Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Collection
2.2. Specific Surface Area
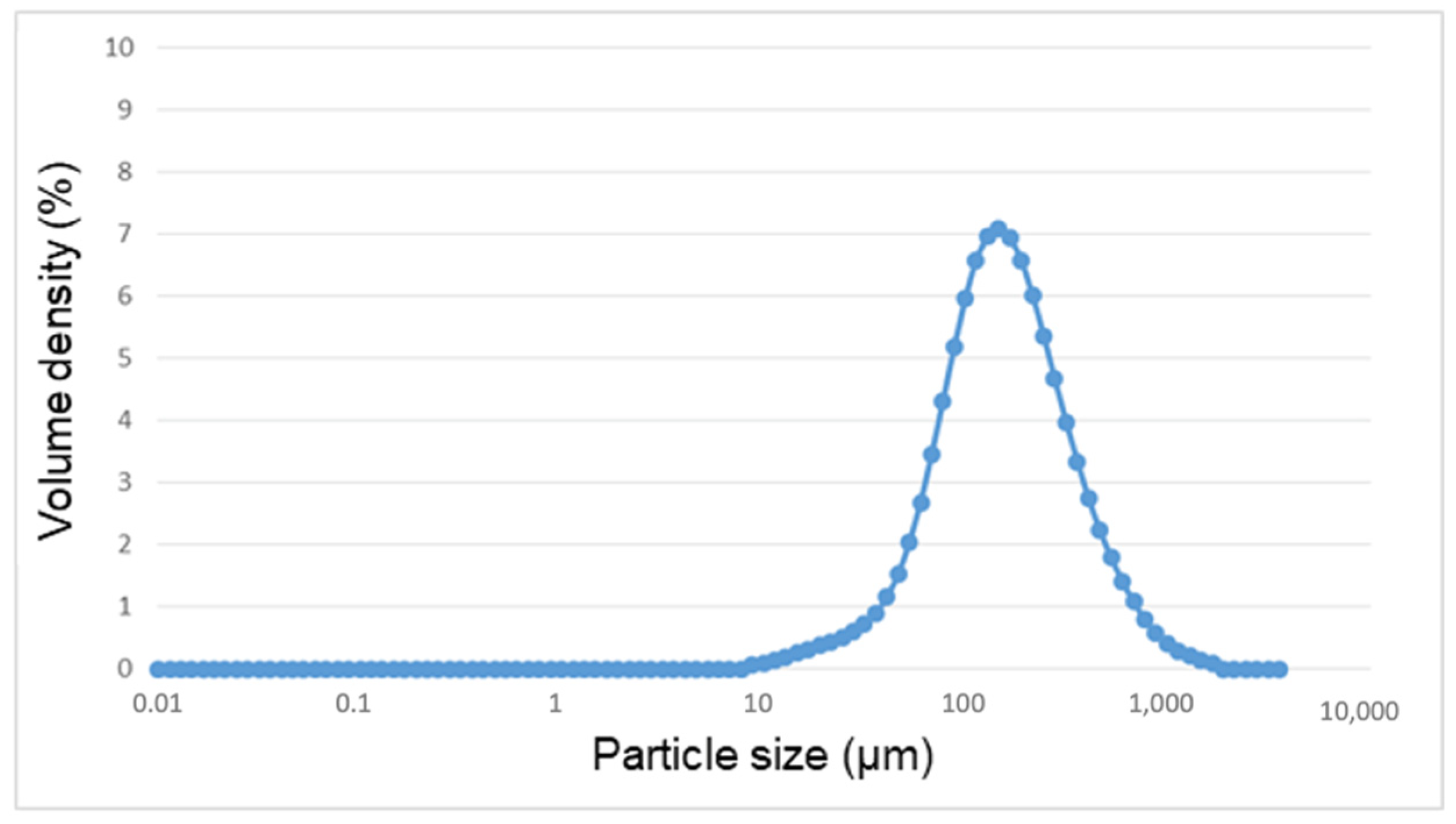
2.3. Granulometry
2.4. Scanning Electron Microscopy
2.5. Ion Release
2.6. X-ray Diffraction
2.7. Tensiometry
2.8. Cytotoxicity Assay
2.9. Statistical Analysis
3. Results
3.1. Specific Surface Area
3.2. Granulometry
3.3. Scanning Electron Microscopy
3.4. Ion Release
3.5. X-ray Diffraction
3.6. Tensiometry
3.7. Citotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Markowska-Szczupak, A.; Endo-Kimura, M.; Paszkiewicz, O.; Kowalska, E. Are titania photocatalysts and titanium implants safe? Review on the toxicity of titanium compounds. Nanomaterials 2020, 10, 2065. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti6Al4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Challa, V.S.; Mali, S.; Misra, R.D. Reduced toxicity and superior cellular response of preosteoblasts to Ti-6Al-7Nb alloy and comparison with Ti6Al4V. J. Biomed. Mater. Res. A 2013, 101, 2083–2089. [Google Scholar] [CrossRef]
- Willis, J.; Crean, S.J.; Barrak, F.N. Is titanium alloy Ti-6Al-4 V cytotoxic to gingival fibroblasts-A systematic review. Clin. Exp. Dent. Res. 2021. [Google Scholar] [CrossRef]
- de Castro, M.C.B.; Couto, A.A.; Almeida, G.F.C.; Massi, M.; de Lima, N.B.; da Sobrinho, A.S.; Castagnet, M.; Xiavier, G.L.; Oliveira, R.R. The effect of plasma nitriding on the fatigue behavior of the Ti6Al4V alloy. Materials 2019, 12, 520. [Google Scholar] [CrossRef] [Green Version]
- Brizuela-Velasco, A.; Pérez-Pevida, E.; Jiménez-Garrudo, A.; Gil-Mur, F.J.; Manero, J.M.; Punset-Fuste, M.; Chávarri-Prado, D.; Diéguez-Pereira, M.; Monticelli, F. Mechanical characterisation and biomechanical and biological behaviours of Ti-Zr binary-alloy dental implants. Biomed. Res. Int. 2017, 2017, 2785863. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindstrom, J.; Hallen, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Heimisdottir, K. Dental implants-Are they better than natural teeth? Eur. J. Oral Sci. 2018, 126, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, 246–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakic, M.; Galindo-Moreno, P.; Monje, A.; Radovanovic, S.; Wang, H.L.; Cochran, D.; Sculean, A.; Canullo , L. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 1805–1816. [Google Scholar] [CrossRef]
- Chan, H.L.; Lin, G.H.; Suarez, F.; MacEachern, M.; Wang, H.L. Surgical management of peri-implantitis: A systematic review and meta-analysis of treatment outcomes. J. Periodontol. 2014, 85, 1027–1041. [Google Scholar] [CrossRef]
- Monje, A.; Pons, R.; Amerio, E.; Wang, H.L.; Nart, J. Resolution of peri-implantitis by means of implantoplasty as adjunct to surgical therapy: A retrospective study. J. Periodontol 2021. [Google Scholar] [CrossRef]
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontol. 2000 2014, 66, 255–273. [Google Scholar] [CrossRef]
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.L.; Giannobile, W.V. Is metal particle release associated with peri-implant bone destruction ? An emerging concept. J. Dent. Res. 2018, 97, 259–265. [Google Scholar] [CrossRef]
- Albrektsson, T.; Canullo, L.; Cochran, D.; De Bruyn, H. “Peri-implantitis”: A complication of a foreign body or a man-made “disease”. Facts and fiction. Clin. Implant. Dent. Relat. Res. 2016, 18, 840–849. [Google Scholar] [CrossRef]
- Canullo, L.; Schlee, M.; Wagner, W.; Covani, U. International brainstorming meeting on etiologic and risk factors of peri-implantitis, montegrotto (Padua, Italy), august 2014. Int J. Oral Maxillofac. Implants 2015, 30, 1093–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto-Alvaredo, J.; Blanco, E.; Bettmer, J.; Hevia, D.; Sainz, R.M.; López Cháves, C.; Sánchez, C.; Llopis, J.; Sanz-Medel, J.; Montes-Bayón, M. Evaluation of the biological effect of Ti generated debris from metal implants: Ions and nanoparticles. Metallomics 2014, 6, 1702–1708. [Google Scholar] [CrossRef]
- Barrak, F.N.; Li, S.; Muntane, A.M.; Jones, J.R. Particle release from implantoplasty of dental implants and impact on cells. Int. J. Implant. Dent. 2020, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Costa-Berenguer, X.; García-García, M.; Sánchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implants Res. 2018, 29, 46–54. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- ISO. 10993-12, Biological Evaluation of Medical Devices. Part 12; Sample Preparation and Reference Materials; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Gutensohn, K.; Beythien, C.; Bau, J.; Fenner, T.; Grewe, P.; Koester, R.; Padmanaban, K.; Kuehnl, P. In vitro analyses of diamond-like carbon coated stents: Reduction of metal ion release, platelet activation, and thrombogenicity. Thromb. Res. 2000, 99, 577–585. [Google Scholar] [CrossRef]
- Washburn, W.E. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- ISO. 10993-5, Biological Evaluation of Medical Devices. Part 5. Test for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Ramel, C.F.; Lüssi, A.; Özcan, M.; Jung, R.E.; Hämmerle, C.H.F.; Thoma, D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin. Oral Implants Res. 2016, 27, 776–781. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T.; Chrcanovic, B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018, 11, 123–136. [Google Scholar]
- Berglundh, T.; Gotfredsen, K.; Zitzmann, N.U.; Lang, N.P.; Lindhe, J. Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness: An experimental study in dogs. Clin. Oral Implants Res. 2007, 18, 655–661. [Google Scholar] [CrossRef]
- Lang, N.P.; Berglundh, T.; Heitz-Mayfield, L.J.; Pjetursson, B.E.; Salvi, G.E.; Sanz, M. Consensus statements and recommended clinical procedures regarding implant survival and complications. Int. J. Oral Maxillofac. Implants 2004, 19, 150–154. [Google Scholar]
- Schliephake, H.; Sicilia, A.; Al Nawas, B.; Donos, N.; Gruber, R.; Jepsen, S.; Milinkovic, I.; Mombelli, A.; Navarro, J.M.; Quirynen, M. Drugs and diseases: Summary and consensus statements of group 1. The 5th EAO Consensus Conference 2018. Clin. Oral Implants Res. 2018, 29, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombelli, A.; Hashim, D.; Cionca, N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin. Oral Implants Res. 2018, 29, 37–53. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.; Romanos, G. Potential causes of titanium particle and ion release in implant dentistry: A systematic review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchini, M.; Galarraga-Vinueza, M.; Bedoya, K.; Correa, B.; de Souza Magini, R.; Schwarz, F. Implantoplasty enhancing peri-implant bone stability over a 3-year follow-up: A case series. Int. J. Periodontics Restor. Dent. 2020, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Schwarz, F.; Sader, R.; Becker, J.; Obreja, K. Assessment of peri-implant tissue dimensions following surgical therapy of advanced ligature-induced peri-implantitis defects. Int. J. Implant. Dent. 2021, 7, 4. [Google Scholar] [CrossRef]
- Suárez-López del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implants Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef]
- Holt, G.; Murnaghan, C.; Reilly, J.; Meek, R.M.D. The biology of aseptic osteolysis. Clin. Orthop. Relat. Res. 2007, 460, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.; Ries, M.D.; Paprosky, W. How prevalent are implant wear and osteolysis, and how has the scope of osteolysis changed since 2000? J. Am. Acad. Orthop. Surg. 2008, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, C.H.; Singh, G.; Willert, H.-G.; Buchhorn, G.H. Metallic debris from metal-on-metal total hip arthroplasty regulates periprosthetic tissues. World J. Orthop. 2014, 5, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef]
- Obando-Pereda, G.A.; Fischer, L.; Stach-Machado, D.R. Titanium and zirconia particle-induced pro-inflammatory gene expression in cultured macrophages and osteolysis, inflammatory hyperalgesia and edema in vivo. Life Sci. 2014, 97, 96–106. [Google Scholar] [CrossRef]
- Pajarinen, J.; Kouri, V.P.; Jämsen, E.; Li, T.F.; Mandelin, J.; Konttinen, Y.T. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater. 2013, 9, 9229–9240. [Google Scholar] [CrossRef] [PubMed]
- Farokhzadeh, K.; Edrisy, A. Fatigue improvement in low temperature plasma nitrided Ti6Al4V alloy. Mater. Sci. Eng. A 2015, 620, 435–444. [Google Scholar] [CrossRef]
- Malinov, S.; Sha, W.; Guo, Z.; Tang, C.C.; Long, A.E. Synchrotron X-ray diffraction study of the phase transformations in titanium alloys. Mater. Charact. 2002, 48, 279–295. [Google Scholar] [CrossRef] [Green Version]
- Gioka, C.; Bourauel, C.; Zinelis, S.; Eliades, T.; Silikas, N.; Eliades, G. Titanium orthodontic brackets: Structure, composition, hardness and ionic release. Dent. Mater. 2004, 20, 693–700. [Google Scholar] [CrossRef]
- de Morais, L.S.; Serra, G.G.; Albuquerque Palermo, E.F.; Andrade, L.R.; Müller, C.A.; Meyers, M.A.; Elias, C.N. Systemic levels of metallic ions released from orthodontic mini-implants. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Dalal, A.; Pawar, V.; Mcallister, K.; Weaver, C.; Hallab, N.J. Orthopedic implant cobalt-alloy particles produce greater toxicity and inflammatory cytokines than titanium alloy and zirconium alloy-based particles in vitro, in human osteoblasts, fibroblasts, and macrophages. J. Biomed. Mater. Res. A 2012, 100, 2147–2158. [Google Scholar] [CrossRef]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; van den Bogaart, G. Modulation of immune responses by particle size and shape. Front. Immunol. 2021, 11, 607945. [Google Scholar] [CrossRef]





| Assay | Specific Surface Area (m2/g) (SD) | C | Correlation Coeficient |
|---|---|---|---|
| 1 | 0.3893 (0.0212) | 23.21 | 0.9998 |
| 2 | 0.3550 (0.0316) | 24.82 | 0.9997 |
| 3 | 0.2441 (0.0165) | 22.07 | 0.9998 |
| Equivalent Diameter (µm) | |
|---|---|
| Mode | 152 |
| 10th percentile | 61 |
| 50th percentile | 159 |
| 90th percentile | 433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledano-Serrabona, J.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I. Materials 2021, 14, 6507. https://doi.org/10.3390/ma14216507
Toledano-Serrabona J, Gil FJ, Camps-Font O, Valmaseda-Castellón E, Gay-Escoda C, Sánchez-Garcés MÁ. Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I. Materials. 2021; 14(21):6507. https://doi.org/10.3390/ma14216507
Chicago/Turabian StyleToledano-Serrabona, Jorge, Francisco Javier Gil, Octavi Camps-Font, Eduard Valmaseda-Castellón, Cosme Gay-Escoda, and Maria Ángeles Sánchez-Garcés. 2021. "Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I" Materials 14, no. 21: 6507. https://doi.org/10.3390/ma14216507







