New Organophilic Montmorillonites with Lactic Acid Oligomers and Other Environmentally Friendly Compounds and Their Effect on Mechanical Properties of Polylactide (PLA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Sodium Montmorillonite
2.3. Obtaining a PLA Composite with Selected Modified Montmorillonites
2.4. Test Methods and Apparatus
- The carbon and hydrogen content of the samples was determined using a Perkin Elmer CHNS/O II 2400 instrument (Perkin Elmer, Waltham, MA, USA).
- Fourier transform infrared spectra were recorded on a Perkin-Elmer Paragon 1000 spectrometer (Perkin Elmer, Waltham, MA, USA) equipped with a PIKE MIRacle ATR attachment with an AMTIR crystal from Pike Technologies. The wave number range from 500 to 4000 cm−1 was used with automatic background correction. Measurement control and data acquisition were performed using Perkin-Elmer’s Spectrum® 5.3 software.
- The XRD measurements of the powder samples were carried out on a D8 Discover diffractometer (Bruker, Billerica, MA, USA) equipped with a ceramic X-ray tube with a copper anode (radiation wavelength 1.54 Å). The diffractometer operates with a parallel beam system, a monochromator—a Goebel mirror (parabolic), and a position sensitive Vantec 1 detector. The tests were performed at room temperature.
- Thermogravimetric analysis was performed using the SDT Q600 V20.9 Build 23 instrument (TA Instruments, New Castle, DE, USA) from TA Instruments. Measurements were made in air or nitrogen atmospheres in the temperature range of 25–800 °C, the heating rate was 20 °C/min.
- The morphology of the samples was analyzed using an ULTRA Plus field emission scanning electron microscope (Zeiss, Jena, Germany) with a Zeiss GEMINI column (Zeiss, Jena, Germany), equipped with 2 secondary electron detectors: a standard one in the SE2 chamber and an in-column InLens. Prior to SEM measurement, the samples were vacuum sprayed with a conductive carbon layer.
- The swelling capacity was measured at room temperature by immersing a sample of dry powder weighing about 0.25 g in 100 cm3 of deionized water for 72 h. The samples were pre-dried at 110 °C for 3 h. After equilibrium was reached, excess water was removed from the sample by filtration and the material was dried to a constant weight. The swelling capacity (Seq) was determined gravimetrically using the following equation:where: Seq—swelling capacity, mwet—mass of wet sample, mdry—mass of dried sample, mwater—mass of absorbed water.
- Apparatus for strength measurements—Instron 5566 (Instron, Norwood, MA, United States). Measurements of mechanical parameters of rigid or flexible materials in the tensile or bending mode; the possibility of measuring samples of various shapes (foil, line/cord, bar).
3. Results and Discussion
3.1. Elemental Analyses
3.2. FTIR Spectroscopy of Sodium Mt and Organophilic Montmorillonites
3.3. X-ray Diffraction of Modified Montmorillonites
3.4. Thermal Stability of Modified Montmorillonites
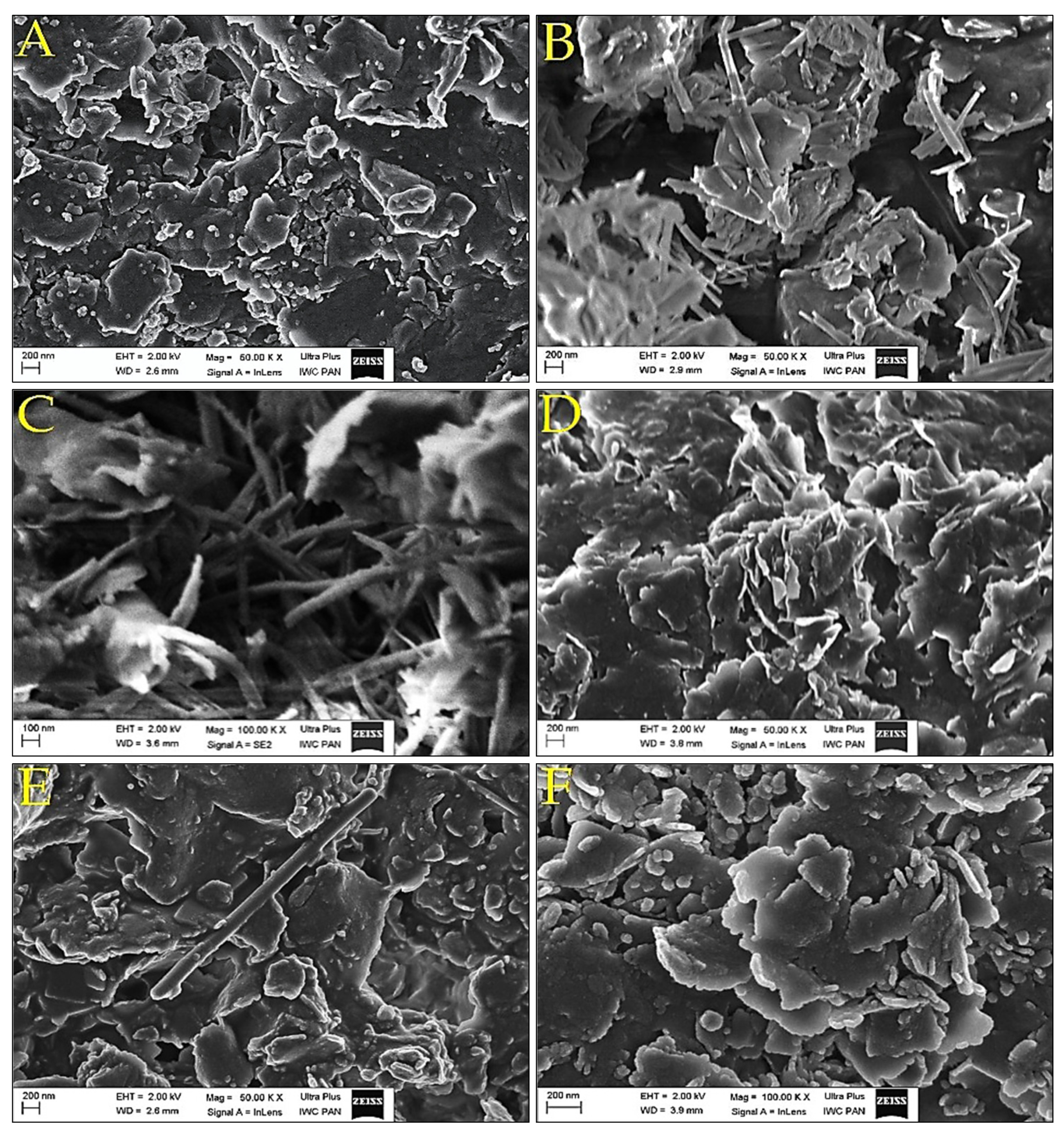
3.5. SEM Investigations
3.6. Swelling Capacity
3.7. Possibility to Use Montmorillonites with Lactic Acid Oligomers and Other Environmentally Friendly Compounds to Modify Mechanical Properties of PLA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uddin, F. Montmorillonite: An Introduction to Properties and Utilization. In Current Topics in the Utilization of Clay in Industrial and Medical Applications; IntechOpen: London, UK, 2018; pp. 1–23. [Google Scholar]
- Tsipursky, S.I.; Drits, V.A. The distribution of octahedral cations in the 2:1 layers of dioctahedral smectites studied by oblique-texture electron diffraction. Clay Miner. 1984, 19, 177–193. [Google Scholar] [CrossRef]
- Huang, J.; Tong, X.; Yang, J.; Wang, Z.; Zhang, M.; Wang, X.; Yang, J. Synthesis of poly (hexamethylene terephthalamide)-co-polycaprolactam/modified montmorillonite nanocomposites with enhanced mechanical properties and lower water absorption rate by in-situ polymerization. J. Polym. Res. 2020, 27, 138. [Google Scholar] [CrossRef]
- Kurleto, Ż.; Grabowska, B.; Kaczmarska, K.; Szymański, Ł. Chemical bonds in montmorillonite. Arch. Foundry Eng. 2015, 15, 79–82. [Google Scholar]
- Chanra, J.; Budiant, E.; Soegijono, B. Surface modification ofmontmorillonite by the use of organic cations via conventional ion exchange method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012057. [Google Scholar] [CrossRef]
- Yingyi, N.; Jinlong, S.; Meng, F.; Huiwen, C.; Zepeng, Z. Effects of cationic modifier type on the structure and morphology of organo-montmorillonite and its application properties in a high-temperature white oil system. Appl. Clay Sci. 2021, 203, 105995. [Google Scholar]
- Wang, Z.; Lu, X.; Liang, X.; Ji, J. Improving the Stability and Efficiency of Dimeric Fatty Acids Production by Increasing the Brønsted Acidity and Basal Spacing of Montmorillonite. Eur. J. Lipid Sci. 2020, 122, 1900342. [Google Scholar] [CrossRef]
- Yang, Z.; Li, B.; Tang, F. Influence of Cu2+-organic montmorillonites on thermal decomposition and smoke emission of poly(vinyl chloride) by cone calorimetric study. J. Vinyl Addit. Technol. 2007, 13, 31–39. [Google Scholar] [CrossRef]
- Zhu, A.; Cai, A.; Zhou, W.; Shi, Z. Effect of flexibility of grafted polymer on the morphology and property of nanosilica/PVC composites. Appl. Surf. Sci. 2008, 254, 3745–3752. [Google Scholar] [CrossRef]
- Betega de Paiva, L.; Morales, A.R.; Valenzuela Diaz, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Boukerrou, A.; Duchet, J.; Fellachi, S.; Kaci, M. Morphology and mechanical and viscoelastic properties of rubbery epoxy/organoclay montmorillonite nanocomposites. J. Appl. Polym. Sci. 2007, 103, 3547–3552. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, M.; Wang, F.; Li, P.; Shi, M. Adsorption properties and mechanism of montmorillonite modified by two Gemini surfactants with different chain lengths for three benzotriazole emerging contaminants: Experimental and theoretical study. Appl. Clay Sci. 2021, 207, 106086. [Google Scholar] [CrossRef]
- Hedley, C.B.; Yuan, G.; Theng, B.K.G. Thermal analysis of montmorillonites modified with quaternary phosphonium and ammonium surfactants. Appl. Clay Sci. 2007, 35, 180–188. [Google Scholar] [CrossRef]
- Madejová, J.; Barlog, M.; Jankovič, Ľ.; Slaný, M.; Pálková, H. Comparative study of alkylammonium- and alkylphosphoniumbased analogues of organo-montmorillonites. Appl. Clay Sci. 2021, 200, 105894. [Google Scholar] [CrossRef]
- Qiu, J.; Cui, K.; Chen, G.; Wang, Y.; Liu, D.; Jiang, S.; Wang, Y.; Wu, P.; Liu, X.; Wang, G.; et al. Micro-structure and gel performance of octadecyl trimethyl ammonium chloride intercalated montmorillonite. Coll. Surf. A Physicochem. Eng. Asp. 2021, 610, 125710. [Google Scholar] [CrossRef]
- Shuai, C.; Yu, L.; Feng, P.; Zhong, Y.; Zhao, Z.; Chen, Z.; Yang, W. Organic montmorillonite produced an interlayer locking effect in a polymer scaffold to enhance interfacial bonding. Mater. Chem. Front. 2020, 4, 2398–2408. [Google Scholar] [CrossRef]
- Wójcik-Bania, M.; Matusik, J. The Effect of Surfactant-Modified Montmorillonite on the Cross-Linking Efficiency of Polysiloxanes. Materials 2021, 14, 2623. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Wasim, M.; Naeem, M.A.; Khan, M.R.; Yue, S.; Saba, H.; Hussain, T.; Siddiqui, M.Q.; Farooq, A.; Wei, Q. Composite of PLA Nanofiber and Hexadecyl Trimethyl-Ammonium Chloride-Modified Montmorillonite Clay: Fabrication and Morphology. Coatings 2020, 10, 484. [Google Scholar] [CrossRef]
- Albozahid, M.; Naji, H.Z.; Alobad, Z.K.; Saiani, A. Effect of OMMT reinforcement on morphology and rheology properties of polyurethane copolymer nanocomposites. J. Elastomers Plast. 2021, 00952443211006160. [Google Scholar] [CrossRef]
- Atigh, Z.B.Q.; Sardari, P.; Moghiseh, E.; Lajayer, B.A.; Hursthouse, A.S. Purified montmorillonite as a nano-adsorbent of potentially toxic elements from environment: An overview. Nanotechnol. Environ. Eng. 2021, 6, 12. [Google Scholar] [CrossRef]
- Shakeri, F.; Nodehi, A.; Atai, M. PMMA/double-modified organoclay nanocomposites as fillers for denture base materials with improved mechanical properties. J. Mech. Behav. Biomed. Mater. 2019, 90, 11–19. [Google Scholar] [CrossRef]
- Hızall, J.; Yılmazoğlu, M. Montmorillonite Clay Composite for Heavy Metal Removal from Water. In Green Adsorbents to Remove Metals, Dyes and Boron from Polluted Water; Environmental Chemistry for a Sustainable World Book Series; Springer: Cham, Switzerland, 2021; Volume 49, pp. 93–112. [Google Scholar]
- Opafola, O.T.; David, A.O.; Ajibade, F.O.; Adeyemi, H.O.; Solana, O.I.; Odugbose, B.D. The utilization of bentonite enhanced termite mound soil mixture as filter for the treatment of paint industrial effluent. SN Appl. Sci. 2021, 3, 415. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Martín-Alfonso, M.J.; Valencia, C.; Cuberes, M.T. Rheological and tribological approaches as a tool for the development of sustainable lubricating greases based on nano-montmorillonite and castor oil. Friction 2021, 9, 415–428. [Google Scholar] [CrossRef]
- Lopes Alves, J.; de Rosa, T.V.; Realinho, V.; Antunes, M.; Velasco, J.I.; Morales, A.R. The effect of Brazilian organic-modified montmorillonites on the thermal stability and fire performance of organoclay-filled PLA nanocomposites. Appl. Clay Sci. 2020, 194, 105697. [Google Scholar] [CrossRef]
- Bezerra Lima, E.M.; Middea, A.; Marconcini, J.M.; Corrêa, A.C.; Fernandes Pereira, J.; Vieira Guimaraes, A.; Firmino de Lima, J.; Ramos dos Anjos, M.; Miranda de Castro, I.; Nunes Oliveira, R.; et al. Biodegradable PLA based nanocomposites for packaging applications: The effects of organo-modified bentonite concentration. J. Appl. Polym. Sci. 2021, 138, 50907. [Google Scholar] [CrossRef]
- Lopes Alves, J.; de Rosa, T.V.; de Redondo Realinho, V.C.; de Sousa Pais Antunes, M.; Velasco, J.I.; Morales, A.R. Single and hybrid organoclay-filled PLA nanocomposites: Mechanical properties, viscoelastic behavior and fracture toughening mechanism. J. Appl. Polym. Sci. 2021, 138, 50784. [Google Scholar] [CrossRef]
- Bijarimi, M.; Syuhada, A.; Zulaini, N.; Shahadah, N.; Alhadadi, W.; Ahmad, M.N.; Ramli, A.; Normaya, E. Poly(lactic acid)/Acrylonitrile Butadiene Styrene Nanocomposites with Hybrid Graphene Nanoplatelet/Organomontmorillonite: Effect of Processing Temperatures. Int. Polym. Proc. 2020, 35, 355–366. [Google Scholar] [CrossRef]
- Rucińska, K. Inorganic-Organic Composites Containing Lactic Acid Oligomers. Ph.D. Thesis, Warsaw University of Technology, Warsaw, Poland, 2019. [Google Scholar]
- Frydrych, A.; Florjańczyk, Z.; Kundys, A.; Plichta, A. New route to segmental star-shaped copolymers of lactic acid. Mol. Cryst. Liquid Cryst. 2014, 603, 89–98. [Google Scholar] [CrossRef]
- Florjańczyk, Z.; Frydrych, A.; Chudzik, A.; Rucińska, K.; Basamon, M. Synthesis of star shaped copolymers obtained from lactic acid and hetetocyclic monomers. Polimery 2017, 62, 291–297. [Google Scholar] [CrossRef]
- Florjańczyk, Z.; Chudzik, A.; Frydrych, A.; Rucinska, K. Synthesis and characterization of lactic acid-citric acid copolymers. Polimery 2017, 62, 335–343. [Google Scholar] [CrossRef]




| Sample | Elemental Analysis | Seq (g/g) | d(001) (nm) | |
|---|---|---|---|---|
| %C | %H | |||
| Mt | 0.78 | 1.56 | 5.651 | 1.28 |
| Mt-LAc | 17.64 | 2.77 | 2.297 | 1.77 |
| Mt-LAc-CAc | 13.23 | 2.05 | 3.176 | 1.91 |
| Mt-LAc-MA | 21.43 | 3.06 | 3.131 | 2.01 |
| Mt-LAc-PT | 14.81 | 2.09 | 2.828 | 1.83 |
| Mt-LAc-SAc | 12.79 | 2.25 | 1.356 | 1.79 |
| Mt-LAc-CAP | 19.99 | 3.37 | 1.489 | 1.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rucińska, K.; Florjańczyk, Z.; Dębowski, M.; Gołofit, T.; Malinowski, R. New Organophilic Montmorillonites with Lactic Acid Oligomers and Other Environmentally Friendly Compounds and Their Effect on Mechanical Properties of Polylactide (PLA). Materials 2021, 14, 6286. https://doi.org/10.3390/ma14216286
Rucińska K, Florjańczyk Z, Dębowski M, Gołofit T, Malinowski R. New Organophilic Montmorillonites with Lactic Acid Oligomers and Other Environmentally Friendly Compounds and Their Effect on Mechanical Properties of Polylactide (PLA). Materials. 2021; 14(21):6286. https://doi.org/10.3390/ma14216286
Chicago/Turabian StyleRucińska, Katarzyna, Zbigniew Florjańczyk, Maciej Dębowski, Tomasz Gołofit, and Rafał Malinowski. 2021. "New Organophilic Montmorillonites with Lactic Acid Oligomers and Other Environmentally Friendly Compounds and Their Effect on Mechanical Properties of Polylactide (PLA)" Materials 14, no. 21: 6286. https://doi.org/10.3390/ma14216286






