Strength and Leaching Behavior of Contaminated Mining Sludge at High Water Content Stabilized with Lime Activated GGBS
Abstract
:1. Introduction
2. Material and Method
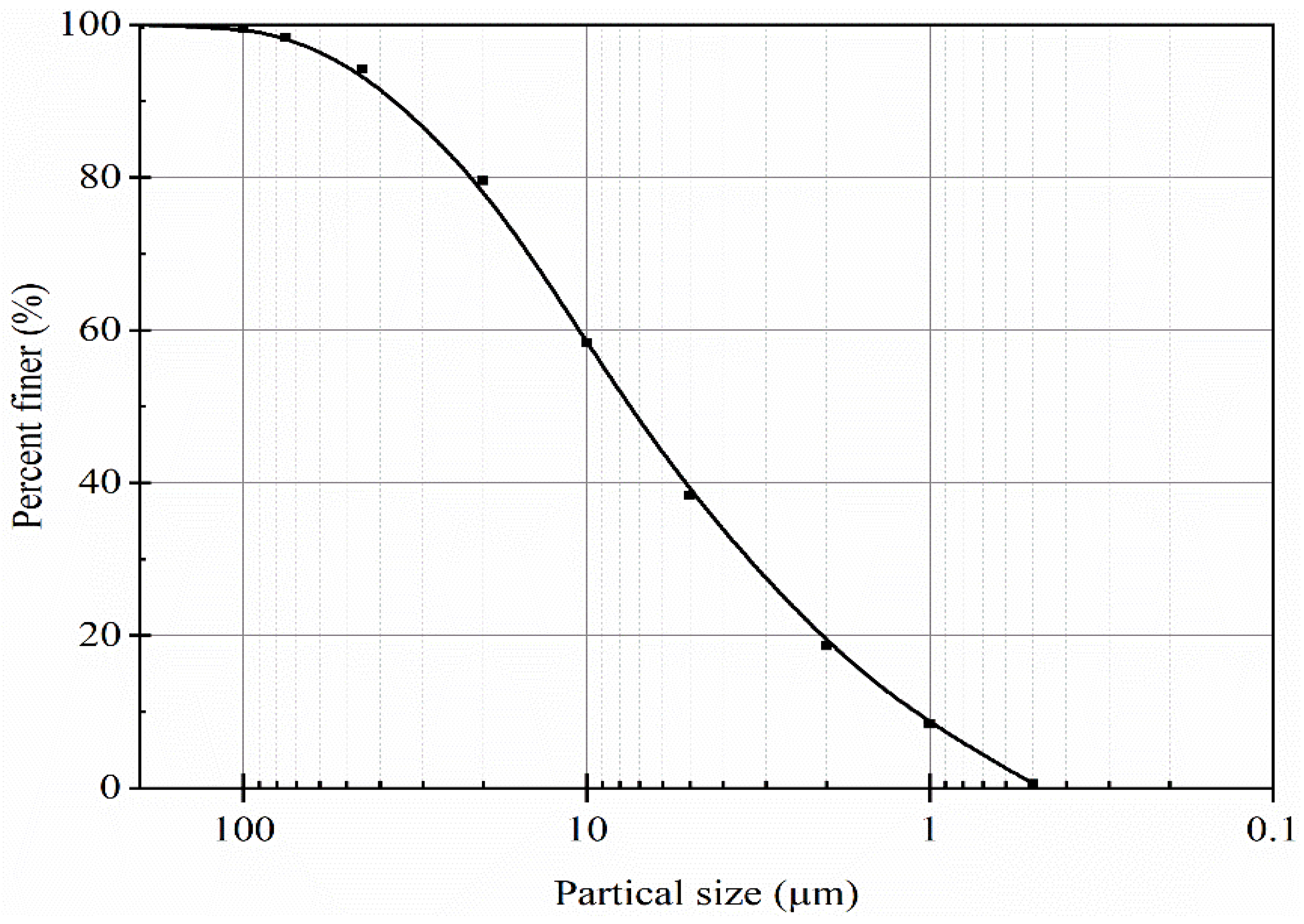
2.1. Materials
2.2. Preparation of Contaminated Mining Sludge (CMS) at High Water Content
2.3. Testing Procedure
3. Results and Discussion
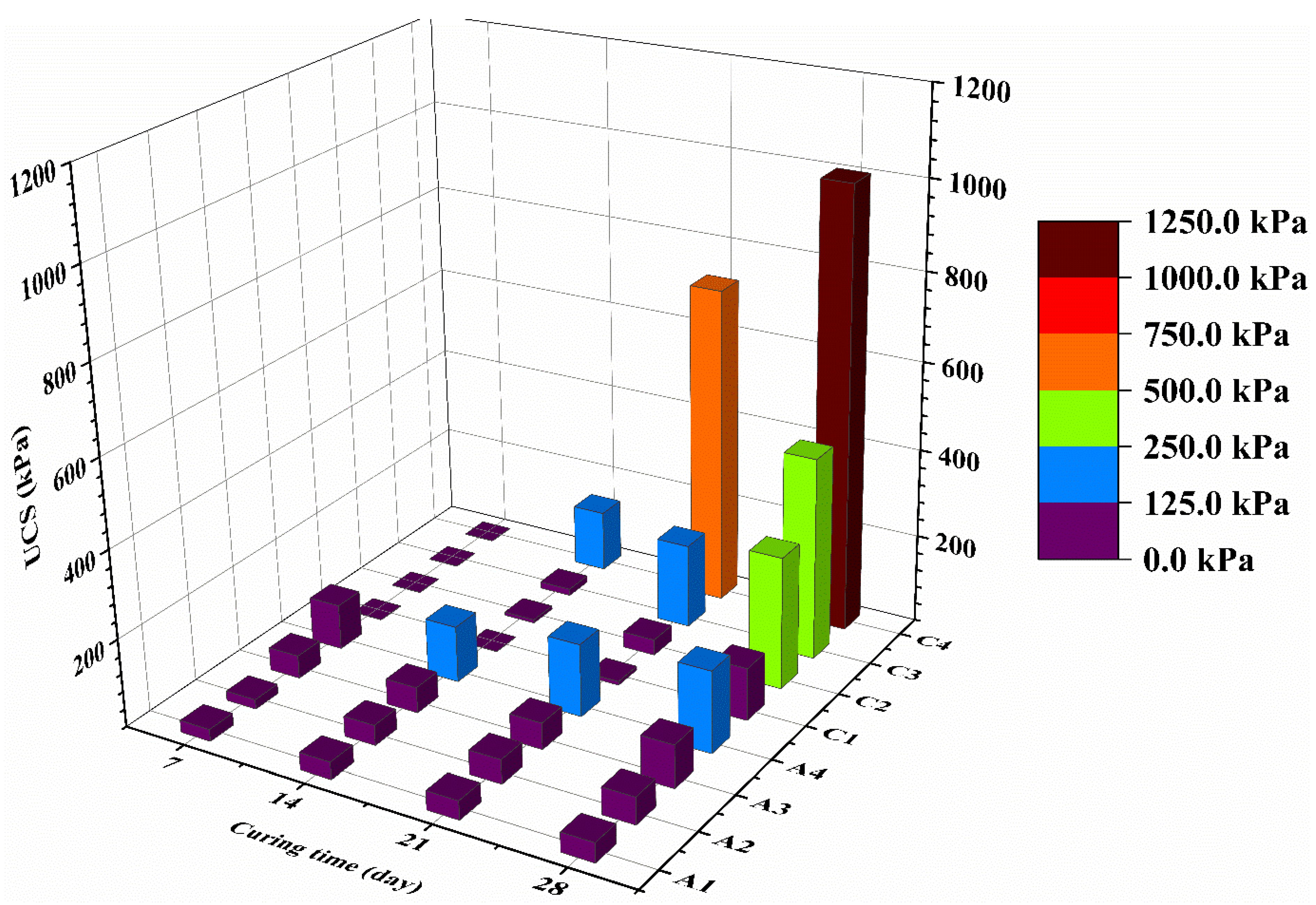
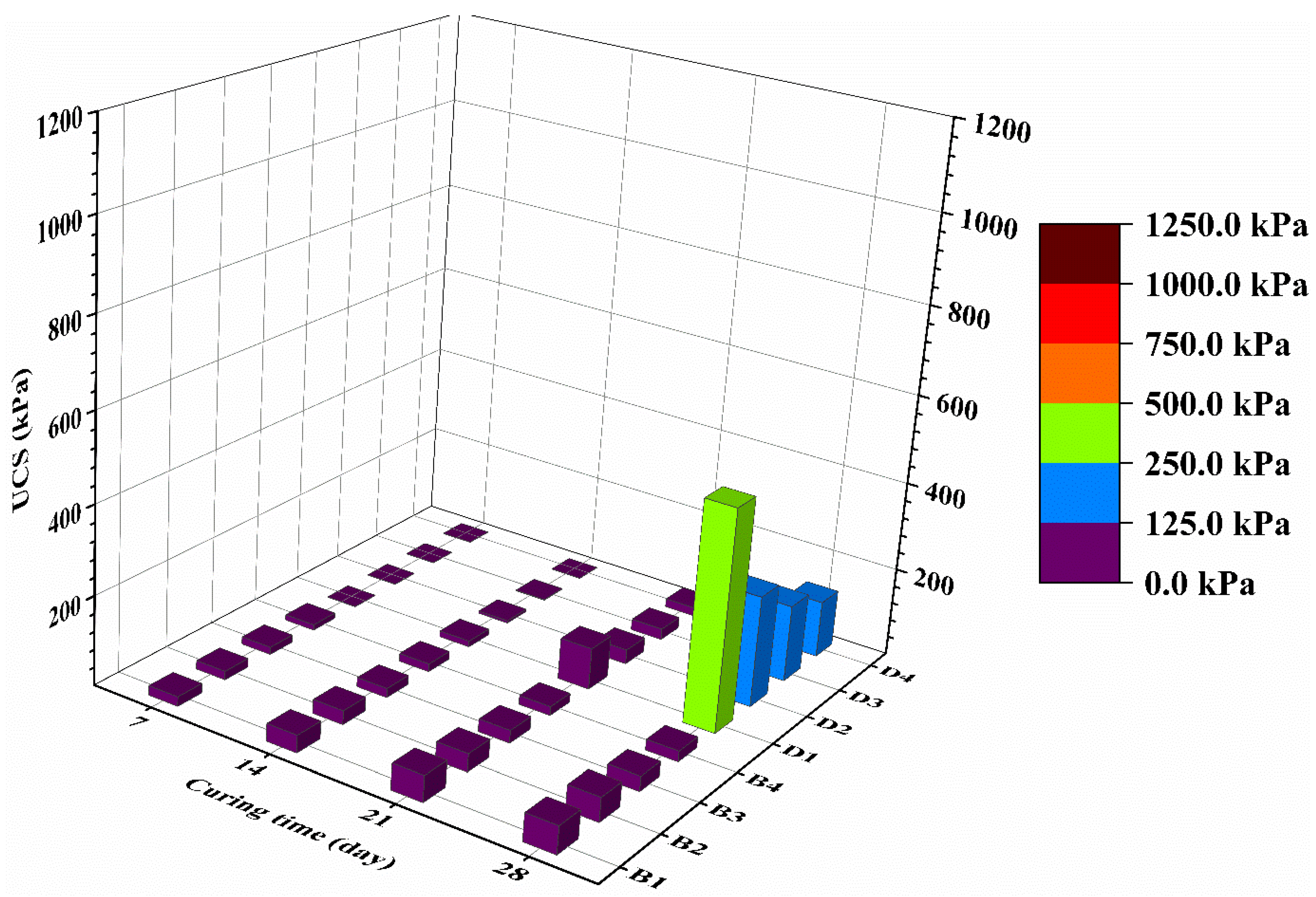
3.1. Strength Characteristics of Treated Mining Sludge at High Water Content
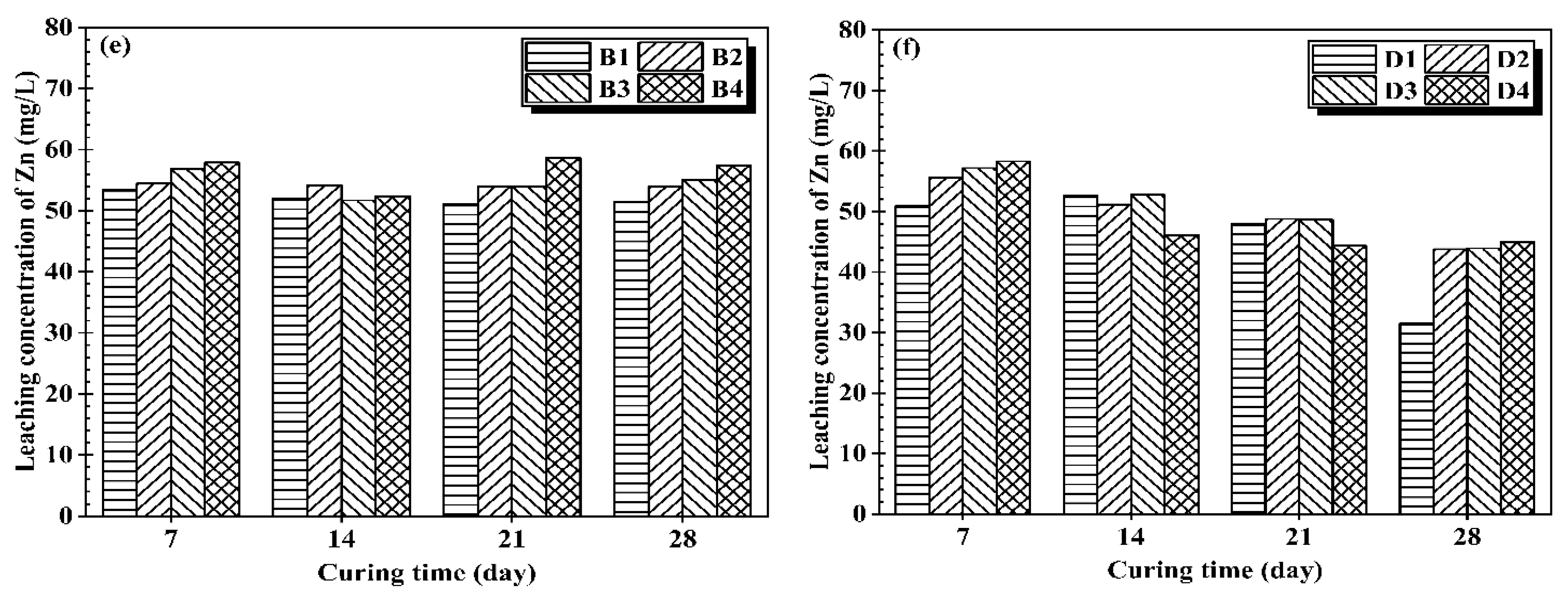
3.2. Heavy Metal Leaching Behavior of Treated Mining Sludge at High Water Content

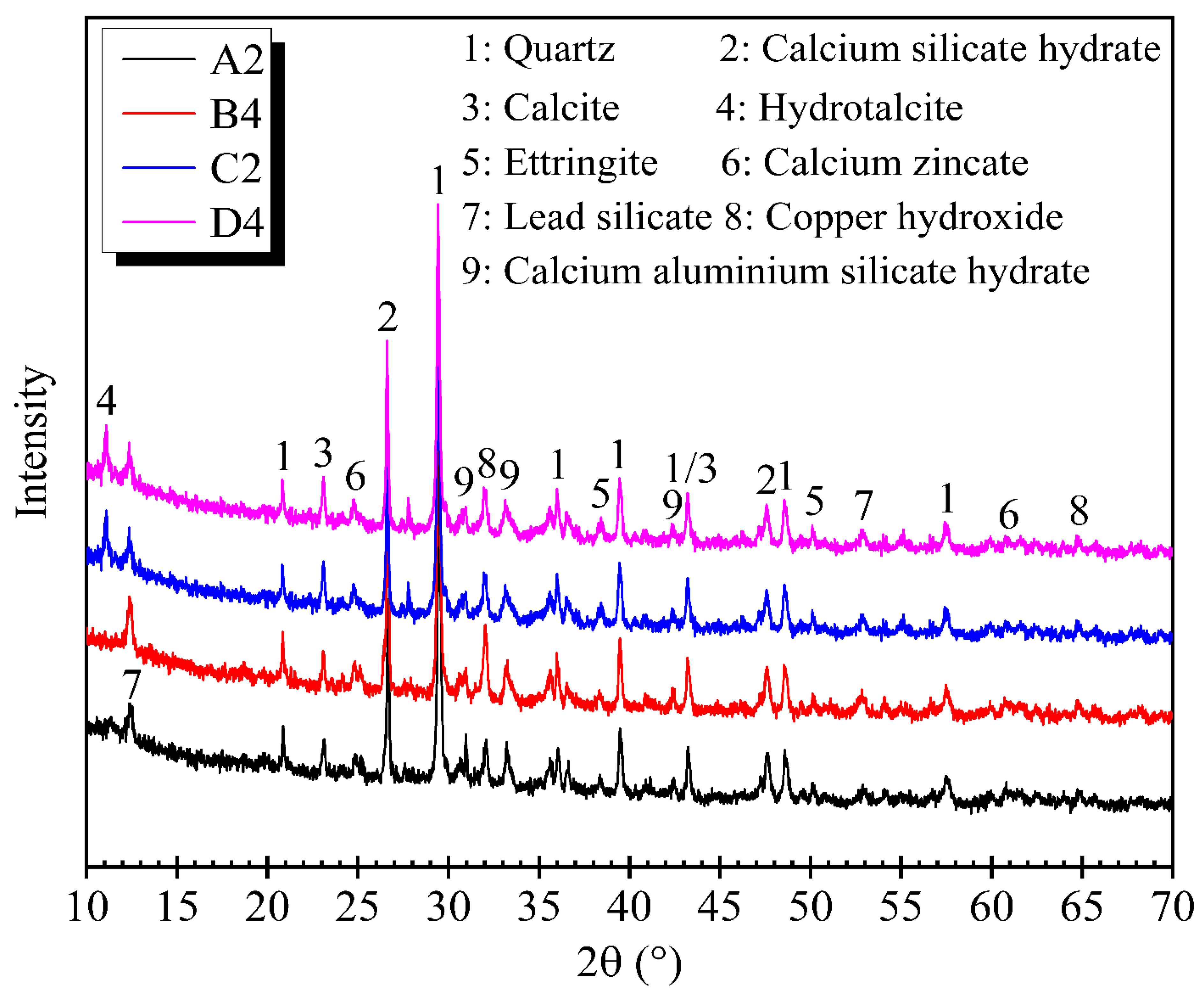
3.3. XRD Analysis of Treated Mining Sludge at High Water Content

3.4. SEM Images for Treated Mining Sludge at High Water Content

4. Conclusions
- Lime-activated GGBS has substantially better performance than OPC in the aspect of strength development of treated mining sludge. At 28-day, the UCS of CG stabilized CMS showed 5.44 times higher UCS than OPC stabilized CMS at the same water content and binder content.
- Both CG and OPC samples exhibit a decrease in the leaching concentration of heavy metal with an increase in curing time. However, CG stabilized samples show comparable capability of heavy metal stabilization in contrast to OPC.
- XRD patterns showed that the main hydration products of both CG and OPC mixes were CSH, CASH, and ettringite. The hydrotalcite produced in the CG mix was the only difference between the hydration products of CG and OPC mixes.
- SEM micrographs exhibited that CG mix developed dense microstructure due to the formation of more voluminous hydration products such as hydrotalcite, filling the pores between CMS particles more effectively, resulting in a dense stabilized matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CMS | contaminated mining sludge |
| S/S | solidification/stabilization |
| U.C.S. | unconfined compressive strength |
| TCLP | toxicity characteristics leaching procedure |
| ASTM | American standard of testing material |
| EPA | environment protection agency |
| W | water content |
| Aw | binder content |
| OPC | ordinary Portland cement |
| CG | lime activated GGBS |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
| SEM | scanning electron microscopy |
| Ht | hydrotalcite |
References
- Young, G.; Chen, Y.; Yang, M. Concentrations, distribution, and risk assessment of heavy metals in the iron tailings of Yeshan National Mine Park in Nanjing, China. Chemosphere 2021, 271, 129546. [Google Scholar] [CrossRef] [PubMed]
- Glotov, V.E.; Chlachula, J.; Glotova, L.P.; Little, E. Causes and environmental impact of the gold-tailings dam failure at Karamken, the Russian Far East. Eng. Geol. 2018, 245, 236–247. [Google Scholar] [CrossRef]
- Xia, W.-Y.; Du, Y.-J.; Li, F.-S.; Guo, G.-L.; Yan, X.-L.; Li, C.-P.; Arulrajah, A.; Wang, F.; Wang, S. Field evaluation of a new hydroxyapatite based binder for ex-situ solidification/stabilization of a heavy metal contaminated site soil around a Pb-Zn smelter. Constr. Build. Mater. 2019, 210, 278–288. [Google Scholar] [CrossRef]
- Peng, W.; Chaosheng, T.; Kaiqiang, S.; Zhiguo, C.; Shikang, X.; Bin, S. Advances on solidification/stabilization of sludge disposal. Gongcheng Dizhixue Bao J. Eng. Geol. 2016, 24, 649–660. [Google Scholar]
- Yi, Y.; Liska, M.; Jin, F.; Al-Tabbaa, A. Mechanism of reactive magnesia—Ground granulated blastfurnace slag (GGBS) soil stabilization. Can. Geotech. J. 2016, 53, 773–782. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Zhong, G.; Zeng, H. A comparison study on heavy metal/metalloid stabilization in Maozhou River sediment by five types of amendments. J. Soils Sediments 2019, 19, 3922–3933. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Yi, Y.; Al-Tabbaa, A. Process envelopes for stabilisation/solidification of contaminated soil using lime-slag blend. Environ. Sci. Pollut. Res. 2011, 18, 1286–1296. [Google Scholar] [CrossRef]
- Feng, Y.-S.; Du, Y.-J.; Xia, W.-Y.; Reddy, K.R. Pilot-scale field investigation of ex situ solidification/stabilization of soils with inorganic contaminants using two novel binders. Acta Geotech. 2019, 15, 1467–1480. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Zhou, Y.; Rinklebe, J.; Song, H.; Kwon, E.E.; Baek, K.; Ok, Y.S. Mechanistic insights into red mud, blast furnace slag, or metakaolin-assisted stabilization/solidification of arsenic-contaminated sediment. Environ. Int. 2019, 133, 105247. [Google Scholar] [CrossRef]
- Keramatikerman, M.; Chegenizadeh, A.; Nikraz, H. Effect of GGBFS and lime binders on the engineering properties of clay. Appl. Clay Sci. 2016, 132–133, 722–730. [Google Scholar] [CrossRef]
- Zhang, W.-L.; Zhao, L.-Y.; McCabe, B.A.; Chen, Y.-H.; Morrison, L. Science of the Total Environment Dredged marine sediments stabilized/solidified with cement and GGBS: Factors affecting mechanical behaviour and leachability. Sci. Total Environ. 2020, 733, 138551. [Google Scholar] [CrossRef]
- Goodarzi, A.R.; Movahedrad, M. Stabilization/solidification of zinc-contaminated kaolin clay using ground granulated blast-furnace slag and different types of activators. Appl. Geochem. 2017, 81, 155–165. [Google Scholar] [CrossRef]
- Yi, Y.; Zheng, X.; Liu, S.; Al-Tabbaa, A. Comparison of reactive magnesia- and carbide slag-activated ground granulated blastfurnace slag and Portland cement for stabilisation of a natural soil. Appl. Clay Sci. 2015, 111, 21–26. [Google Scholar] [CrossRef]
- Jin, F.; Al-Tabbaa, A. Evaluation of novel reactive MgO activated slag binder for the immobilisation of lead and zinc. Chemosphere 2014, 117, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Lang, L.; Chen, B.; Li, N. Utilization of lime/carbide slag-activated ground granulated blast-furnace slag for dredged sludge stabilization. Mar. Georesour. Geotechnol. 2020, 39, 659–669. [Google Scholar] [CrossRef]
- Gu, K.; Jin, F.; Al-Tabbaa, A.; Shi, B.; Liu, C.; Gao, L. Incorporation of reactive magnesia and quicklime in sustainable binders for soil stabilisation. Eng. Geol. 2015, 195, 53–62. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Cement and Concrete Research Use of CaO as an activator for producing a price-competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Jin, F.; Al-Tabbaa, A. The performance of blended conventional and novel binders in the in-situ stabilisation/solidification of a contaminated site soil. J. Hazard. Mater. 2015, 285, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Kogbara, R.B.; Al-Tabbaa, A. Mechanical and leaching behaviour of slag-cement and lime-activated slag stabilised/solidified contaminated soil. Sci. Total Environ. 2011, 409, 2325–2335. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency (USEPA). Report: Recent Developments for In Situ Treatment of Metals Contaminated Soils; United States Environmental Protection Agency, Office of Solid Waste and Emergency Response: Washington, DC, USA, 1996.
- Luo, Y. Environmental problems in the mining of metal minerals. IOP Conf. Ser. Earth Environ. Sci. 2019, 384, 012195. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.; Gu, L.; Liu, S.; Puppala, A.J. Carbide slag-activated ground granulated blastfurnace slag for soft clay stabilization. Can. Geotech. J. 2015, 52, 656–663. [Google Scholar] [CrossRef]
- Beijing HL Consulting Company. Market Research Report on Magnesia (MgO) Industry in China; Beijing HL Consulting Company: Beijing, China, 2009. [Google Scholar]
- Du, Y.-J.; Jiang, N.-J.; Liu, S.-Y.; Jin, F.; Singh, D.; Puppala, A.J. Engineering properties and microstructural characteristics of cement-stabilized zinc-contaminated kaolin. Can. Geotech. J. 2014, 51, 289–302. [Google Scholar] [CrossRef]
- China National Environmental Monitoring Center (CNEMC). The Soil Background Value in China; CNEMC: Beijing, China, 1990. [Google Scholar]
- Yi, Y.; Liska, M.; Al-Tabbaa, A. Properties of Two Model Soils Stabilized with Different Blends and Contents of GGBS, MgO, Lime, and PC. J. Mater. Civ. Eng. 2014, 26, 267–274. [Google Scholar] [CrossRef]
- Yi, Y.; Gu, L.; Liu, S. Microstructural and mechanical properties of marine soft clay stabilized by lime-activated ground granulated blastfurnace slag. Appl. Clay Sci. 2015, 103, 71–76. [Google Scholar] [CrossRef]
- Mastoi, A.K.; Pu, H.; Chen, X.; Nyanzi, A.S.; Jhatial, A.A. Physico-mechanical and microstructural behaviour of high-water content zinc-contaminated dredged sediment treated with integrated approach PHDVPSS. Environ. Sci. Pollut. Res. 2021, 28, 58331–58341. [Google Scholar] [CrossRef]
- Li, Y.-C.; Min, X.-B.; Chai, L.-Y.; Shi, M.-Q.; Tang, C.-J.; Wang, Q.-W.; Liang, Y.-J.; Lei, J.; Liyang, W.-J. Co-treatment of gypsum sludge and Pb/Zn smelting slag for the solidification of sludge containing arsenic and heavy metals. J. Environ. Manag. 2016, 181, 756–761. [Google Scholar] [CrossRef]
- Makusa, G.P.; Mattson, H.; Knutsson, S. Shear strength evaluation of preloaded stabilized dredged sediments using CTP. In Proceedings of the 3rd International Symposium on Cone Penetration Testing, Las Vegas, NV, USA, 12–14 May 2014; pp. 753–759. [Google Scholar]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Li, W.; Ni, P.; Yi, Y. Comparison of reactive magnesia, quick lime, and ordinary Portland cement for stabilization/solidification of heavy metal-contaminated soils. Sci. Total Environ. 2019, 671, 741–753. [Google Scholar] [CrossRef]
- Reginald, B.; Kogbara, A.A.-T.; Yi, Y.; Stegeman, J.A. Cement–fly ash stabilisation/solidification of contaminated soil: Performance properties and initiation of operating envelopes. Appl. Geochem. 2013, 33, 64–75. [Google Scholar]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Oti, J.E.; Bai, J. Developing unfired stabilised building materials in the UK. In Engineering Sustainability; ICE Publishing: London, UK, 2008; pp. 211–218. [Google Scholar] [CrossRef]
- Niu, M.; Li, G.; Wang, Y.; Li, Q.; Han, L.; Song, Z. Comparative study of immobilization and mechanical properties of sulfoaluminate cement and ordinary Portland cement with different heavy metals. Constr. Build. Mater. 2018, 193, 332–343. [Google Scholar] [CrossRef]
- Nidzam, R.M.; Kinuthia, J.M. Sustainable soil stabilisation with blastfurnace slag—A review. Proc. Inst. Civ. Eng. Constr. Mater. 2010, 163, 157–165. [Google Scholar] [CrossRef]
- Pu, H.; Mastoi, A.K.; Chen, X.; Song, D.; Qiu, J.; Yang, P. An integrated method for the rapid dewatering and solidification/stabilization of dredged contaminated sediment with a high water content. Front. Environ. Sci. Eng. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Arulrajah, A.; Yaghoubi, M.; Miri, M. ScienceDirect Evaluation of fly ash- and slag-based geopolymers for the improvement of a soft marine clay by deep soil mixing. Soils Found. 2018, 58, 1358–1370. [Google Scholar] [CrossRef]
| Property | Value | |
|---|---|---|
| Nature moisture content, % | 50 | |
| pH | 7.8 | |
| Specific gravity | 2.61 | |
| Liquid limit LL, % | 41.45 | |
| Plastic limit PL, % | 24.43 | |
| Plasticity Index | 17.02 | |
| Particle Size Distribution | Sand fraction (0.075–2 mm), % | 1.68 |
| Silt fraction (0.002–0.075 mm), % | 79.74 | |
| Clay and colloid fraction (<0.002 mm), % | 18.58 | |
| Soil Classification | Lean clay CL | |
| Total Cu concentration, mg/kg | 609.92 | |
| Total Pb concentration, mg/kg | 15.6 | |
| Total Zn concentration, mg/kg | 274.9 | |
| Composition | CaO (wt.%) | SiO2 (wt.%) | Al2O3 (wt.%) | Fe2O3 (wt.%) | MgO (wt.%) | K2O (wt.%) | SO3 (wt.%) | Na2O (wt.%) | Others (wt.%) | Loss on Ignition (wt.%) |
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 59.81 | 22.33 | 6.26 | 2.54 | 3.41 | 0.70 | 4.02 | 0.68 | - | 0.25 |
| CaO | 92.1 | 1.46 | 0.68 | 0.101 | 4.79 | 0.017 | 0.19 | - | 0.152 | - |
| GGBS | 38.00 | 36.3 | 14.29 | 0.24 | 7.74 | 0.43 | 2.33 | 0.22 | - | 0.45 |
| CMS | 48.38 | 21.59 | 6.56 | 15.80 | 3.39 | 0.65 | 1.20 | - | 1.79 | 0.64 |
| Mix Type | Binder Type | Group | Case No. | W (%) | Aw (%) | Curing Time | No. of Specimens Prepared | Testing Items |
|---|---|---|---|---|---|---|---|---|
| OPC | Ordinary Portland cement | A | A1 | 120 | 10 | 7, 14, 21 and 28 days | 8 | UCS at 7,14, 21 and 28-day for all specimens TCLP at 7, 14, 21, and 28-day for all specimens XRD at 28-day for selected specimens SEM at 28-day for selected specimens |
| A2 | 120 | 12 | 8 | |||||
| A3 | 120 | 15 | 8 | |||||
| A4 | 120 | 20 | 8 | |||||
| B | B1 | 100 | 12 | 8 | ||||
| B2 | 120 | 12 | 8 | |||||
| B3 | 140 | 12 | 8 | |||||
| B4 | 160 | 12 | 8 | |||||
| CG | CaO-GGBS (1:3) | C | C1 | 120 | 10 | 8 | ||
| C2 | 120 | 12 | 8 | |||||
| C3 | 120 | 15 | 8 | |||||
| C4 | 120 | 20 | 8 | |||||
| D | D1 | 100 | 12 | 8 | ||||
| D2 | 120 | 12 | 8 | |||||
| D3 | 140 | 12 | 8 | |||||
| D4 | 160 | 12 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdoul Fatah, T.; Zhang, R.; Huang, X.; Zheng, J.; Miao, Y.; Mastoi, A.K. Strength and Leaching Behavior of Contaminated Mining Sludge at High Water Content Stabilized with Lime Activated GGBS. Materials 2021, 14, 6524. https://doi.org/10.3390/ma14216524
Abdoul Fatah T, Zhang R, Huang X, Zheng J, Miao Y, Mastoi AK. Strength and Leaching Behavior of Contaminated Mining Sludge at High Water Content Stabilized with Lime Activated GGBS. Materials. 2021; 14(21):6524. https://doi.org/10.3390/ma14216524
Chicago/Turabian StyleAbdoul Fatah, Traore, Rongjun Zhang, Xiaosong Huang, Junjie Zheng, Yu Miao, and Aamir Khan Mastoi. 2021. "Strength and Leaching Behavior of Contaminated Mining Sludge at High Water Content Stabilized with Lime Activated GGBS" Materials 14, no. 21: 6524. https://doi.org/10.3390/ma14216524
APA StyleAbdoul Fatah, T., Zhang, R., Huang, X., Zheng, J., Miao, Y., & Mastoi, A. K. (2021). Strength and Leaching Behavior of Contaminated Mining Sludge at High Water Content Stabilized with Lime Activated GGBS. Materials, 14(21), 6524. https://doi.org/10.3390/ma14216524






