Surface Structure Analysis of Initial High-Temperature Oxidation of SS441 Stainless Steel
Abstract
:1. Introduction
2. Experimental Methods
2.1. Heat Treatment
2.2. Surface Morphology
2.3. Microscale Surface Roughness
2.4. Oxide Scale Composition and Raman Spectra
2.5. Surface Impedance
3. Results
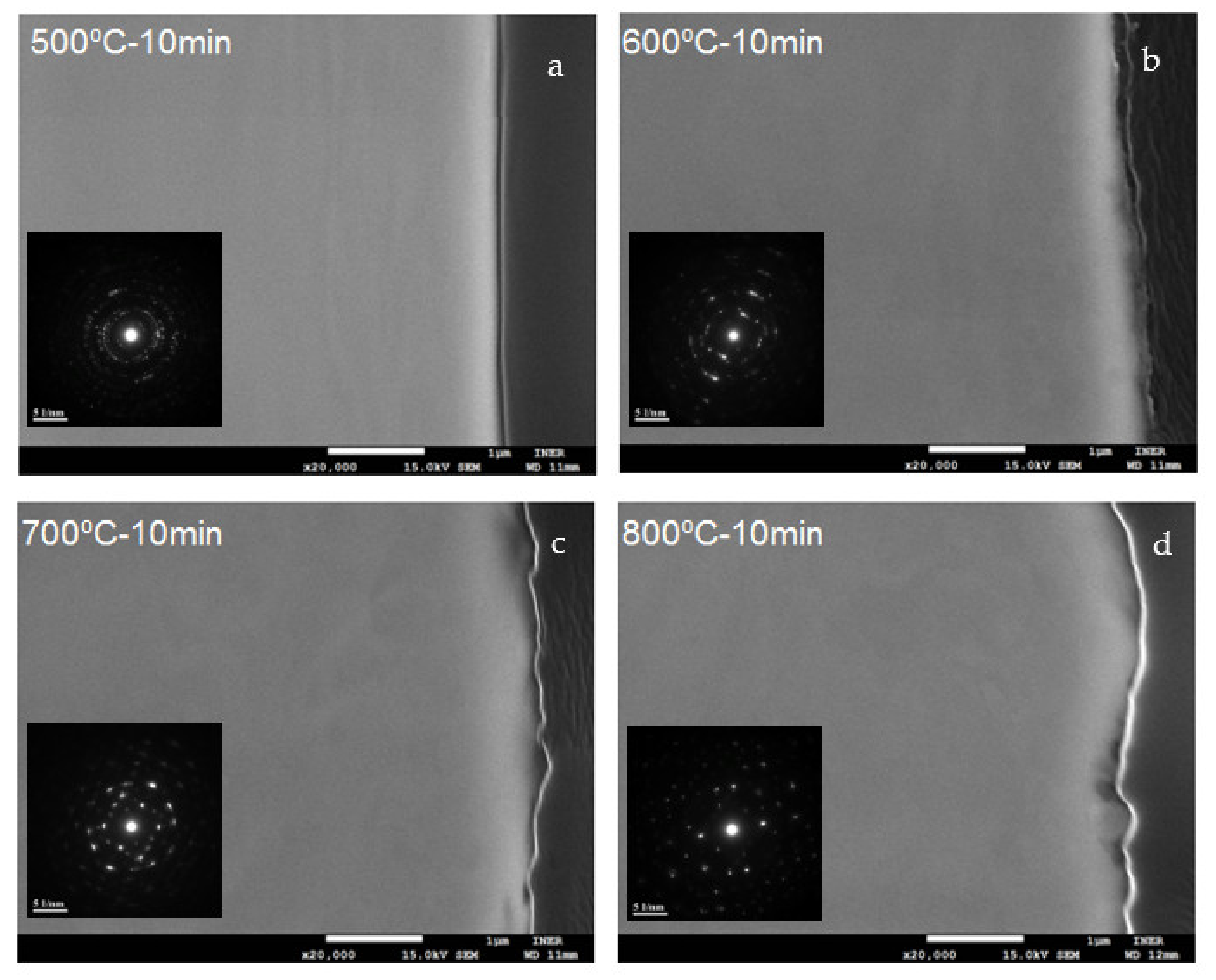
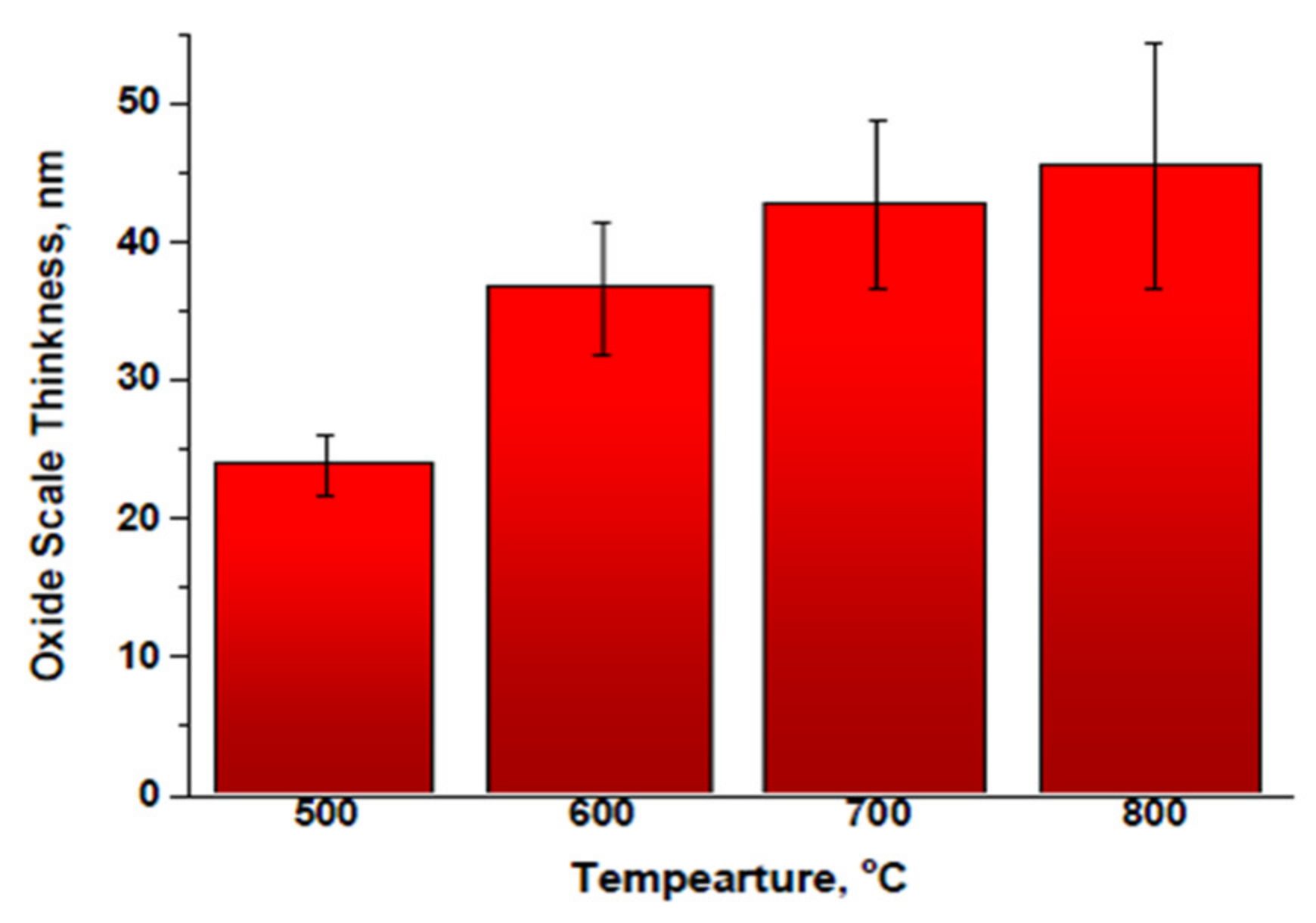
3.1. Oxide Scale Morphology and Elemental Analysis
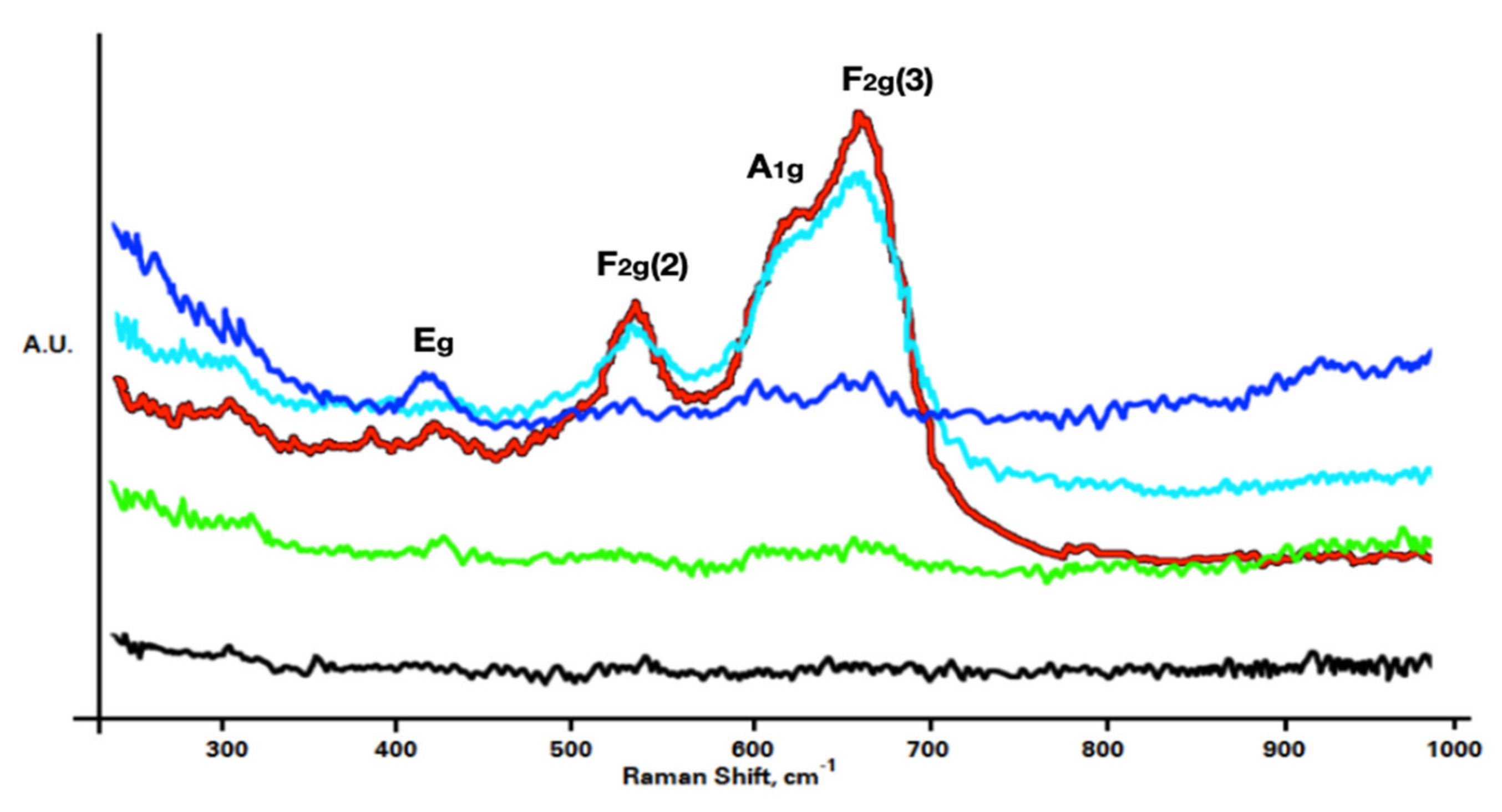
3.2. Oxide Scale Crystalline Size and Chemical Environment
3.3. Electrochemical Properties of Oxide Scales
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sazali, N. Emerging Technologies by Hydrogen: A Review. Int. J. Hydrog. Energy 2012, 45, 18753–18771. [Google Scholar] [CrossRef]
- Baratto, F.; Diwekar, U.M. Life Cycle Assessment of Fuel Cell-Based APUs. J. Power Sources 2005, 139, 188–196. [Google Scholar] [CrossRef]
- Fernandes, M.; Andrade, S.T.D.P.; Bistritzki, V.; Fonseca, R.M.; Zacarias, L.; Gonçalves, H.; de Castro, A.; Domingues, R.; Matencio, T. SOFC-APU Systems for Aircraft: A Review. Int. J. Hydrog. Energy 2018, 43, 16311–16333. [Google Scholar] [CrossRef]
- Staffell, I.; Ingram, A.; Kendall, K. Energy and Carbon Payback Times for Solid Oxide Fuel Cell Based Domestic CHP. Int. J. Hydrog. Energy 2012, 37, 2509–2523. [Google Scholar] [CrossRef]
- Minh, N. Solid Oxide Fuel Cell Technology—Features and Applications. Solid State Ionics 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Kendall, K. Progress in Solid Oxide Fuel Cell Materials. Int. Mater. Rev. 2013, 50, 257–264. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in Material Selection for Solid Oxide Fuel Cell Technology: A Review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Grolig, J.G.; Froitzheim, J.; Svensson, J.E. Coated Stainless Steel 441 as Interconnect Material for Solid Oxide Fuel Cells: Oxidation Performance and Chromium Evaporation. J. Power Sources 2014, 248, 1007–1013. [Google Scholar] [CrossRef] [Green Version]
- Saunders, S.R.J.; Monteiro, M.; Rizzo, F. The Oxidation Behavior of Metals and Alloys at High Temperatures in Atmospheres Containing Water Vapour: A Review. Prog. Mater. Sci. 2008, 53, 775–837. [Google Scholar] [CrossRef]
- Mah, J.C.; Muchtar, A.; Somalu, M.R.; Ghazali, M.J. Metallic Interconnects for Solid Oxide Fuel Cell: A Review on Protective Coating and Deposition Techniques. Int. J. Hydrog. Energy 2017, 42, 9219–9229. [Google Scholar] [CrossRef]
- Talic, B.; Molin, S.; Hendriksen, P.V.; Lein, H.L. Effect of Pre-oxidation on the Oxidation Resistance of Crofer 22 APU. Corros. Sci. 2018, 138, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Barelli, L.; Barluzzi, E.; Bidini, G. Diagnosis Methodology and Technique for Solid Oxide Fuel Cells: A Review. Int. J. Hydrog. Energy 2013, 38, 5060–5074. [Google Scholar] [CrossRef]
- Gazzarri, J.; Kesler, O. Short-stack modeling of degradation in solid oxide fuel cells: Part, I. Contact degradation. J. Power Sources 2008, 176, 138–154. [Google Scholar] [CrossRef]
- Alnegren, P.; Sattari, M.; Svensson, J.-E.; Froitzheim, J. Temperature Dependence of Corrosion of Ferritic Stainless Steel in Dual Atmosphere at 600–800 °C. J. Power Sources 2018, 392, 129–138. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, M.; Wang, N.; Ma, C.; Wang, J.; Han, M. A Short Review of Cathode Poisoning and Corrosion in Solid Oxide Fuel Cell. Int. J. Hydrog. Energy 2017, 42, 24948–24959. [Google Scholar] [CrossRef]
- Jiang, S.P.; Chen, X. Chromium Deposition and Poisoning of Cathodes of Solid Oxide Fuel Cells: A Review. Int. J. Hydrog. Energy 2014, 39, 505–531. [Google Scholar] [CrossRef]
- Key, C.; Eziashi, J.; Froitzheim, J.; Amendola, R.; Smith, R.; Gannon, P. Methods to Quantify Reactive Chromium Vaporization from Solid Oxide Fuel Cell Interconnects. J. Electrochem. Soc. 2014, 161, C373–C381. [Google Scholar] [CrossRef] [Green Version]
- Sachitanand, R.; Svensson, J.-E.; Froitzheim, J. The Influence of Cr Evaporation on Long Term Cr Depletion Rates in Ferritic Stainless Steels. Oxid. Met. 2015, 84, 241–257. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.-J.; Li, F.-S.; Chou, K.-C. Oxidation Mechanism of Fe–16Cr alloy as SOFC Interconnect in Dry/Wet Air. J. Alloy. Compd. 2013, 574, 437–442. [Google Scholar] [CrossRef]
- Magdefrau, N.; Chen, L.; Anidow, M. Effects of Alloy Heat Treatment on Oxidation Kinetics and Scale Morphology for Crofer 22 APU. J. Power Sources 2013, 241, 756–767. [Google Scholar] [CrossRef]
- Kofstad, P. High Temperature Corrosion; Elsevier Applied Science Publishers Ltd.: New York, NY, USA, 1988. [Google Scholar]
- Falk-Windisch, H.; Svensson, J.E.; Froitzheim, J. The Effect of Temperature on Chromium Vaporization and Oxide Scale Growth on Interconnect Steels for Solid Oxide Fuel Cells. J. Power Sources 2015, 287, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, S.; Combemale, L.; Popa, I.; Chandra-Ambhorn, S.; Chandra-Ambhorn, W.; Promdirek, P.; Wongpromrat, P. CHAPTER 6 Development of SOFC Interconnect Stainless Steels. Solid State Phenom. 2020, 300, 135–156. [Google Scholar] [CrossRef] [Green Version]
- Froitzheim, J.; Ravash, H.; Larsson, E.; Johansson, L.G.; Svensson, J.E. Investigation of Chromium Volatilization from FeCr Interconnects by Denuder Technique. J. Electrochem. Soc. 2010, 157, B1295. [Google Scholar] [CrossRef]
- Ghiara, G.; Piccardo, P.; Bongiorno, V.; Geipei, C.; Spotorno, R. Characterization of Metallic Interconnects Extracted from Solid Oxide Fuel Cell Stacks Operated up to 20,000 h in Real Life Conditions: The Air Side. Energies 2020, 13, 6487. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Wang, R.; Lau, G.Y.; Karimaghaloo, A.; Lee, M.H.; Tucker, M.C. Progress in Durability of MS-SOFCs with Infiltrated Electrodes. J. Power Sources 2019, 437, 226935. [Google Scholar] [CrossRef] [Green Version]
- Żurek, J.; Wessel, E.; Niewolak, L.; Schmitz, F.; Kern, T.-U.; Singheiser, L.; Quadakkers, W.J. Anomalous Temperature Dependence of Oxidation Kinetics During Steam Oxidation of Ferritic Steels in the Temperature Range 550–650 °C. Corros. Sci. 2004, 46, 2301–2317. [Google Scholar] [CrossRef]
- Sánchez, L.; Hierro, M.P.; Perez-Trujillo, F.J. Effect of Chromium Content on the Oxidation Behaviour of Ferritic Steels for Applications in Steam Atmospheres at High Temperatures. Oxid. Met. 2009, 71, 173–186. [Google Scholar] [CrossRef]
- Young, D.; Zurek, J.; Singheiser, L.; Quadakkers, W. Temperature Dependence of Oxide Scale Formation on High-Cr Ferritic Steels in Ar–H2–H2O. Corros. Sci. 2011, 53, 2131–2141. [Google Scholar] [CrossRef]
- Goebel, C.; Alnegren, P.; Faust, R.; Svensson, J.-E.; Froitzheim, J. The Effect of Pre-Oxidation Parameters on the Corrosion Behavior of AISI 441 in Dual Atmosphere. Int. J. Hydrog. Energy 2018, 43, 14665–14674. [Google Scholar] [CrossRef]
- Wang, R.; Pal, U.B.; Gopalan, S.; Basu, S.N. Chromium Poisoning Effects on Performance of (La,Sr)MnO3-Based Cathode in Anode-Supported Solid Oxide Fuel Cells. J. Electrochem. Soc. 2017, 164, F740–F747. [Google Scholar] [CrossRef]
- Cheng, F.; Cui, J.; Wang, L.; Li, S.; Sun, J. Performance of Co Ni O Spinel Oxide Coating on AISI 430 Stainless Steel as Interconnect for Intermediate Temperature Solid Oxide Fuel Cell. Int. J. Hydrog. Energy 2017, 42, 12477–12484. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of Spinels. J. Am. Ceram. Soc. 1999, 82, 3279–3292. [Google Scholar] [CrossRef]
- Biagioni, C.; Pasero, M. The Systematics of the Spinel-Type Minerals: An Overview. Am. Miner. 2014, 99, 1254–1264. [Google Scholar] [CrossRef]
- Veronica, D.; Giovanni, B.A.; Danilo, B.; Pier, P.L. Raman Fingerprint of Chromate, Aluminate and Ferrite Spinels. J. Raman Spectro. 2015, 46, 1255–1264. [Google Scholar]
- Yung, T.-Y.; Tseng, H.-P.; Yang, P.; Liu, L.-K. The Effects of Thermal Aging on Metallic Interconnects of Solid Oxide Fuel Cells. Mater. Res. Innov. 2013, 17, s129–s135. [Google Scholar] [CrossRef]




| Element | Cr | Mn | Nb | C | Si | Ti | S | Fe |
|---|---|---|---|---|---|---|---|---|
| Wt% | 17.7 | 0.30 | 0.37 | 0.015 | 0.55 | 0.15 | 0.002 | Bal. |
| Atomic % | 500 °C-10 min | 600 °C-10 min | 700 °C-10 min | 800 °C-10 min |
|---|---|---|---|---|
| Fe | 63.99 | 63.69 | 62.85 | 69.10 |
| Cr | 16.01 | 15.92 | 14.7 | 17.41 |
| Nb | 0.39 | 0.27 | 0.23 | 0.39 |
| Mn | - | - | 0.40 | 0.45 |
| O | 5.71 | 6.34 | 7.7 | 11.46 |
| Entry | Peak Position (2 θ) | FWHM | Size (nm) |
|---|---|---|---|
| SS441 | 44.65° | 0.3324° | 25.12 |
| 500 °C-10 min | 44.61° | 0.3601° | 31.80 |
| 600 °C-10 min | 44.65° | 0.2951° | 39.84 |
| 700 °C-10 min | 44.65° | 0.2827° | 41.71 |
| 800 °C-10 min | 44.64° | 0.2441° | 47.79 |
| Fe | Fe-Cr | Fe19Mn | |
|---|---|---|---|
| 500 °C, 10 min | 48.4% | 39.0% | 12.5% |
| 600 °C, 10 min | 9.2% | 69.3% | 21.5% |
| 700 °C, 10 min | 15.8% | 62.9% | 21.3% |
| 800 °C, 10 min | 16.6% | 60.1% | 23.3% |
| Rs (kΩ) | R1 (kΩ) | Capacitor (mF) | R2 (MΩ) | CPE1/n (μMho) | R3 (kΩ) | CPE2/n (μMho) | |
|---|---|---|---|---|---|---|---|
| 500 °C-10 min | 0.17 | 4.58 | 0.794 | 2.96 | 2.25/0.581 | - | - |
| 600 °C-10 min | 0.37 | 9.18 | 1.01 | 4.56 | 3.83/0.636 | 1.26 | 8.02/0.703 |
| 700 °C-10 min | 1.39 | 1.02 | 2.23 | 0.73 | 14.8/0.784 | 2.36 | 21.9/0.589 |
| 800 °C-10 min | 1.46 | 2.51 | 4.45 | 1.10 | 11.3/0.589 | 2.05 | 76.6/0.526 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yung, T.-Y.; Tseng, H.-P.; Lu, W.-F.; Tsai, K.-C.; Shen, T.; Cheng, H.-M.; Chen, J.-S.; Chen, P.-T. Surface Structure Analysis of Initial High-Temperature Oxidation of SS441 Stainless Steel. Materials 2021, 14, 6136. https://doi.org/10.3390/ma14206136
Yung T-Y, Tseng H-P, Lu W-F, Tsai K-C, Shen T, Cheng H-M, Chen J-S, Chen P-T. Surface Structure Analysis of Initial High-Temperature Oxidation of SS441 Stainless Steel. Materials. 2021; 14(20):6136. https://doi.org/10.3390/ma14206136
Chicago/Turabian StyleYung, Tung-Yuan, Hui-Ping Tseng, Wen-Feng Lu, Kun-Chao Tsai, Tien Shen, Hsin-Ming Cheng, Jeng-Shiung Chen, and Po-Tuan Chen. 2021. "Surface Structure Analysis of Initial High-Temperature Oxidation of SS441 Stainless Steel" Materials 14, no. 20: 6136. https://doi.org/10.3390/ma14206136
APA StyleYung, T.-Y., Tseng, H.-P., Lu, W.-F., Tsai, K.-C., Shen, T., Cheng, H.-M., Chen, J.-S., & Chen, P.-T. (2021). Surface Structure Analysis of Initial High-Temperature Oxidation of SS441 Stainless Steel. Materials, 14(20), 6136. https://doi.org/10.3390/ma14206136







