The Electrical and Thermal Transport Properties of La-Doped SrTiO3 with Sc2O3 Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Phase and Microstructure Characterization
2.3. Thermoelectric Performance Measurements
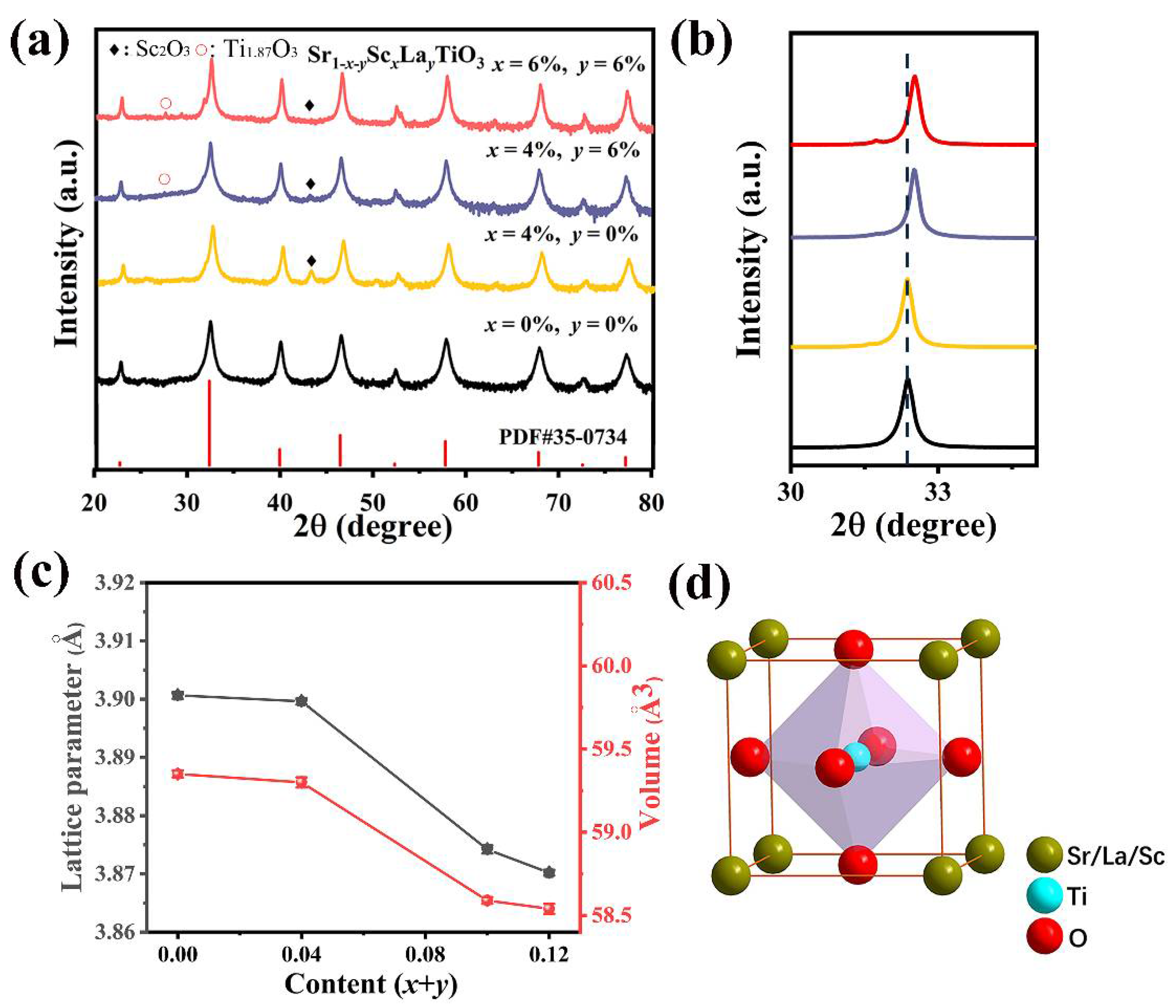
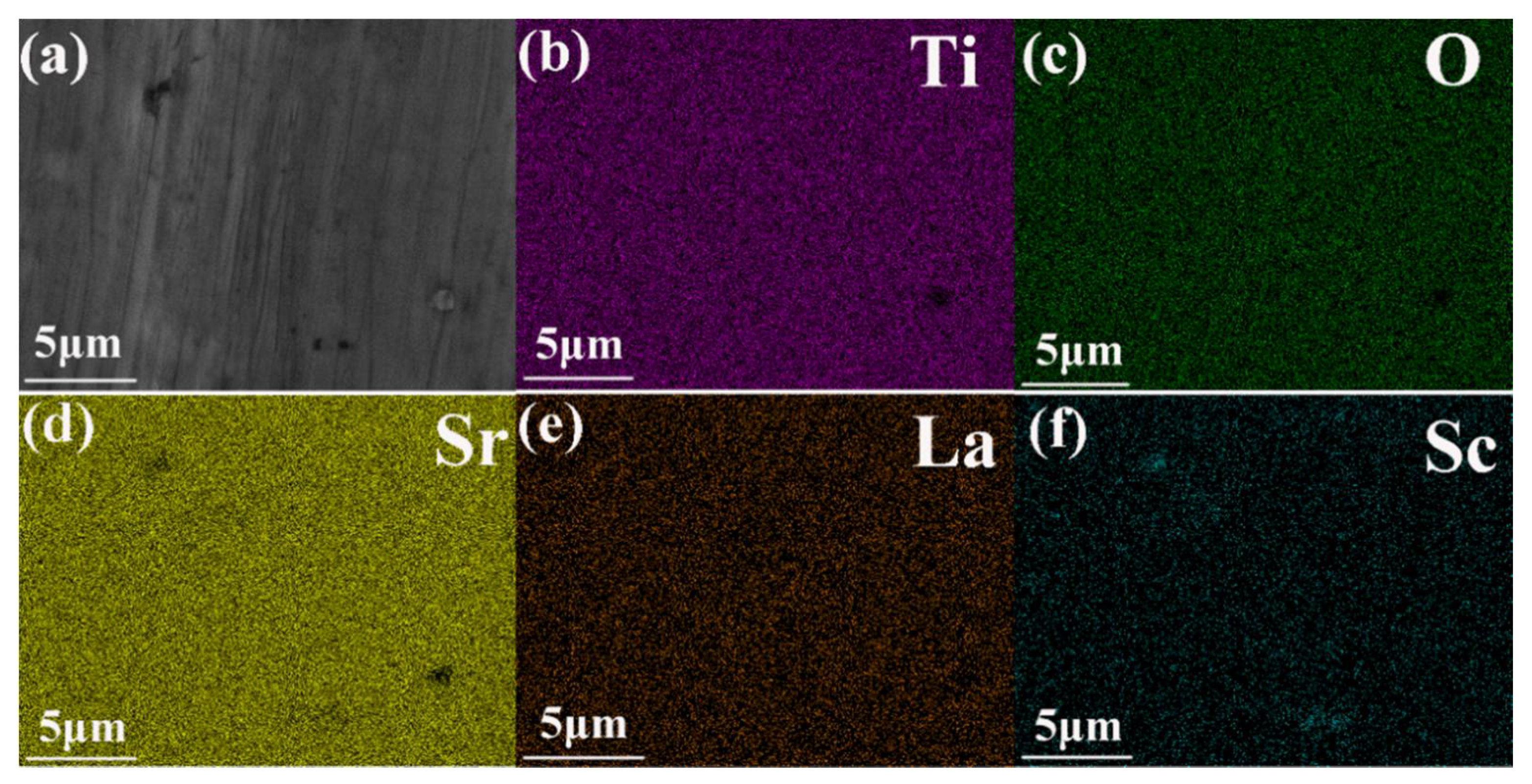
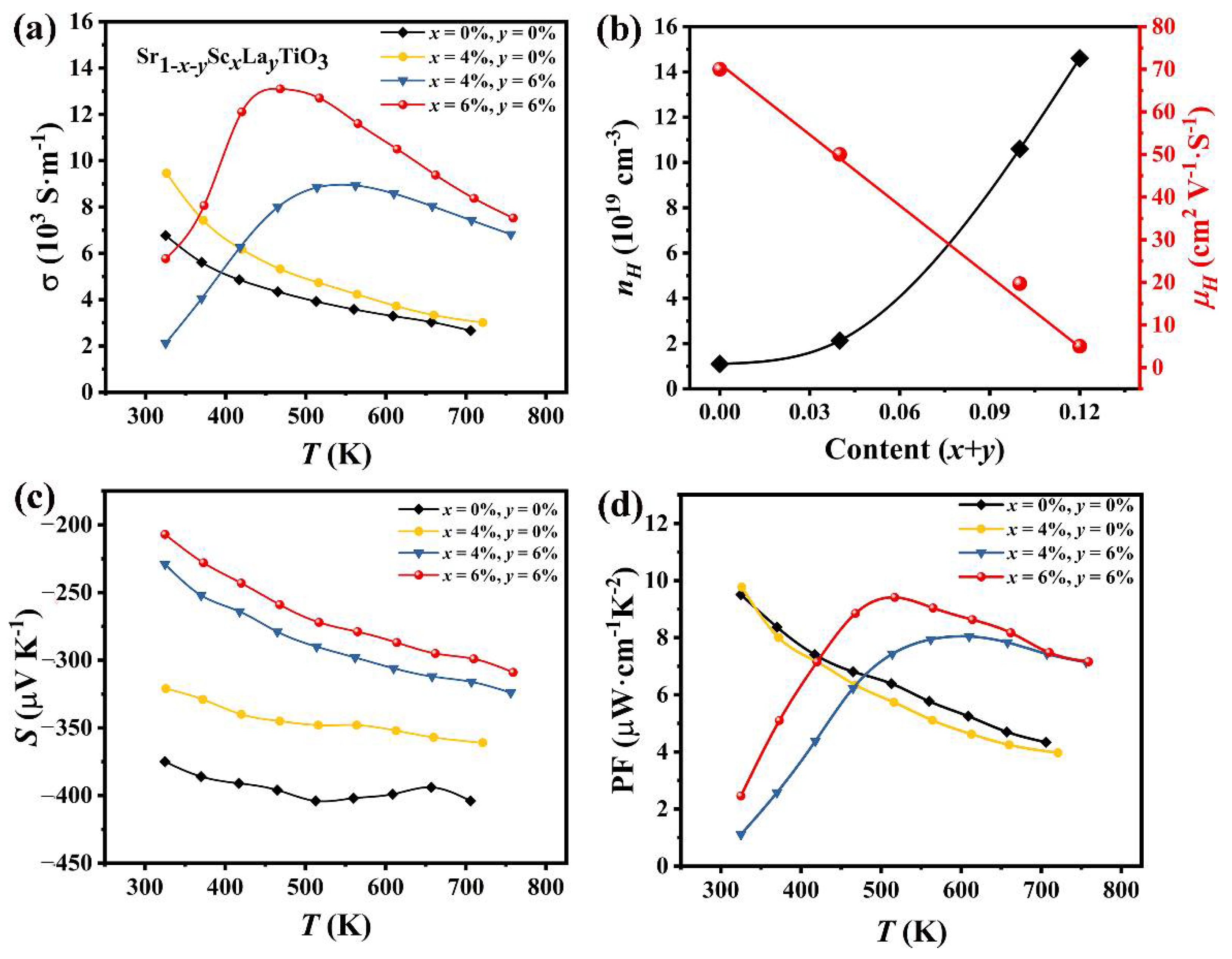
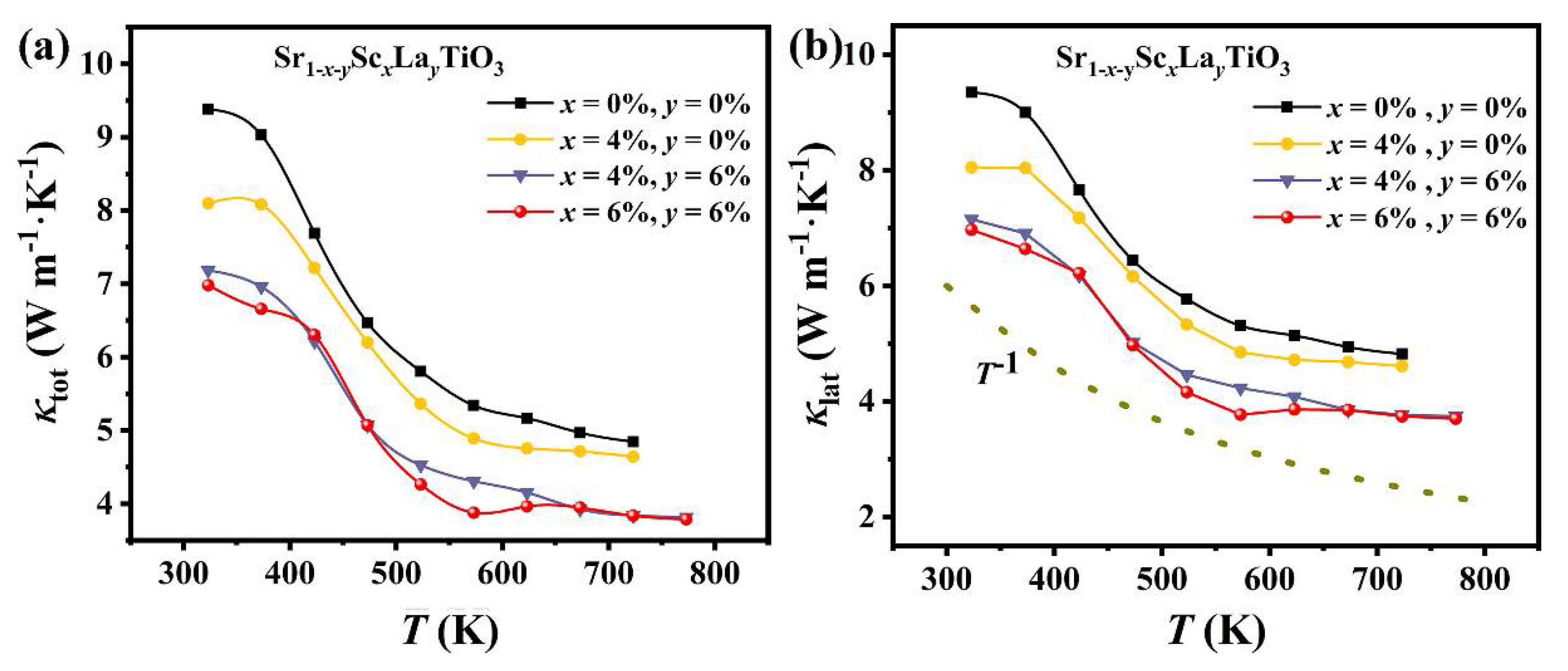
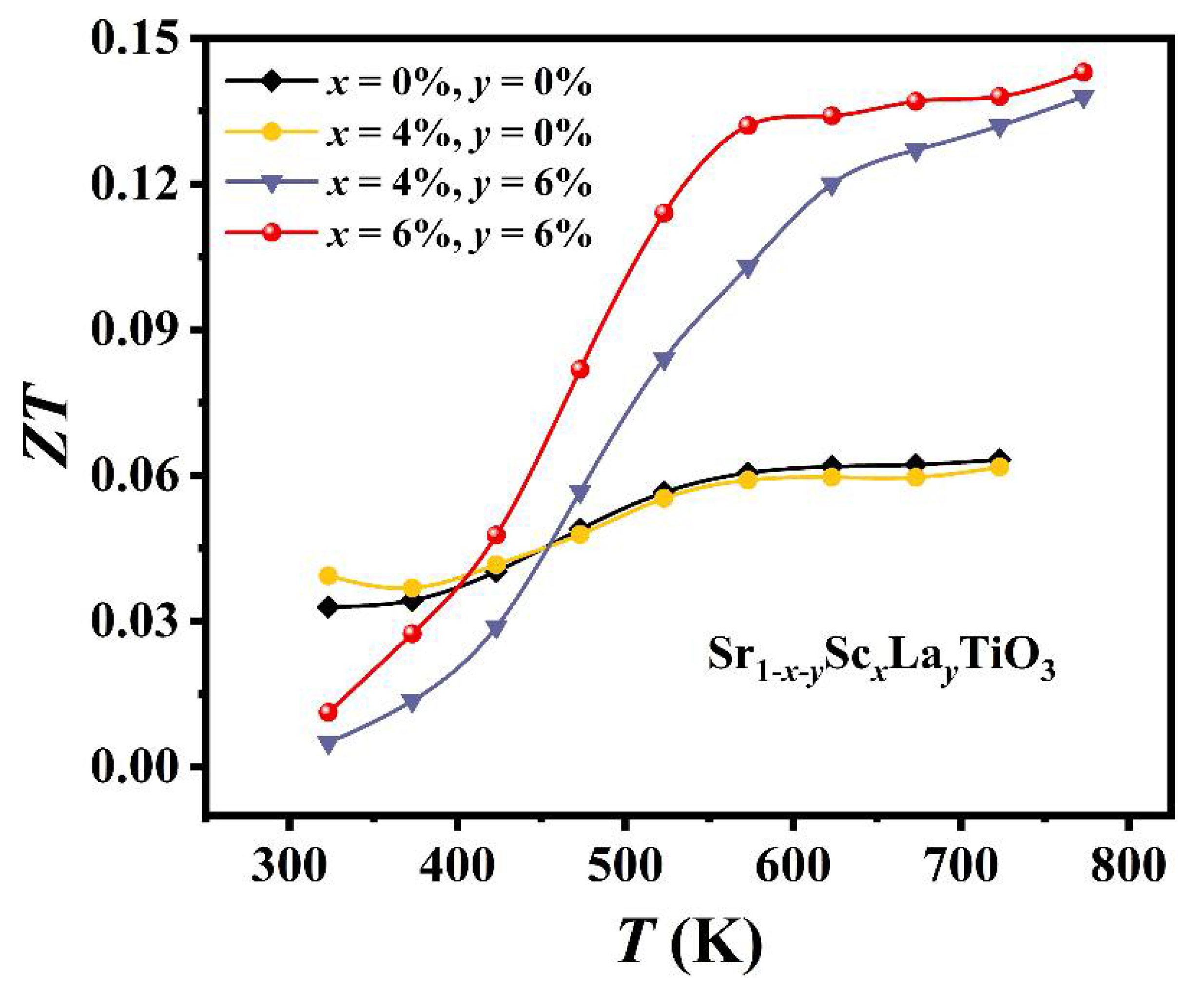
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tritt, T.M. Holey and unholey semiconductors. Science 1999, 283, 804–805. [Google Scholar] [CrossRef]
- Disalvo, F.J. Thermoelectric cooling and power generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, L.D. Thermoelectric materials: Energy conversion between heat and electricity. J. Mater. 2015, 1, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Bourgès, C.; Srinivasan, B.; Fontaine, B.; Sauerschnig, P.; Minard, A.; Halet, J.-F.; Miyazaki, Y.; Berthebaud, D.; Mori, T. Tailoring the thermoelectric and structural properties of Cu–Sn based thiospinel compounds [CuM1+xSn1−xS4 (M = Ti, V, Cr, Co)]. J. Mater. Chem. C 2020, 8, 16368–16383. [Google Scholar] [CrossRef]
- Muchtar, A.R.; Srinivasan, B.; Le Tonquesse, S.; Singh, S.; Soelami, N.; Yuliarto, B.; Berthebaud, D.; Mori, T. Physical insights on the lattice softening driven mid-temperature range thermoelectrics of Ti/Zr-inserted SnTe—an outlook beyond the horizons of conventional phonon scattering and excavation of Heikes’ Equation for estimating carrier properties. Adv. Energy Mater. 2021, 11, 2101122. [Google Scholar] [CrossRef]
- Li, J.F.; Liu, W.S.; Zhao, L.D.; Zhou, M. High-performance nanostructured thermoelectric materials. NPG Asia Mater. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Nan, Y.C.; Minnich, A.J.; Chen, G.; Ren, Z.F. Enhancement of thermoelectric figure-of-merit by a bulk nanostructuring approach. Adv. Funct. Mater. 2010, 20, 357–376. [Google Scholar]
- Pei, Y.; Shi, X.; LaLonde, A.; Wang, H.; Chen, L.; Snyder, G.J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature 2011, 473, 66–69. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, T.; Wang, H.; Hu, L.; Xie, H.; Jiang, G.; Snyder, G.J.; Zhao, X. Low electron scattering potentials in high performance Mg2Si0.45Sn0.55 based thermoelectric solid solutions with band convergence. Adv. Energy Mater. 2013, 3, 1238–1244. [Google Scholar] [CrossRef]
- Tan, G.; Shi, F.; Hao, S.; Chi, H.; Zhao, L.-D.; Uher, C.; Wolverton, C.; Dravid, V.P.; Kanatzidis, M.G. Codoping in SnTe: Enhancement of thermoelectric performance through synergy of resonance levels and band convergence. J. Am. Chem. Soc. 2015, 137, 5100–5112. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.H.; Mun, H.A.; Kim, H.S.; Hwang, S.W.; Roh, J.W.; Yang, D.J.; Shin, W.H.; Li, X.S.; Lee, Y.H.; et al. Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 2015, 348, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Meng, X.; Qin, D.; Cui, B.; Wu, H.; Zhang, Y.; Pennycook, S.J.; Cai, W.; Sui, J. New insights into the role of dislocation engineering in N-type filled skutterudite CoSb3. J. Mater. Chem. C 2019, 7, 13622–13631. [Google Scholar] [CrossRef]
- Hu, L.; Zhu, T.; Liu, X.; Zhao, X. Point defect engineering of high-performance bismuth-telluride-based thermoelectric materials. Adv. Funct. Mater. 2014, 24, 5211–5218. [Google Scholar] [CrossRef]
- Zhao, K.; Zhu, C.; Qiu, P.; Blichfeld, A.B.; Eikeland, E.; Ren, D.; Iversen, B.B.; Xu, F.; Shi, X.; Chen, L. High thermoelectric performance and low thermal conductivity in Cu2−yS1/3Se1/3Te1/3 liquid-like materials with nanoscale mosaic structures. Nano Energy 2017, 42, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Viciu, L.; Li, L.; Wang, Y.; Foo, M.L.; Watauchi, S.; Pascal JR, R.A.; Cava, R.J.; Ong, N.P. Large enhancement of the thermopower in NaxCoO2 at high Na doping. Nat. Mater. 2006, 5, 537–540. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, I.; Sasago, Y.; Uchinokura, K. Large Thermoelectric power in NaCo2O4 crystals. Phys. Rev. B 1997, 56, 12685–12687. [Google Scholar] [CrossRef]
- Masset, A.C.; Michel, C.; Maignan, A.; Hervieu, M.; Toulemonde, O.; Studer, F.; Raveau, B.; Hejtmanek, J. Misfit-layered cobaltite with an anisotropic giant magnetoresistance: Ca3Co4O9. Phys. Rev. B 2000, 62, 166–175. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Shi, Z.; Nan, C.-W. Preparation of Ca3Co4O9 and improvement of its thermoelectric properties by spark plasma sintering. J. Am. Ceram. Soc. 2005, 88, 1337–1340. [Google Scholar] [CrossRef]
- Ohta, H.; Sugiura, K.; Koumoto, K. Recent progress in oxide thermoelectric materials: P-type Ca3Co4O9 and n-type SrTiO3. Inorg. Chem. 2008, 47, 8429–8436. [Google Scholar] [CrossRef] [PubMed]
- Boston, R.; Schmidt, W.L.; Lewin, G.D.; lyasara, A.C.; Lu, Z.; Zhang, H.; Sinclair, D.C.; Reaney, I.M. Protocols for the fabrication, characterization, and optimization of n-type thermoelectric ceramic oxides. Chem. Mater. 2016, 29, 265–280. [Google Scholar] [CrossRef] [Green Version]
- Fergus, J.W. Oxide materials for high temperature thermoelectric energy conversion. J. Eur. Ceram. Soc. 2012, 32, 525–540. [Google Scholar] [CrossRef]
- Ito, M.; Furumoto, D. Microstructure and thermoelectric properties of NaxCo2O4/Ag composite synthesized by the polymerized complex method. J. Alloys Compd. 2008, 450, 517–520. [Google Scholar] [CrossRef]
- Muta, H.; Kurosaki, K.; Yamanaka, S. Thermoelectric properties of rare earth doped SrTiO3. J. Alloys Compd. 2003, 350, 292–295. [Google Scholar] [CrossRef]
- Ravichandran, J.; Siemons, W.; Oh, D.-W.; Kardel, J.T.; Chari, A.; Heijmerikx, H.; Scullin, M.L.; Majumdar, A.; Ramesh, R.; Cahill, D.G. High temperature thermoelectric response of double-doped SrTiO3 epitaxial films. Phys. Rev. B 2010, 82, 165126. [Google Scholar] [CrossRef] [Green Version]
- Shirai, K.; Yamanaka, K. Mechanism behind the high thermoelectric power factor of SrTiO3 by calculating the transport coefficients. J. Appl. Phys. 2013, 113, 188–576. [Google Scholar] [CrossRef]
- Okuda, T.; Nakanishi, K.; Miyasaka, S.; Tokura, Y. Large thermoelectric response of metallic perovskites: Sr1-xLaxTiO3 (0 < x < 0.1). Phys. Rev. B 2001, 63, 113104. [Google Scholar]
- Daniels, L.M.; Ling, S.; Savvin, S.N.; Pitcher, M.J.; Dyer, M.S.; Claridge, J.B.; Slater, B.; Corà, F.; Alaria, J.; Rosseinsky, M.J. A and B site doping of a phonon-glass perovskite oxide thermoelectric. J. Mater. Chem. A 2018, 6, 15640–15652. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, C.L.; Li, Y.; Su, W.B. Influence of rare earth doping on thermoelectric properties of SrTiO3 ceramics. J. Appl. Phys. 2013, 114, 223174. [Google Scholar] [CrossRef]
- Daniels, L.M.; Savvin, S.N.; Pitcher, M.J.; Dyer, M.S.; Claridge, J.B.; Ling, S.; Slater, B.; Cora, F.; Alaria, J.; Rosseinsky, M. Phonon-glass electron-crystal behaviour by A site disorder in n-type thermoelectric oxides. Energy Environ. Sci. 2017, 10, 1917–1922. [Google Scholar] [CrossRef] [Green Version]
- Akselrud, L.; Grin, Y. WinCSD: Software package for crystallographic calculations (Version 4). J. Appl. Crystallogr. 2014, 47, 803–805. [Google Scholar] [CrossRef]
- Hu, T.-Y.; Yao, M.-Y.; Hu, D.-L.; Gu, H.; Wang, Y.-J. Effect of mechanical alloying on sinterability and phase evolution in pressure-less sintered TiB2‒TiC ceramics. J. Mater. 2019, 5, 670–678. [Google Scholar] [CrossRef]
- Tkach, A.; Vilarinho, P.M. Scandium doped strontium titanate ceramics: Structure, microstructure, and dielectric properties. Bol. Soc. Esp. Artic. Cerámica Y Vidr. 2008, 47, 238–241. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Luo, D.; Huang, K. Electrical conductivity and stability of A-site deficient (La, Sc) co-doped SrTiO3 mixed ionic-electronic conductor. Mater. Lett. 2011, 65, 2624–2627. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Crystallogr. A 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Collignon, C.; Bourges, P.; Fauqué, B.; Behnia, K. Heavy nondegenerate electrons in doped strontium titanate. Phys. Rev. X 2020, 10, 031025. [Google Scholar] [CrossRef]
- Wang, Y.; Fujinami, K.; Zhang, R.; Wan, C.; Wang, N.; Ba, Y.; Koumoto, K. Interfacial thermal resistance and thermal conductivity in nanograined SrTiO3. Appl. Phys. Express 2010, 3, 031101. [Google Scholar] [CrossRef]
- Ahmed, A.; Hossain, M.S.A.; Islam, S.M.K.N.; Yun, F.; Yang, G.; Hossain, R.; Khan, A.; Na, J.; Eguchi, M.; Yamauchi, Y.; et al. Significant improvement in electrical conductivity and figure of merit of nanoarchitectured porous SrTiO3 by Ladoping optimization. ACS Appl. Mater. Interfaces 2020, 12, 28057–28064. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Reid, K.M.; Yu, X.; Leitner, D.M. Change in vibrational entropy with change in protein volume estimated with mode Gruneisen parameters. J. Chem. Phys. 2021, 154, 055102. [Google Scholar] [CrossRef]
- Bosoni, E.; Sosso, G.C.; Bernasconi, M. Grüneisen parameters and thermal conductivity in the phase change compound GeTe. J. Comput. Electron. 2017, 16, 997–1002. [Google Scholar] [CrossRef]
- Ren, G.-K.; Lan, J.-L.; Ventura, K.J.; Tan, X.; Lin, Y.-H.; Nan, C.-W. Contribution of point defects and nano-grains to thermal transport behaviours of oxide-based thermoelectrics. npj Comput. Mater. 2016, 2016, 16023. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Meisner, G.P.; Chen, L. Strain field fluctuation effects on lattice thermal conductivity of ZrNiSn-based thermoelectric compounds. Appl. Phys. Lett. 2004, 85, 1140–1142. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, H.; Lei, W.; Sinclair, D.C.; Reaney, I.M. High-figure-of-merit thermoelectric La-doped A-site-deficient SrTiO3 ceramics. Chem. Mater. 2016, 28, 925–935. [Google Scholar] [CrossRef] [Green Version]
| Nominal Comp. | Ti (%) | O (%) | Sr (%) | La (%) | Sc (%) | Real Comp. |
|---|---|---|---|---|---|---|
| SrTiO3 | 20.01 ± 0.27 | 60.04 ± 0.12 | 19.95 ± 0.25 | - | - | SrTi0.997O3 |
| Sr0.96Sc0.04TiO3 | 20.03 ± 0.16 | 60.05 ± 0.13 | 19.20 ± 0.15 | - | 0.72 ± 0.28 | Sr0.959Sc0.036Ti1.001O3 |
| Sr0.9Sc0.04La0.06TiO3 | 19.88 ± 0.21 | 60.18 ± 0.31 | 17.98 ± 0.19 | 1.14 ± 0.09 | 0.82 ± 0.32 | Sr0.897Sc0.040La0.058Ti0.99O3 |
| Sr0.88Sc0.06La0.06TiO3 | 20.02 ± 0.18 | 59.92 ± 0.21 | 17.69 ± 0.20 | 1.21 ± 0.06 | 1.16 ± 0.21 | Sr0.885Sc0.058La0.061Ti1.002O3 |
| Sr1-x-yScxLayTiO3 | Average Grain Size (μm) | Real Density (g cm−3) | Thermal Expansion Coefficients (10−5 K−1, 500–800 K) |
|---|---|---|---|
| SrTiO3 | 1.77 ± 0.07 | 5.105 | 1.00 ± 0.01 |
| Sr0.96Sc0.04TiO3 | 1.87 ± 0.01 | 4.997 | 1.01 ± 0.01 |
| Sr0.9Sc0.04La0.06TiO3 | 1.35 ± 0.07 | 5.124 | 1.02 ± 0.01 |
| Sr0.88Sc0.06La0.06TiO3 | 1.35 ± 0.05 | 5.134 | 1.08 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, K.; Yang, F.; Weng, T.; Chen, J.; Zhang, J.; Luo, J.; Li, H.; Rao, G.; Zhao, J. The Electrical and Thermal Transport Properties of La-Doped SrTiO3 with Sc2O3 Composite. Materials 2021, 14, 6279. https://doi.org/10.3390/ma14216279
Guo K, Yang F, Weng T, Chen J, Zhang J, Luo J, Li H, Rao G, Zhao J. The Electrical and Thermal Transport Properties of La-Doped SrTiO3 with Sc2O3 Composite. Materials. 2021; 14(21):6279. https://doi.org/10.3390/ma14216279
Chicago/Turabian StyleGuo, Kai, Fan Yang, Tianyao Weng, Jianguo Chen, Jiye Zhang, Jun Luo, Han Li, Guanghui Rao, and Jingtai Zhao. 2021. "The Electrical and Thermal Transport Properties of La-Doped SrTiO3 with Sc2O3 Composite" Materials 14, no. 21: 6279. https://doi.org/10.3390/ma14216279
APA StyleGuo, K., Yang, F., Weng, T., Chen, J., Zhang, J., Luo, J., Li, H., Rao, G., & Zhao, J. (2021). The Electrical and Thermal Transport Properties of La-Doped SrTiO3 with Sc2O3 Composite. Materials, 14(21), 6279. https://doi.org/10.3390/ma14216279






