Histologic and Histomorphometric Evaluation of a New Bioactive Liquid BBL on Implant Surface: A Preclinical Study in Foxhound Dogs
Abstract
1. Introduction
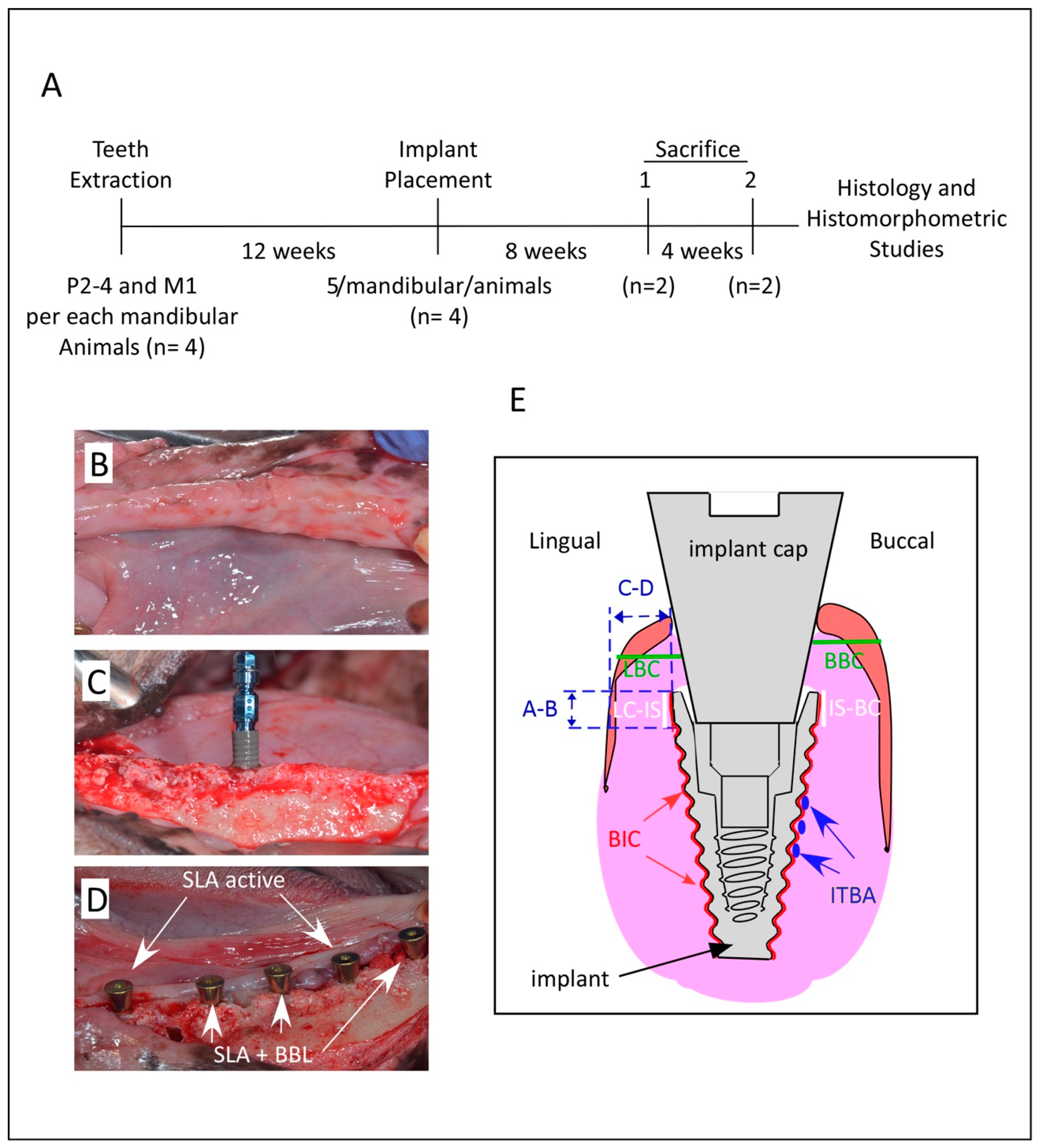
2. Material and Methods
2.1. Statement of Compliance and Declaration of the Research Ethics
2.2. Bone Bioactive Liquid and Implant Design
2.3. Tooth Extraction, Surgical Procedure, and Animal Care
2.4. Termination
2.5. Histologic Processing
2.6. Histomorphometric Analysis and Examination
2.7. Statistical Analysis
3. Results
3.1. Bone Integration and Density
3.2. Crestal Bones Loss and Tissue Thickness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fugazzotto, P.A. Success and Failure Rates of 1,344 6- to 9-mm-Length Rough-Surface Implants Placed at the Time of Transalveolar Sinus Elevations, Restored with Single Crowns, and Followed for 60 to 229 Months in Function. Int. J. Oral Maxillofac. Implant. 2017, 32, 1359–1363. [Google Scholar] [CrossRef][Green Version]
- Scarano, A.; Carinci, F.; Assenza, B.; Piattelli, M.; Murmura, G.; Piattelli, A. Vertical ridge augmentation of atrophic posterior mandible using an inlay technique with a xenograft without miniscrews and miniplates: Case series. Clin. Oral Implant. Res. 2011, 22, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, E.A.; Janal, M.N.; Granato, R.; Marin, C.; Suzuki, M.; Tovar, N.; Coelho, P.G. Buccal and lingual bone level alterations after immediate implantation of four implant surfaces: A study in dogs. Clin. Oral Implant. Res. 2012, 24, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Domínguez, M.; Ortega-Asensio, V.; Fuentes-Numancia, E.; Aragoneses, J.M.; Barbu, H.M.; Ramírez-Fernández, M.P.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Samet, N.; Gehrke, S.A. Can the Macrogeometry of Dental Implants Influence Guided Bone Regeneration in Buccal Bone Defects? Histomorphometric and Biomechanical Analysis in Beagle Dogs. J. Clin. Med. 2019, 8, 618. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Treichel, T.L.E.; Pérez-Díaz, L.; Calvo-Guirado, J.L.; Júnior, J.A.; Mazón, P.; De Aza, P.N. Impact of Different Titanium Implant Thread Designs on Bone Healing: A Biomechanical and Histometric Study with an Animal Model. J. Clin. Med. 2019, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Baldan, N. Scanning electron microscopy of the bone-bioactive implant interface. J. Biomed. Mater. Res. 1997, 36, 429–440. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, S.-M.; Yang, H.-W.; Choi, B.-H. Effect of Ultraviolet Irradiation on Osseointegration of Dental Implants: A Comparative Histomorphometric Study in Canine Models. Appl. Sci. 2020, 10, 4216. [Google Scholar] [CrossRef]
- Yoon, W.-J.; Kim, S.-G.; Oh, J.-S.; You, J.-S.; Jeong, K.-I.; Lim, S.-C.; Jeong, M.-A. Comparative study on the osseointegration of implants in dog mandibles according to the implant surface treatment. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 345–351. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Blus, C.; Schwarz, D.M.; Orrù, G. Characterization of a Macro- and Micro-Textured Titanium Grade 5 Alloy Surface Obtained by Etching Only without Sandblasting. Materials 2020, 13, 5074. [Google Scholar] [CrossRef]
- Rizo-Gorrita, M.; Fernandez-Asian, I.; Garcia-De-Frenza, A.; Vazquez-Pachon, C.; Serrera-Figallo, M.-A.; Torres-Lagares, D.; Gutierrez-Perez, J.-L. Influence of Three Dental Implant Surfaces on Cell Viability and Bone Behavior. An In Vitro and a Histometric Study in a Rabbit Model. Appl. Sci. 2020, 10, 4790. [Google Scholar] [CrossRef]
- Pippenger, B.; Rottmar, M.; Kopf, B.S.; Stübinger, S.; Torre, F.H.D.; Berner, S.; Maniura-Weber, K. Surface modification of ultrafine-grained titanium: Influence on mechanical properties, cytocompatibility, and osseointegration potential. Clin. Oral Implant. Res. 2018, 30, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Salomó-Coll, O.; De Val, J.E.M.-S.; Ramírez-Fernandez, M.P.; Satorres-Nieto, M.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Osseoinductive elements for promoting osseointegration around immediate implants: A pilot study in the foxhound dog. Clin. Oral Implant. Res. 2015, 27, e167–e175. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; López-Valverde, A.; Aragoneses, J.; de Sousa, B.M.; Rodrigues, M.; Ramírez, J. Systematic Review and Meta-Analysis of the Effectiveness of Calcium-Phosphate Coating on the Osseointegration of Titanium Implants. Materials 2021, 14, 3015. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; Pardal-Peláez, B.; López-Valverde, A.; Ramírez, J. Role of Melatonin in Bone Remodeling around Titanium Dental Implants: Meta-Analysis. Coatings 2021, 11, 271. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Choi, K.S.; Ji, M.-K.; Kim, S.; Gwon, Y.; Park, C.; Kim, J.; Lim, H.-P. Graphene–Chitosan Hybrid Dental Implants with Enhanced Antibacterial and Cell-Proliferation Properties. Appl. Sci. 2020, 10, 4888. [Google Scholar] [CrossRef]
- Kiran, A.S.K.; Kumar, T.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and Bioactive Surface Modifications of Titanium Implants by PCL/TiO2 Nanocomposite Coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef]
- Ito, A.; Senda, K.; Sogo, Y.; Oyane, A.; Yamazaki, A.; Legeros, R.Z. Dissolution rate of zinc-containing beta-tricalcium phosphate ceramics. Biomed. Mater. 2006, 1, 134–139. [Google Scholar] [CrossRef]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res. A 2017, 105, 2218–2227. [Google Scholar] [CrossRef]
- Yue, J.; Jin, Z.; Poon, H.L.E.; Shang, G.; Liu, H.; Wang, D.; Qi, S.; Chen, F.; Xu, Y. Osteogenic and Antibacterial Activity of a Plasma-Sprayed CeO2 Coating on a Titanium (Ti)-Based Dental Implant. Coatings 2020, 10, 1007. [Google Scholar] [CrossRef]
- Odatsu, T.; Kuroshima, S.; Sato, M.; Takase, K.; Valanezhad, A.; Naito, M.; Sawase, T. Antibacterial Properties of Nano-Ag Coating on Healing Abutment: An In Vitro and Clinical Study. Antibiotics 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, M.; Aldemir, S.D.; Gümüşderelioğlu, M.; Anticellular, P.E.O. Coatings on titanium surfaces by sequential electrospinning and crosslinking processes. Emergent Mater. 2019, 2, 169–179. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Lorusso, F.; Orsini, T.; Morra, M.; Iviglia, G.; Valbonetti, L. Biomimetic Surfaces Coated with Covalently Immobilized Collagen Type I: An X-Ray Photoelectron Spectroscopy, Atomic Force Microscopy, Micro-CT and Histomorphometrical Study in Rabbits. Int. J. Mol. Sci. 2019, 20, 724. [Google Scholar] [CrossRef]
- Kulkarni, M.; Patil-Sen, Y.; Junkar, I.; Kulkarni, C.V.; Lorenzetti, M.; Iglič, A. Wettability studies of topologically distinct titanium surfaces. Colloids Surf. B Biointerfaces 2015, 129, 47–53. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Bang, S.-M.; Moon, H.-J.; Kwon, Y.-D.; Yoo, J.-Y.; Pae, A.; Kwon, I.K. Osteoblastic and osteoclastic differentiation on SLA and hydrophilic modified SLA titanium surfaces. Clin. Oral Implant. Res. 2013, 25, 831–837. [Google Scholar] [CrossRef]
- Romero-Ruiz, M.M.; Gil-Mur, F.J.; Ríos-Santos, J.V.; Lázaro-Calvo, P.; Ríos-Carrasco, B.; Herrero-Climent, M. Influence of a Novel Surface of Bioactive Implants on Osseointegration: A Comparative and Histomorfometric Correlation and Implant Stability Study in Minipigs. Int. J. Mol. Sci. 2019, 20, 2307. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yang, D.J.; Yeo, S.; An, H.W.; Ryoo, K.H.; Park, K.B. The cytocompatibility and osseointegration of the Ti implants with XPEED(R) surfaces. Clin. Oral Implant. Res. 2012, 23, 1283–1289. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, T.; Qian, S.; Liu, X.; Sun, J.; Li, B. Silicon-Doped Titanium Dioxide Nanotubes Promoted Bone Formation on Titanium Implants. Int. J. Mol. Sci. 2016, 17, 292. [Google Scholar] [CrossRef]
- Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Z. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings 2019, 9, 612. [Google Scholar] [CrossRef]
- Salomó-Coll, O.; De Val, J.E.M.-S.; Ramírez-Fernández, M.P.; Hernández-Alfaro, F.; Gargallo-Albiol, J.; Calvo-Guirado, J.L. Topical applications of vitamin D on implant surface for bone-to-implant contact enhance: A pilot study in dogs part II. Clin. Oral Implant. Res. 2015, 27, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Orive, G.; Pla, R.; Roman, P.; Serrano, V.; Andía, I. The effects of PRGF on bone regeneration and on titanium implant osseointegration in goats: A histologic and histomorphometric study. J. Biomed. Mater. Res. Part A 2008, 91A, 158–165. [Google Scholar] [CrossRef]
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implant. 1999, 14, 529–535. [Google Scholar]
- Anitua, E.; Muruzabal, F.J.; Alcalde, I.; Merayo-Lloves, J.; Orive, G. Plasma rich in growth factors (PRGF-Endoret) stimulates corneal wound healing and reduces haze formation after PRK surgery. Exp. Eye Res. 2013, 115, 153–161. [Google Scholar] [CrossRef]
- Anitua, E.; Prado, R.; Orive, G.; Tejero, R. Effects of calcium-modified titanium implant surfaces on platelet activation, clot formation, and osseointegration. J. Biomed. Mater. Res. Part A 2014, 103, 969–980. [Google Scholar] [CrossRef]
- Anitua, E.; Cerqueira, A.; Romero-Gavilán, F.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Goñi, I.; et al. Influence of calcium ion-modified implant surfaces in protein adsorption and implant integration. Int. J. Implant. Dent. 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Bragança, L.K.; Velasco-Ortega, E.; Calvo-Guirado, J.L. Evaluation of dimensional behavior of peri-implant tissues in implants immediately exposed or submerged in fresh extraction and healed sites: A histological study in dogs. Int. J. Implant. Dent. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Discepoli, N.; Vignoletti, F.; Laino, L.; de Sanctis, M.; Muñoz, F.; Sanz, M. Early healing of the alveolar process after tooth extraction: An experimental study in the beagle dog. J. Clin. Periodontol. 2013, 40, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Benítez-García, J.A.; de Val, J.E.M.S.; Martínez, C.P.-A.; Gehrke, S.A.; Delgado-Ruiz, R.; Moses, O. Socket-shield technique: The influence of the length of the remaining buccal segment of healthy tooth structure on peri-implant bone and socket preservation. A study in dogs. Ann. Anat. Anat. Anz. 2018, 221, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced Bone Apposition to a Chemically Modified SLA Titanium Surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, J.M.; Amara, H.B.; Lee, Y.-M.; Lim, Y.-J.; Kim, H.; Koo, K.-T. Bone healing dynamics associated with 3 implants with different surfaces: Histologic and histomorphometric analyses in dogs. J. Periodontal Implant. Sci. 2019, 49, 25–38. [Google Scholar] [CrossRef]
- Park, Y.-S.; Yi, K.-Y.; Lee, I.-S.; Jung, Y.-C. Correlation between microtomography and histomorphometry for assessment of implant osseointegration. Clin. Oral Implant. Res. 2005, 16, 156–160. [Google Scholar] [CrossRef]
- Stadlinger, B.; Lode, A.T.; Eckelt, U.; Range, U.; Schlottig, F.; Hefti, T.; Mai, R. Surface-conditioned dental implants: An animal study on bone formation. J. Clin. Periodontol. 2009, 36, 882–891. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.M.; Sousa, B.M.d.; Herrero-Hernández, S.; López-Valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef]
- Oates, T.W.; Valderrama, P.; Bischof, M.; Nedir, R.; Jones, A.; Simpson, J.; Toutenburg, H.; Cochran, D.L. Enhanced implant stability with a chemically modified SLA surface: A randomized pilot study. Int. J. Oral Maxillofac. Implant. 2007, 22, 755–760. [Google Scholar]
- Lech, A.; Butruk-Raszeja, B.A.; Ciach, T.; Lawniczak-Jablonska, K.; Kuzmiuk, P.; Bartnik, A.; Wachulak, P.; Fiedorowicz, H. Surface Modification of PLLA, PTFE and PVDF with Extreme Ultraviolet (EUV) to Enhance Cell Adhesion. Int. J. Mol. Sci. 2020, 21, 9679. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Ni, J.; Zheng, K.; Shen, Y.; Wang, X.; He, G.; Jin, S.; Tang, T. Dual effects and mechanism of TiO2 nanotube arrays in reducing bacterial colonization and enhancing C3H10T1/2 cell adhesion. Int. J. NanoMed. 2013, 8, 3093–3105. [Google Scholar]
- Anitua, E.; Muruzabal, F.J.; De la Fuente, M.; Merayo-Lloves, J.; Orive, G. Effects of heat-treatment on plasma rich in growth factors-derived autologous eye drop. Exp. Eye Res. 2014, 119, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Tejero, R.; Zalduendo, M.M.; Orive, G. Plasma Rich in Growth Factors Promotes Bone Tissue Regeneration by Stimulating Proliferation, Migration, and Autocrine Secretion in Primary Human Osteoblasts. J. Periodontol. 2013, 84, 1180–1190. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Munaron, L.; Petrillo, S.; Erovigni, F.; Carossa, S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016, 27, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Inchingolo, F.; Murmura, G.; Traini, T.; Piattelli, A.; Lorusso, F. Three-Dimensional Architecture and Mechanical Properties of Bovine Bone Mixed with Autologous Platelet Liquid, Blood, or Physiological Water: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1230. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Aslani, A.; Sambito, M.A.; Kalkhoran, N.M.; Slamovich, E.B.; Webster, T.J. Nanocrystalline hydroxyapatite/titania coatings on titanium improves osteoblast adhesion. J. Biomed. Mater. Res. Part A 2007, 84A, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-M.; Choe, H.-C. Mg-containing hydroxyapatite coatings on Ti-6Al-4V alloy for dental materials. Appl. Surf. Sci. 2018, 432, 294–299. [Google Scholar] [CrossRef]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regi, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef] [PubMed]
- Cesarz-Andraczke, K.; Nowosielski, R.; Basiaga, M.; Babilas, R. Study of the Morphology and Properties of Biocompatible Ca-P Coatings on Mg Alloy. Materials 2019, 13, 2. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrés-Amat, E.; Al Madhoun, A.; Ferrés-Amat, E.; Al Demour, S.; Ababneh, M.A.; Ferrés-Padró, E.; Marti, C.; Carrio, N.; Barajas, M.; Atari, M. Histologic and Histomorphometric Evaluation of a New Bioactive Liquid BBL on Implant Surface: A Preclinical Study in Foxhound Dogs. Materials 2021, 14, 6217. https://doi.org/10.3390/ma14206217
Ferrés-Amat E, Al Madhoun A, Ferrés-Amat E, Al Demour S, Ababneh MA, Ferrés-Padró E, Marti C, Carrio N, Barajas M, Atari M. Histologic and Histomorphometric Evaluation of a New Bioactive Liquid BBL on Implant Surface: A Preclinical Study in Foxhound Dogs. Materials. 2021; 14(20):6217. https://doi.org/10.3390/ma14206217
Chicago/Turabian StyleFerrés-Amat, Eduard, Ashraf Al Madhoun, Elvira Ferrés-Amat, Saddam Al Demour, Mera A. Ababneh, Eduard Ferrés-Padró, Carles Marti, Neus Carrio, Miguel Barajas, and Maher Atari. 2021. "Histologic and Histomorphometric Evaluation of a New Bioactive Liquid BBL on Implant Surface: A Preclinical Study in Foxhound Dogs" Materials 14, no. 20: 6217. https://doi.org/10.3390/ma14206217
APA StyleFerrés-Amat, E., Al Madhoun, A., Ferrés-Amat, E., Al Demour, S., Ababneh, M. A., Ferrés-Padró, E., Marti, C., Carrio, N., Barajas, M., & Atari, M. (2021). Histologic and Histomorphometric Evaluation of a New Bioactive Liquid BBL on Implant Surface: A Preclinical Study in Foxhound Dogs. Materials, 14(20), 6217. https://doi.org/10.3390/ma14206217






