0 | 795.2 | |||||||||||||||||
| 724.7 | 796.8 | |||||||||||||||||||
| 727.0 | 801.5 | |||||||||||||||||||
| 732.6 | 804.2 | |||||||||||||||||||
| qe,exp | Pseudo First Order | Pseudo Second Order | Elovich | Intraparticle Diffusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe, calc | R | k2 | qe, calc | R | α | β | R | ki | R | |||
| (mg g−1) | (min−1) | (mg g−1) | (g mg−1 min−1) | (mg g−1) | (mg g−1 min−1/2) | ||||||||
| C | Pb(II) | 439.0 ± 22.0 | 0.0314 | 429.5 | 0.997 | 0.00179 | 445.8 | 0.988 | 0.105 | 61 × 105 | 0.997 | 17.14 | 0.000 |
| Fe3O4+C | Pb(II) | 280.0 ± 14.0 | 0.0185 | 272.4 | 0.997 | 0.00113 | 290.6 | 0.999 | 0.084 | 393.09 | 0.991 | 10.57 | 0.294 |
| CoFe2O4+C | Pb(II) | 272.0 ± 13.0 | 0.0174 | 267.4 | 0.997 | 0.00109 | 281.7 | 0.999 | 0.081 | 194.07 | 0.994 | 9.54 | 0.508 |
| C | Cd(II) | 177.9 ± 9.0 | 0.0196 | 178.0 | 0.996 | 0.00229 | 182.8 | 0.987 | 0.204 | 23 × 103 | 0.972 | 4.57 | 0.000 |
| Fe3O4+C | Cd(II) | 85.3 ± 4.0 | 0.0154 | 86.5 | 0.994 | 0.00276 | 91.6 | 0.983 | 0.227 | 25.59 | 0.966 | 3.02 | 0.523 |
| CoFe2O4+C | Cd(II) | 77.3 ± 3.8 | 0.0143 | 78.1 | 0.997 | 0.00273 | 83.1 | 0.988 | 0.233 | 13.27 | 0.970 | 2.71 | 0.573 |
| Adsorbent | Adsorbate | qmax exp | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|---|
| qmax | K | R | Kf | n | R | |||
| (mg g−1) | (mg g−1) | (L mg g−1) | ||||||
| C | Pb(II) | 988.59 ± 49.52 | 1202.41 | 0.003207 | 0.9934 | 2.6726 | 1.6974 | 0.9880 |
| Fe3O4+C | Pb(II) | 201.06 ± 12.01 | 317.60 | 0.001028 | 0.9897 | 54.480 | 2.4689 | 0.9669 |
| CoFe2O4+C | Pb(II) | 106.09 ± 8.05 | 122.47 | 0.002911 | 0.9869 | 7.647 | 2.8496 | 0.9759 |
| C | Cd(II) | 165.06 ± 6.60 | 272.77 | 0.002468 | 0.9971 | 4.7957 | 1.8078 | 0.9918 |
| Fe3O4+C | Cd(II) | 120.79 ± 6.03 | 239.88 | 0.001550 | 0.9963 | 1.6506 | 1.5021 | 0.9938 |
| CoFe2O4+C | Cd(II) | 115.02 ± 6.90 | 296.06 | 0.009360 | 0.9997 | 0.9266 | 1.3482 | 0.9997 |
| Precursor | qmax (mg g−1) | Reference | |||
|---|---|---|---|---|---|
| Pb(II) 1st Cycle | Cd(II) 1st Cycle | Pb(II) Last Cycle | Cd(II) Last Cycle | ||
| Argan shells (Fe3O4+C) | 389.5 | 269.0 | 161.6 | 252.0 | Present study |
| Argan shells (CoFe2O4+C) | 248.6 | 264.4 | 159.7 | 155.4 | Present study |
| Oil-tea and camellia | 225.0 | --- | 211.0 | --- | [46] |
| Fresh paulownia tree litter sludge | 263.6 | --- | --- | --- | [47] |
| Sludge | 206.5 | --- | 165.2 | --- | [48] |
| Palm fiber | --- | 197.96 | --- | 161.6 | [49] |
| Rice straw | --- | 10.7 | --- | --- | [50] |
| Palm fiber | 188.18 | --- | 150.5 | --- | [51] |
| Cane | 51.7 | -- | -- | -- | [52] |
| Rice husk | 367.6 | --- | --- | --- | [53] |
| Agricultural wastes | 229.9 | [1] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benjedim, S.; Romero-Cano, L.A.; Hamad, H.; Bailón-García, E.; Slovák, V.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Synthesis of Magnetic Adsorbents Based Carbon Highly Efficient and Stable for Use in the Removal of Pb(II) and Cd(II) in Aqueous Solution. Materials 2021, 14, 6134. https://doi.org/10.3390/ma14206134
Benjedim S, Romero-Cano LA, Hamad H, Bailón-García E, Slovák V, Carrasco-Marín F, Pérez-Cadenas AF. Synthesis of Magnetic Adsorbents Based Carbon Highly Efficient and Stable for Use in the Removal of Pb(II) and Cd(II) in Aqueous Solution. Materials. 2021; 14(20):6134. https://doi.org/10.3390/ma14206134
Chicago/Turabian StyleBenjedim, Safa, Luis A. Romero-Cano, Hesham Hamad, Esther Bailón-García, Václav Slovák, Francisco Carrasco-Marín, and Agustín F. Pérez-Cadenas. 2021. "Synthesis of Magnetic Adsorbents Based Carbon Highly Efficient and Stable for Use in the Removal of Pb(II) and Cd(II) in Aqueous Solution" Materials 14, no. 20: 6134. https://doi.org/10.3390/ma14206134
APA StyleBenjedim, S., Romero-Cano, L. A., Hamad, H., Bailón-García, E., Slovák, V., Carrasco-Marín, F., & Pérez-Cadenas, A. F. (2021). Synthesis of Magnetic Adsorbents Based Carbon Highly Efficient and Stable for Use in the Removal of Pb(II) and Cd(II) in Aqueous Solution. Materials, 14(20), 6134. https://doi.org/10.3390/ma14206134