Analysis of the Quality of Sulfomolybdenum Coatings Obtained by Electrospark Alloying Methods
Abstract
:1. Introduction
- there is volumetric heating of the parts, resulting in the occurrence of changes in their structures and initial geometric parameters (deformations and curvatures);
- there is a need for bulky and expensive technological equipment;
- there is a long process duration;
- there is high energy consumption;
- there are issues regarding marginal cost;
- there are environmental- and technogenic-related concerns, etc.
- An anode material (an alloying one) can form a coating layer on the surface of the cathode (the surface being alloyed), with the coating layer being extremely tightly adhered to the above surface. In this case, not only is there an absence of an interface between the deposited material and the base metal, but there is also an even diffusion of the anode elements into the cathode;
- The alloying process can be carried out in specified places (with the radius range starting from particles the size of a millimeter and larger) without protecting the rest of the surface of the part being alloyed;
- The application ofthe ESAmethod on metal surfaces is very simple, and the necessary equipment is compact and transportable [24].
- there is an inability to form molybdenum disulfide on the surface of the parts;
- there is a decreased possibility of reducing the coefficient of friction;
- there is insufficiently high running-in and wear resistance within the friction pairs, especially without lubrication.
2. Materials and Methods
3. Results and Discussion
4. Conclusions
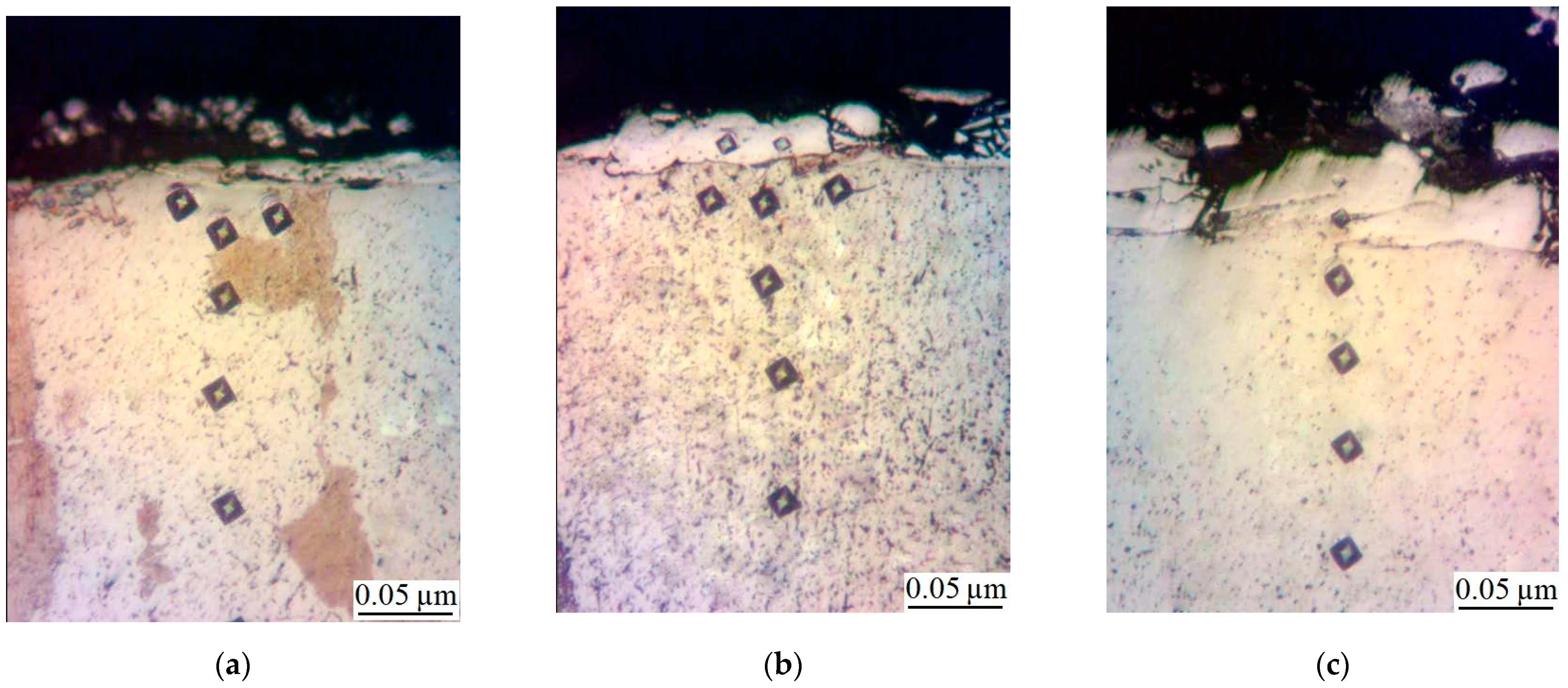
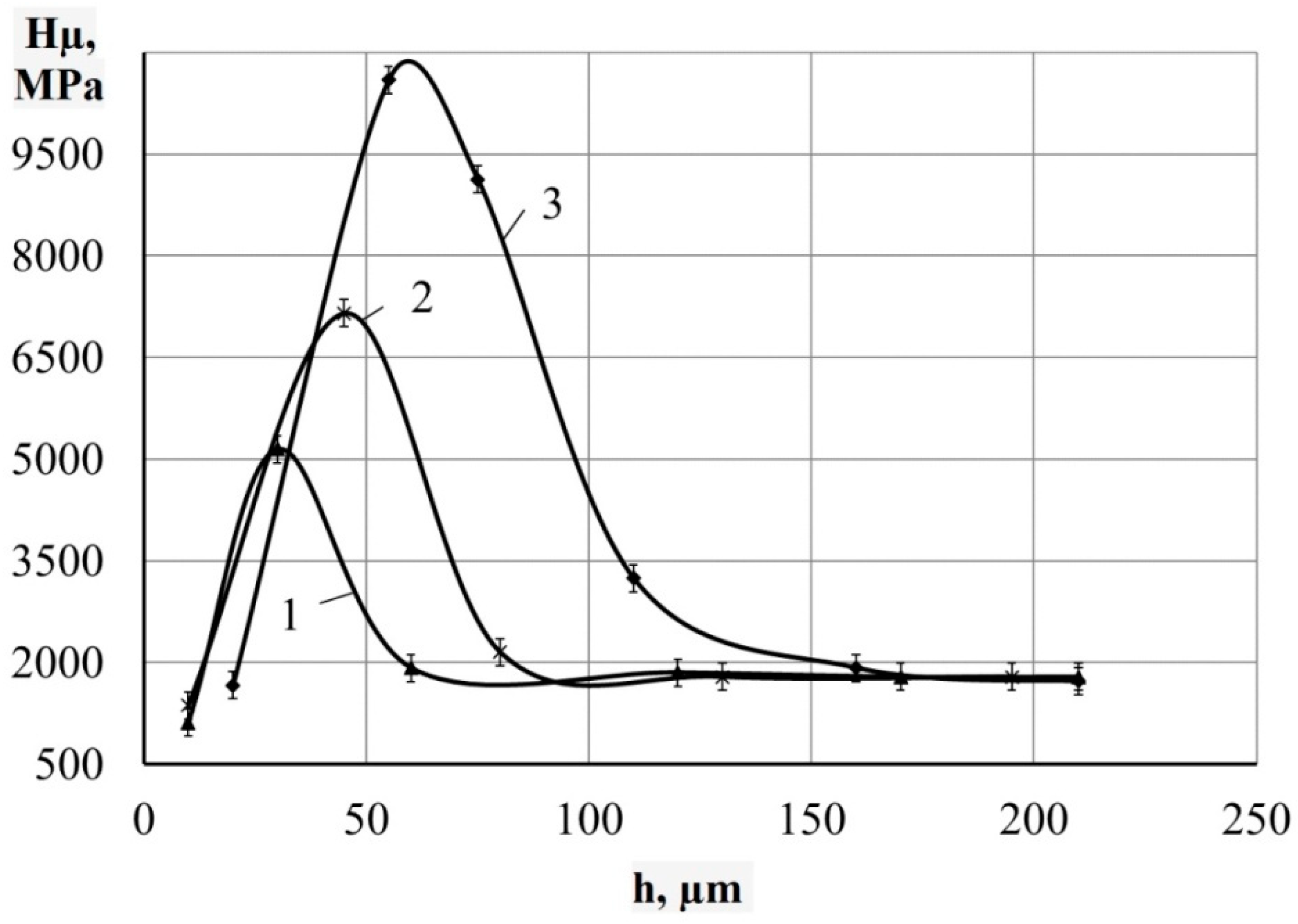
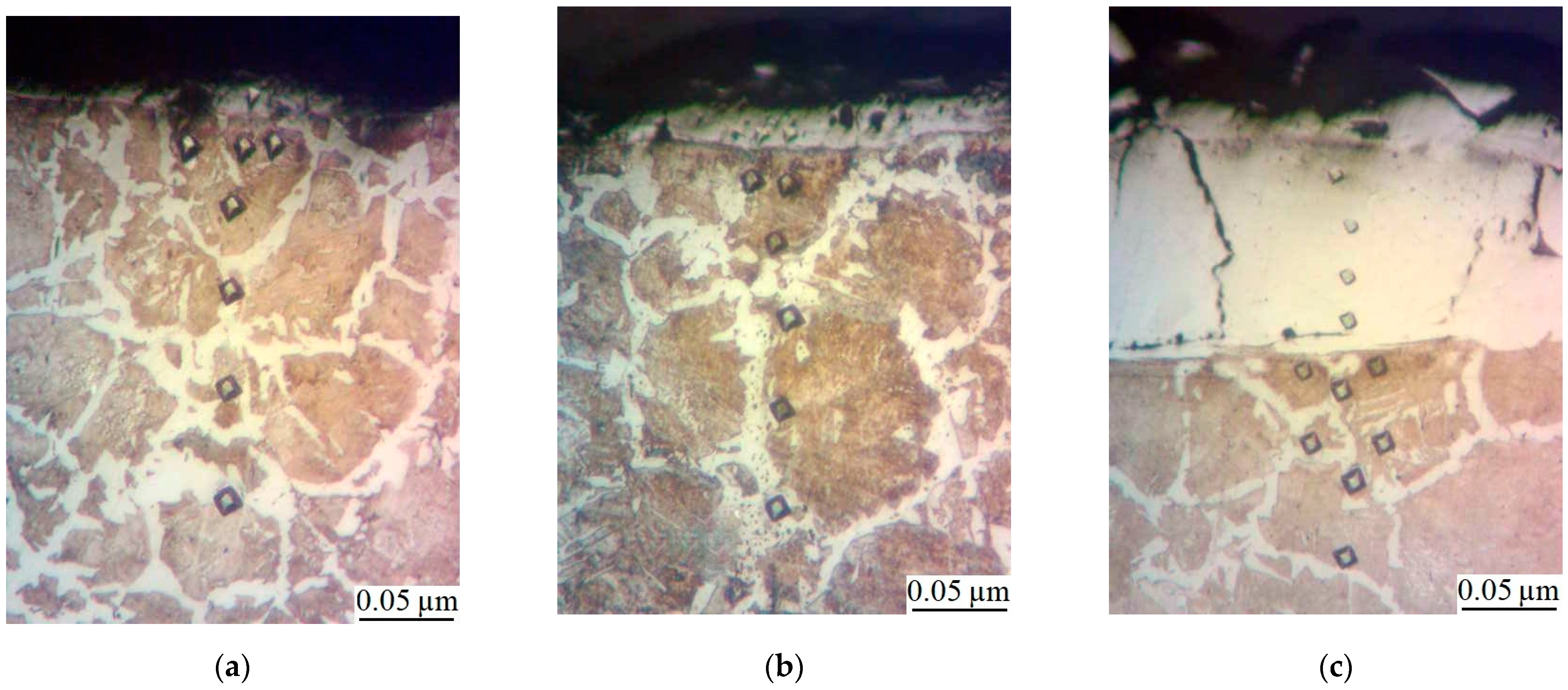
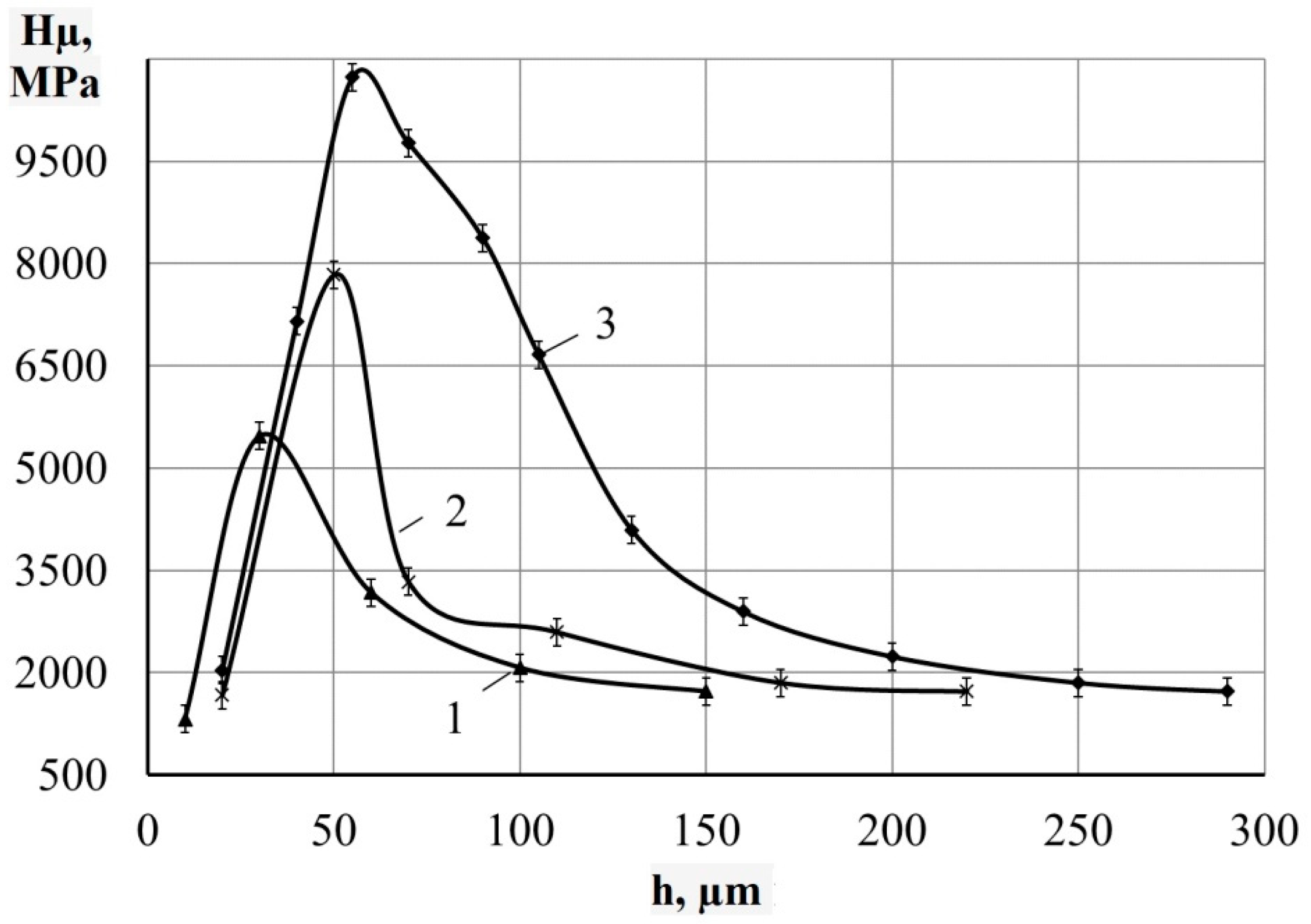
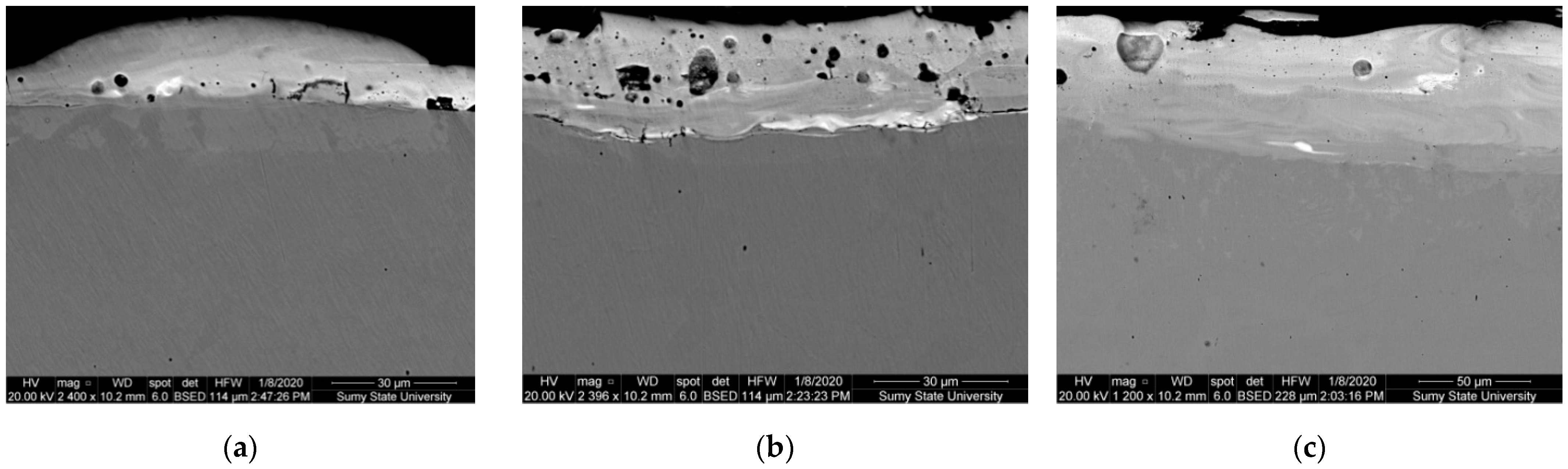
- Metallographic and durametric studies have shown that the sulfomolybdenum coatings consist of the following four zones:
- the upper loose layer has the microhardness Hμ of 1112 to 2040 MPa;
- the “white” strengthened layer has the microhardness Hμ of 5147 to 5474 MPa for Wp = 0.13 J and Hμ of 10596 to 10731 MPa for Wp = 3.4 J;
- the diffusion zone; and
- he base metal.
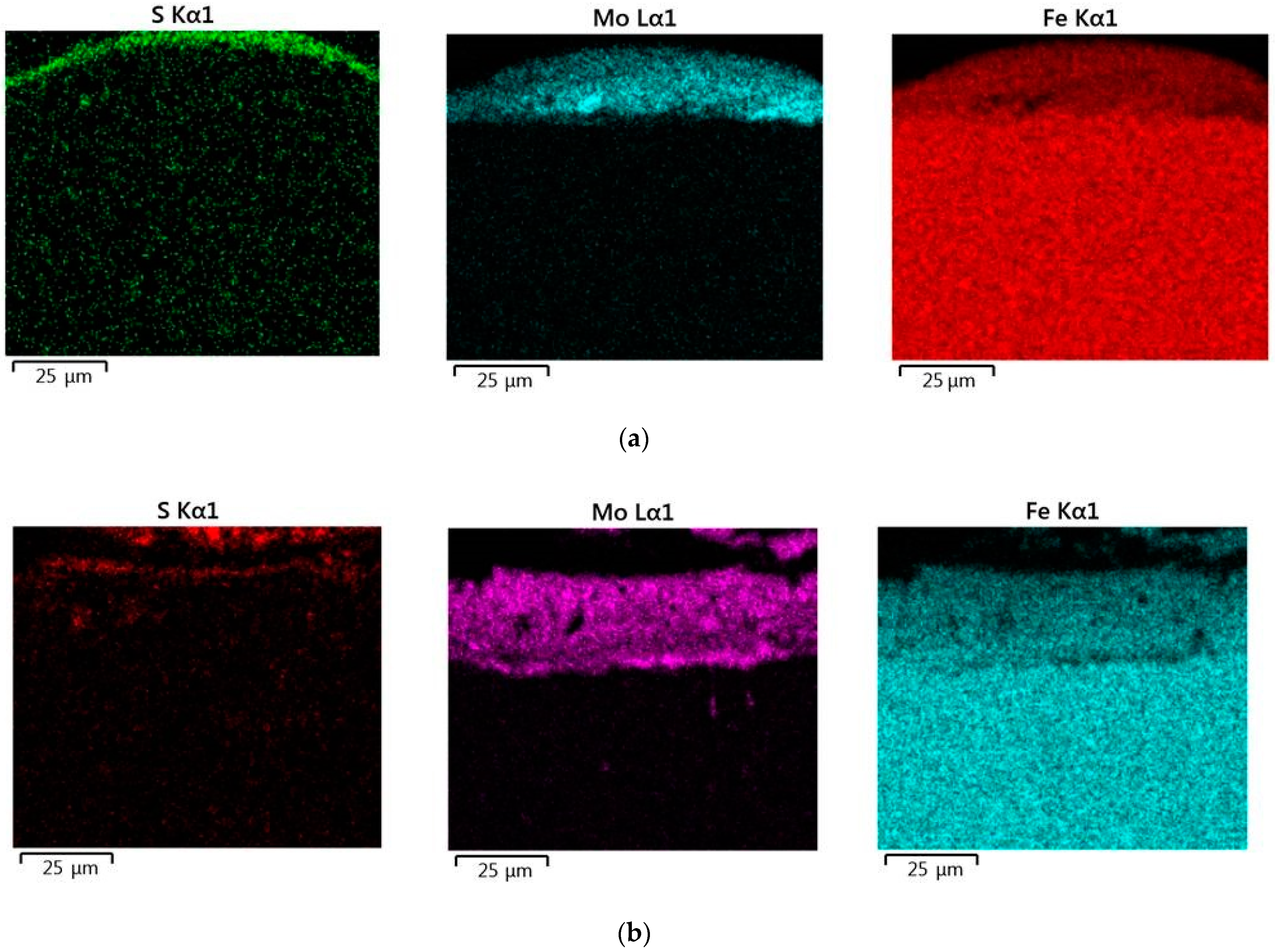
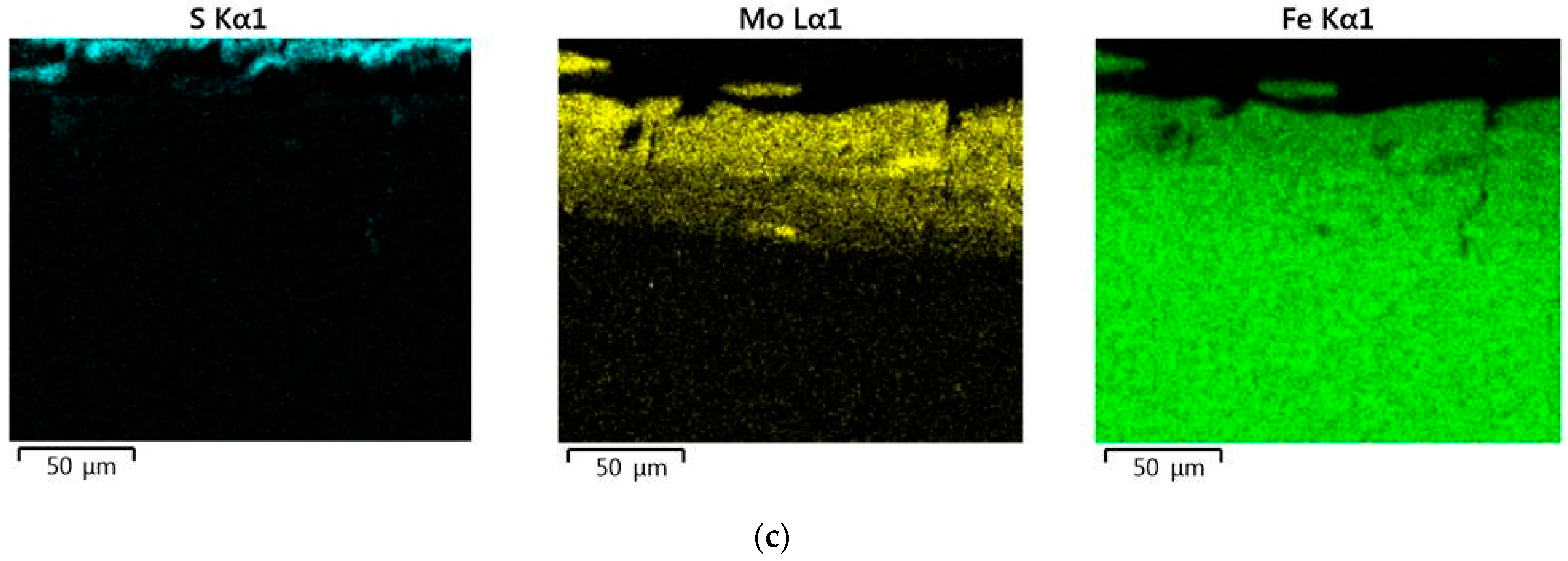
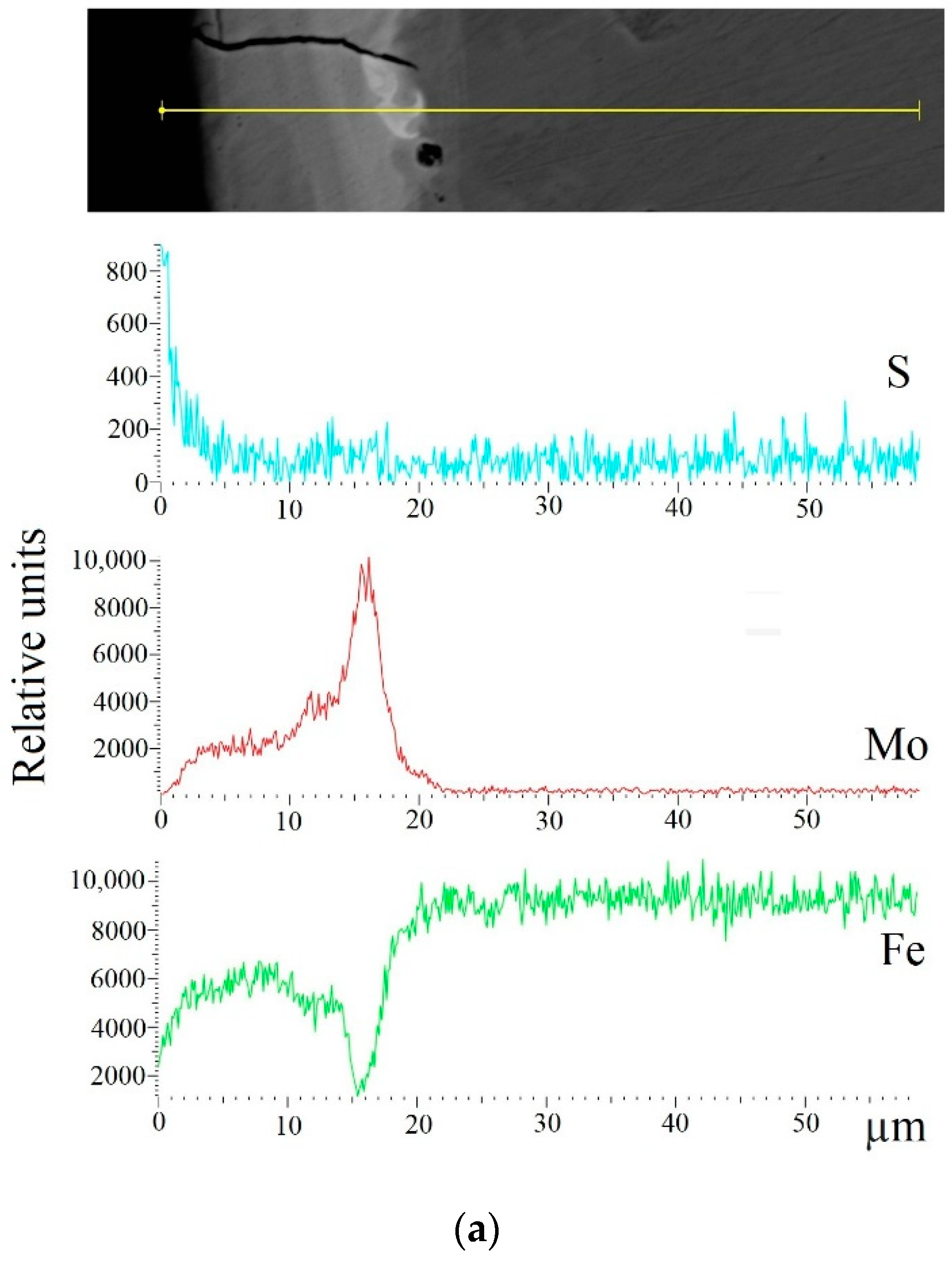
When replacing the substrate of steel C22 with steel C40, there is an increase in the values of the microhardness, the thickness of the strengthened layer, as well as the continuity thereof. - Electron microscopic studies of the obtained coatings showed that the resulting layer has a heterogeneous composition with different concentrations of elements. According to the element distribution maps of the areas of the studied specimens, sulfur is concentrated on the surface and molybdenum is more evenly distributed in the coating. The energy dispersion analysis showed that sulfur and molybdenum are concentrated at depths up to 4 and 19 μm, respectively, at Wp = 0.13 J, up to 5 and 25 μm, respectively, at Wp = 0.55 J, and up to 15 and 70 μm, respectively, at Wp = 3.4 J.
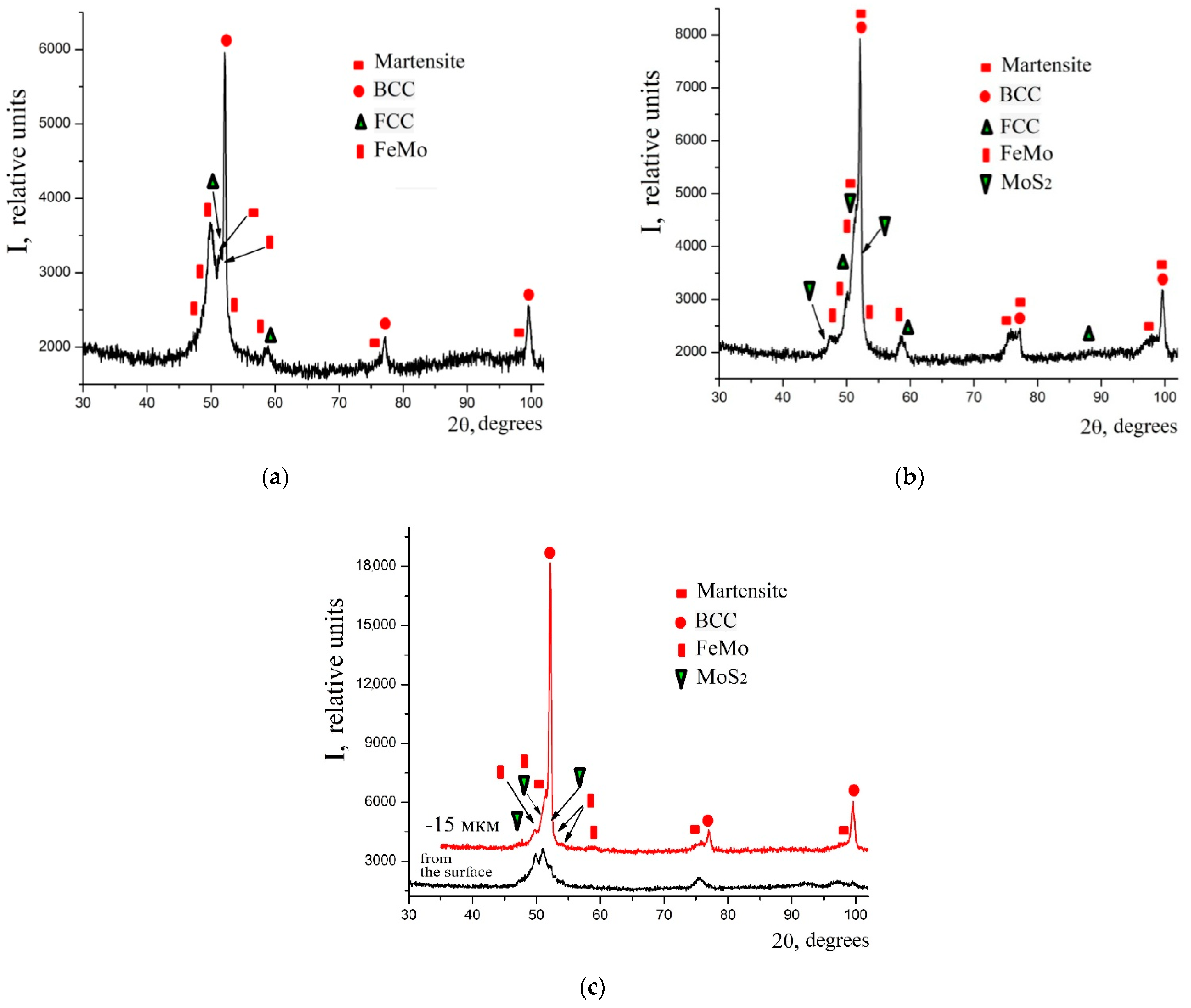
- The X-ray diffraction analysis of the obtained coatings confirms the results of the energy dispersion analysis. At low discharge energies, the phase composition of the coatings on C40 steel is represented by BCC (body centered cubic) solid solution with a lattice period close to ferrite, martensite, FCC (face centered cubic) solid solution, and FeMointermetallide (σ-phase). When replacing the substrate made of C40 steel with the substrate made of C22 steel, the sulfomolybdenum coating reveals a smaller amount of the FCC phase that is residual austenite, at the same discharge energy. Molybdenum disulfide is even formed at the discharge energy Wp = 0.55 J (3.77%); and at Wp = 3.4 J, about 8% thereof is found on the surface, and about 5% thereof is found at a depth down to 15 μm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budnik, O.A.; Sviderskii, V.A.; Budnik, A.F.; Berladir, K.V.; Rudenko, P.V. Composite material for chemical and petrochemical equipment friction assemblies. Chem. Pet. Eng. 2016, 52, 63–68. [Google Scholar] [CrossRef]
- Pavlenko, I.V.; Simonovskiy, V.I.; Demianenko, M.M. Dynamic analysis of centrifugal machines rotors supported on ball bearings by combined application of 3D and beam finite element models. IOP Conf. Ser. Mater. Sci. Eng. 2017, 233, 012053. [Google Scholar] [CrossRef] [Green Version]
- Sviridenok, A.I.; Myshkin, N.K.; Kovaleva, I.N. Latest Developments in Tribology in the Journal of Friction and Wear. J. Frict. Wear 2015, 36, 449–453. [Google Scholar] [CrossRef]
- Jeon, C.H.; Jeong, Y.H.; Seo, J.J.; Tien, H.N.; Hong, S.T.; Yum, Y.J.; Hur, S.H.; Lee, K.J. Material Properties of Graphene/Aluminum Metal Matrix Composites Fabricated by Friction Stir Processing. Int. J. Precis. Eng. Manuf. 2014, 15, 1235–1239. [Google Scholar] [CrossRef]
- Roik, T.A.; Gavrish, A.P.; Kirichok, P.A.; Vitsyuk, Y.Y. Effect of Secondary Structures on the Functional Properties of High Speed Sintered Bearings for Printing Machines. Powder Metall. Met. Ceram. 2015, 54, 119–127. [Google Scholar] [CrossRef]
- Roik, T.A.; Gavrysh, O.A.; Vitsiuk, I.I.; Khmiliarchuk, O.I. New Copper-Based Composites for Heavy-Loaded Friction Units. Powder Metall. Met. Ceram. 2018, 56, 516–522. [Google Scholar] [CrossRef]
- Kostornov, A.G.; Fushchich, O.I. Sintered Antifriction Materials. Powder Metall. Met. Ceram. 2007, 46, 503–512. [Google Scholar] [CrossRef]
- Dachun, L.; Xiumin, C.; Dianjun, L.; Fei, W.; Xiaogang, L.; Bin, Y. Simulation of MoS2 crystal structure and the experimental study of thermal decomposition. J. Mol. Struct. 2010, 980, 66–71. [Google Scholar] [CrossRef]
- Pajovic, S.; Colas, G.; Saulot, A.; Renouf, M.; Filleter, T. Work of Adhesion Measurements of MoS2 Dry Lubricated 440C Stainless Steel Tribological Contacts. Adv. Eng. Mater. 2017, 19, 1700423. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structures, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Presting, H.; König, U. Future nanotechnology developments for automotive applications. Mater. Sci. Eng. C 2003, 23, 737–741. [Google Scholar] [CrossRef]
- Hsu, S.M. Molecular basis of lubrication. Tribol. Int. 2004, 37, 553–559. [Google Scholar]
- Afanasiev, P.; Xia, G.F.; Berhault, G.; Jouguet, B.; Lacroix, M. Surfactant Assisted Synthesis of Highly Dispersed Molybdenum Sulfide. Chem. Mater 1999, 11, 3216. [Google Scholar] [CrossRef]
- Lansdowne, A.R. Molybdenum Disulphide Lubrication; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Bakunin, V.; Suslov, A.; Kuzmina, G.; Parenago, O.; Topchiev, A. Synthesis and Application of Inorganic Nanoparticles as Lubricant Components—A Review. J. Nanoparticle Res. 2004, 6, 273–284. [Google Scholar] [CrossRef]
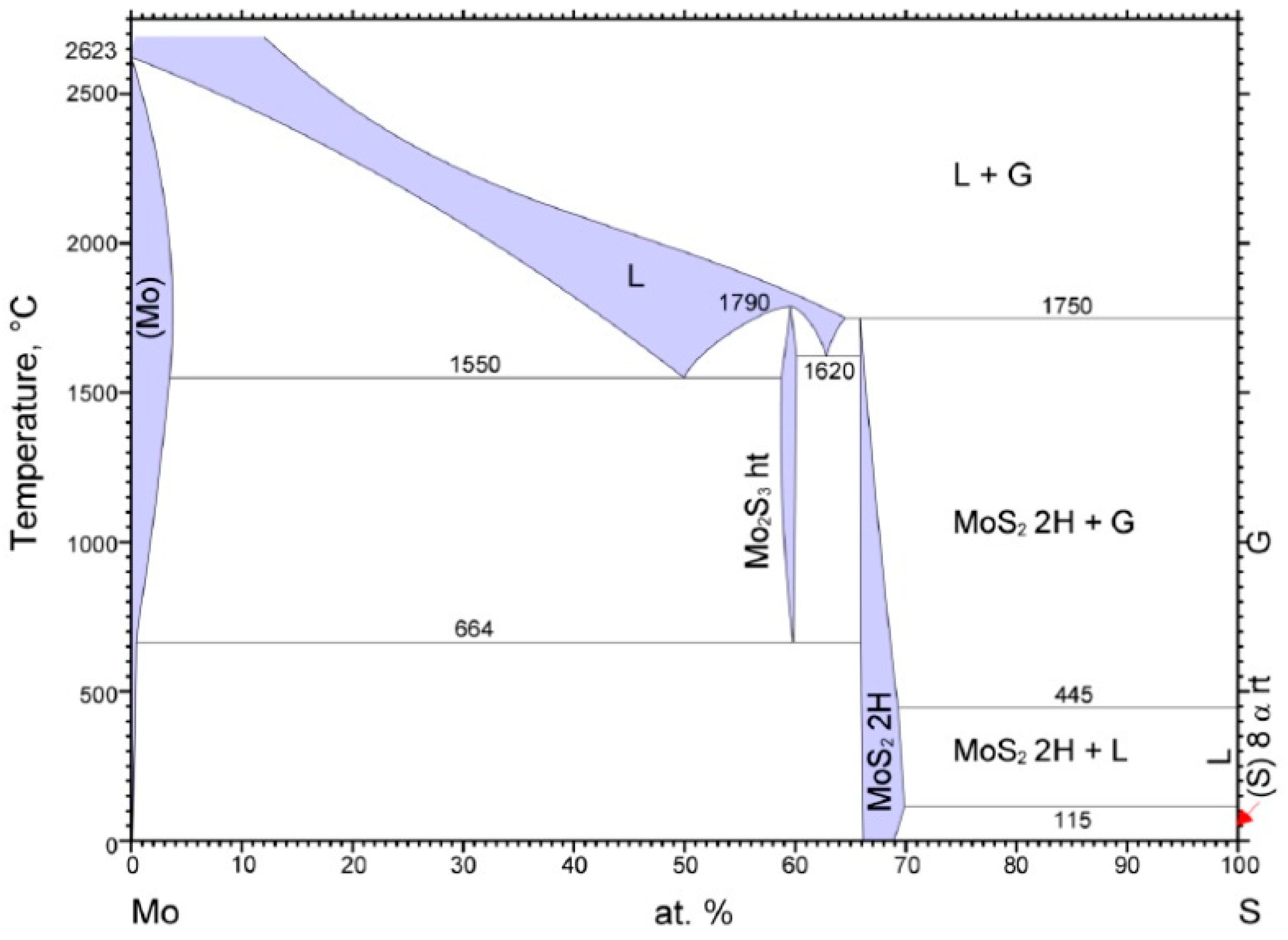
- Massalski, T.B. Binary Alloy. Phase Diagrams; ASM International: Novelty, OH, USA, 1990; p. 370. [Google Scholar]
- Gnezdilova, Y.P.; Serebrovsky, V.V. Strengthening by Sulfocyanization of Electrodeposited Iron-Molybdenum Coatings for the Restoration of Machine Parts; Publishing House of the Kursk State Agricultural Academy (KSAA): Kursk, Russian, 2008; p. 143. Available online: https://elibrary.ru/item.asp?id=19569072& (accessed on 8 June 2021)[in Russian].
- Martsynkovskyy, V.; Tarelnyk, V.; Konoplianchenko, Y.; Gaponova, O.; Dumanchuk, M. Technology support for protecting contacting surfaces of half-coupling—Shaft press joints against fretting wear. In Advances in Design, Simulation and Manufacturing II. DSMIE 2019. Lecture Notes in Mechanical Engineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 216–225. [Google Scholar]
- Timofeev, S.S. Application of Antifriction Coatings Containing Molybdenum Disulphide; Collection of Scientific Works of the Ukrainian State University of Railway Transport (UkrSURT), 2015; pp. 207–210. Available online: http://193.105.7.137/handle/123456789/1174 (accessed on 8 June 2021). [in Russian].
- Smorygo, O.; Voronin, S.; Bertrand, P.; Smurov, I. Fabrication of Thick Molybdenum Disulphide Coatings by Thermal-Diffusion Synthesis. Tribol. Lett. 2004, 17, 723–726. [Google Scholar] [CrossRef]
- Kostyk, K.; Hatala, M.; Kostyk, V.; Ivanov, V.; Pavlenko, I.; Duplakova, D. Simulation of diffusion processes in chemical and thermal processing of machine parts. Processes 2021, 9, 698. [Google Scholar] [CrossRef]
- Tarelnyk, V.B.; Gaponova, O.P.; Konoplyanchenko, I.V.; Evtushenko, N.S.; Herasymenko, V.A. The Analysis of a Structural State of Surface Layer after Electroerosive Alloying. I. Features of Formation of Electroerosive Coatings on Steel 45. Metallofiz. NoveishieTekhnol. 2018, 40, 235–254, [in Russian]. [Google Scholar]
- Tarelnyk, V.B.; Gaponova, O.P.; Konoplyanchenko, I.V.; Yevtushenko, N.S.; Herasymenko, V.O. The Analysis of a Structural State of Surface Layer after Electroerosive Alloying. II. Features of Formation of Electroerosive Coatings on Special Steels and Alloys by Hard Wear-Resistant and Soft Antifriction Materials, [in Russian]. Metallofiz. NoveishieTekhnol. 2018, 40, 795–815. [Google Scholar] [CrossRef]
- Lešnjak, A.; Tušek, J. Processes and properties of deposits in electrospark deposition. Sci. Technol. Weld. Join. 2002, 7, 391–396. [Google Scholar] [CrossRef]
- Ablaev, A.A.; Ratnikov, A.S. Technology of Electrospark Cladding of the Surfaces of the Cylinder-Piston Group of Engines for Transport-Technological Machines [in Russian]. In Problems of Operation and Maintenance of Transport-Technological Machines. Materials of the International Scientific and Technical Conference; Zakharov, N.S., Ed.; The Tyumen Industrial University: Tyumen, Russia, 2009; pp. 12–15. Available online: https://elibrary.ru/item.asp?id=23510188 (accessed on 8 June 2021).
- Tarelnyk, V.; Konoplyanchenko, I.V.; Martsynkovskyy, V.; Dovzhyk, M.; Dumanchuk, M.; Goncharenko, M.; Antoszewski, B.; Gaponova, O. Investigation of Qualitative Parameters of Surface Layers Formed by Stepwise Carburizing and Sulfo-Carburizing of Steel Parts with the Use of Electroerosion Alloying Method. In Proceedings of the 8th IEEE International Conference on Nanomaterials: Applications and Properties, NAP 2018, Zatoka, Ukraine, 9–14 September 2018; p. 03TFNMC26. [Google Scholar] [CrossRef]


| Discharge Energy Wp, J | Productivity, cm2/min |
|---|---|
| 0.13 | 0.8 ÷ 1.0 |
| 0.55 | 1.0 ÷ 1.3 |
| 1.3 | 1.3 ÷ 1.5 |
| 2.6 | 1.5 ÷ 2.0 |
| 3.4 | 2.0 ÷ 2.5 |
| 6.8 | 2.5 ÷ 3.0 |
| Discharge Energy, J | Roughness, μm | Layer of Reduced Microhardness | Strengthened Layer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ra | Rz | Rmax | Hµ, MPa | h, μm | S, % | Hµ, MPa | h, μm | S, % | |
| Steel C22 | |||||||||
| 0.13 | 0.6 | 2.1 | 6.1 | 1112 ± 200 | 20 ± 5 | 45 ± 5 | 5147 ± 200 | 20 ± 5 | 65 ± 5 |
| 0.55 | 1.9 | 3.3 | 14.2 | 1368 ± 200 | 30 ± 5 | 65 ± 5 | 7150 ± 200 | 30 ± 5 | 75 ± 5 |
| 3.40 | 5.5 | 14.7 | 38.5 | 1666 ± 200 | 40 ± 5 | 75 ± 5 | 10596 ± 200 | 50 ± 5 | 90 ± 5 |
| Steel C40 | |||||||||
| 0.13 | 0.8 | 2.3 | 6.5 | 1320 ± 200 | 10 ± 5 | 50 ± 5 | 5474 ± 200 | 25 ± 5 | 75 ± 5 |
| 0.55 | 2.0 | 3.5 | 14.7 | 1670 ± 200 | 20 ± 5 | 70 ± 5 | 7832 ± 200 | 40 ± 5 | 90 ± 5 |
| 3.40 | 5.7 | 14.9 | 38.7 | 2040 ± 200 | 30 ± 5 | 80 ± 5 | 10731 ± 200 | 70 ± 5 | 95 ± 5 |
| Discharge Energy, J | Phase | Lattice Period, a, nm | Phase Content, % (mass.) |
|---|---|---|---|
| 0.55 | BCC solid solution | 2.8720 | 23.90 |
| FCC solid solution | 3.6450 | 25.38 | |
| Martensite | a = 2.8740 c = 2.9200 | 10.98 | |
| FeMo (σ-phase) | a = 9.1280 c = 4.8130 | 39.74 | |
| At a depth down to 15 µm | |||
| 3.4 | BCC solid solution | 2.8720 | 46.36 |
| FCC solid solution | 3.6450 | 6.10 | |
| Martensite | a = 2.8640 c = 2.9200 | 30.14 | |
| FeMo (σ-phase) | a = 9.1280 c = 4.8130 | 12.53 | |
| MoS2 | a = 3.1212 c = 12.2410 | 4.87 | |
| Discharge Energy, J | Phase | Lattice Period, a, nm | Phase Content, % (mass.) |
|---|---|---|---|
| 0.13 | BCC solid solution | 2.8720 | 39.43 |
| FCC solid solution | 3.6450 | 16.15 | |
| Martensite | a = 2.8640 c = 2.9200 | 18.22 | |
| 0.55 | BCC solid solution | 2.8720 | 34.92 |
| FCC solid solution | 3.6450 | 13.72 | |
| Martensite | a = 2.8640 c = 2.9200 | 30.46 | |
| FeMo (σ-phase) | a = 9.1280 c = 4.8130 | 17.14 | |
| MoS2 | a = 3.1212 c = 12.2410 | 3.77 | |
| At a depth down to 15 µm | |||
| 3.4 | BCC solid solution | 2.8800 | 59.86 |
| Martensite | a = 2.8640 c = 2.9200 | 25.46 | |
| FeMo (σ-phase) | a = 9.1280 c = 4.8130 | 9.56 | |
| MoS2 | a = 3.1212 c = 12.2410 | 5.12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaponova, O.P.; Antoszewski, B.; Tarelnyk, V.B.; Kurp, P.; Myslyvchenko, O.M.; Tarelnyk, N.V. Analysis of the Quality of Sulfomolybdenum Coatings Obtained by Electrospark Alloying Methods. Materials 2021, 14, 6332. https://doi.org/10.3390/ma14216332
Gaponova OP, Antoszewski B, Tarelnyk VB, Kurp P, Myslyvchenko OM, Tarelnyk NV. Analysis of the Quality of Sulfomolybdenum Coatings Obtained by Electrospark Alloying Methods. Materials. 2021; 14(21):6332. https://doi.org/10.3390/ma14216332
Chicago/Turabian StyleGaponova, Oksana P., Bogdan Antoszewski, Viacheslav B. Tarelnyk, Piotr Kurp, Oleksandr M. Myslyvchenko, and Nataliia V. Tarelnyk. 2021. "Analysis of the Quality of Sulfomolybdenum Coatings Obtained by Electrospark Alloying Methods" Materials 14, no. 21: 6332. https://doi.org/10.3390/ma14216332







