Contact Reactive Brazing of TC4 Alloy to Al7075 Alloy with Deposited Cu Interlayer
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
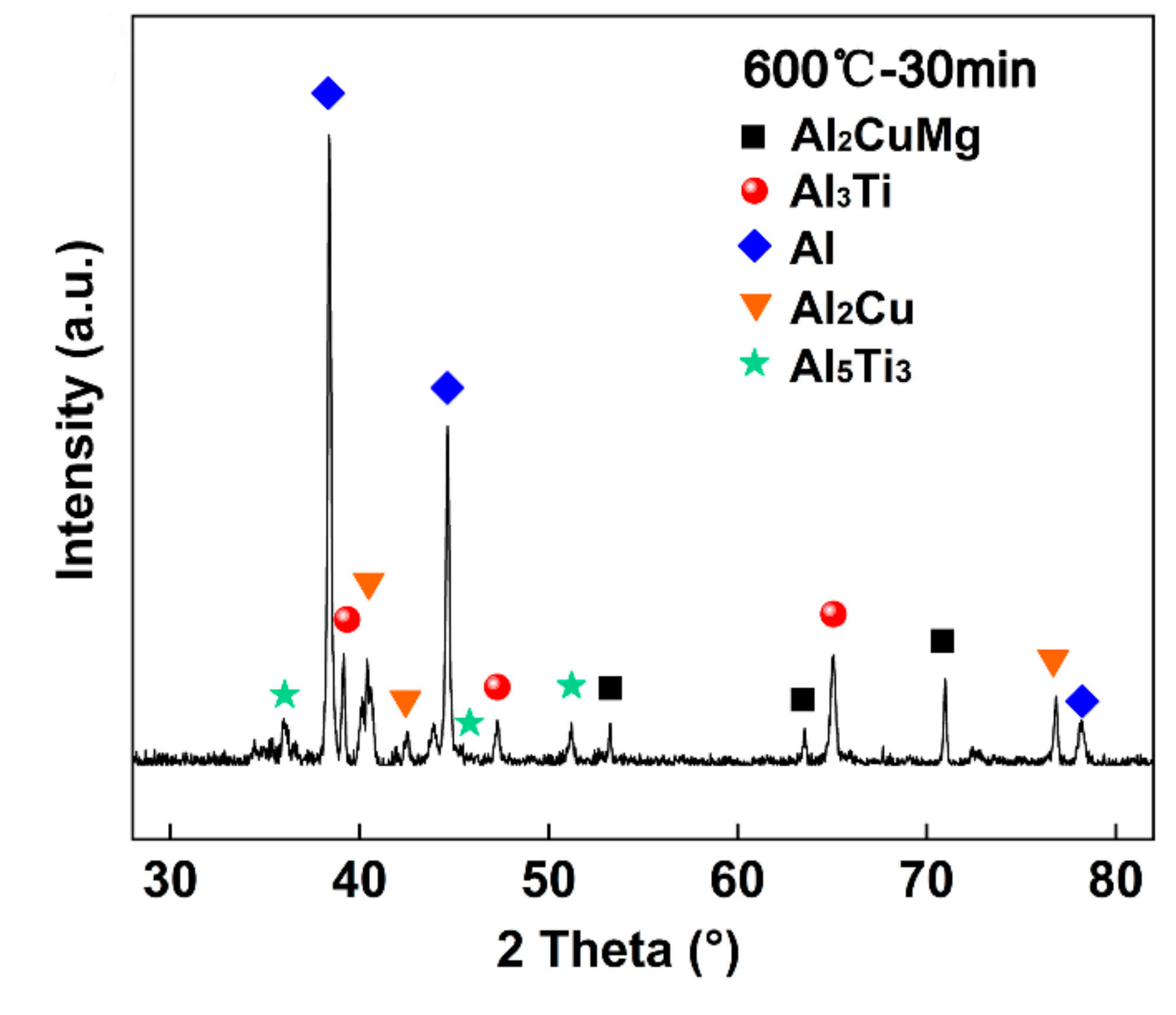
3.1. Typical Microstructure of TC4/Cu Layer/Al7075 Brazed Joint
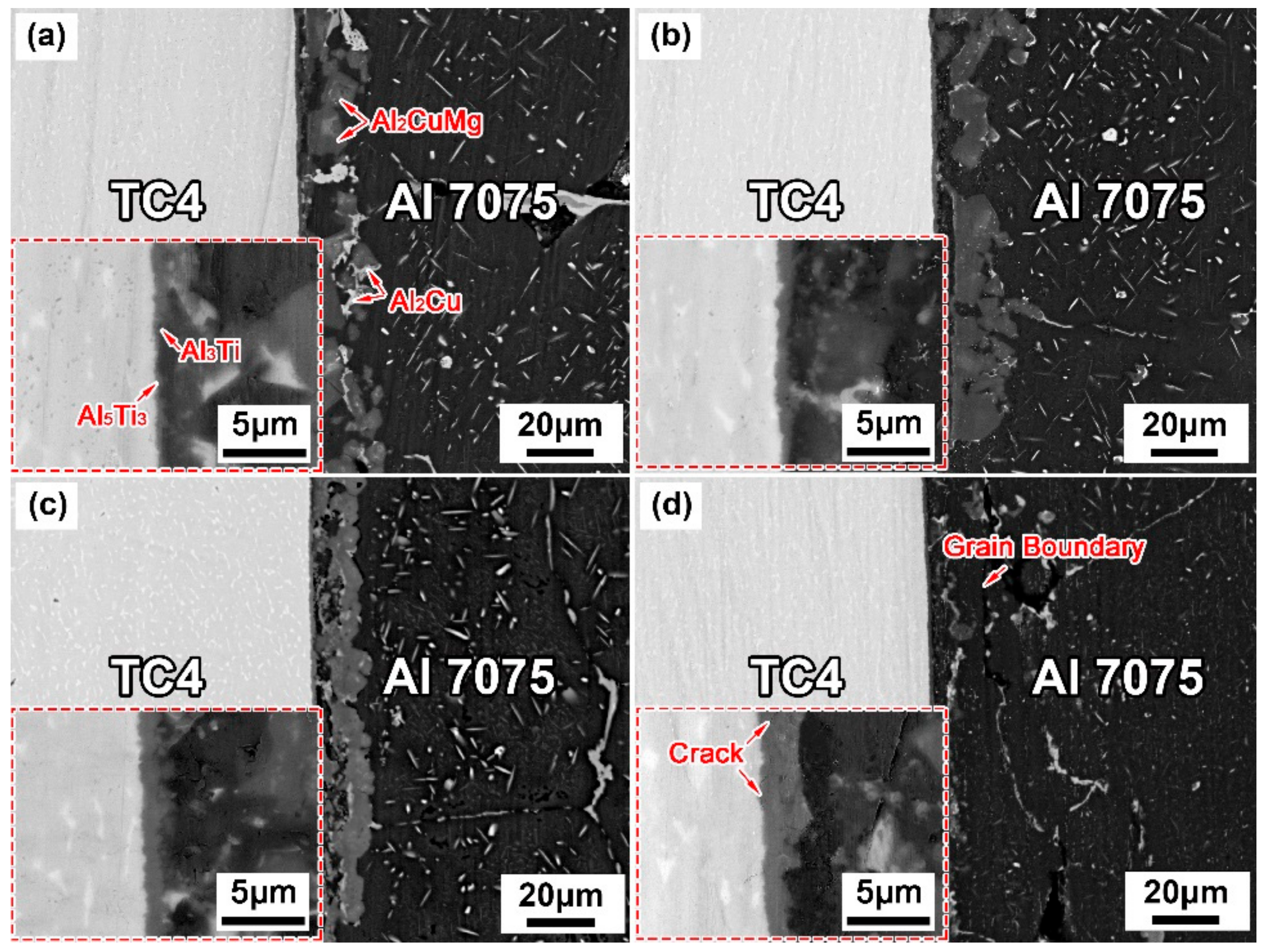
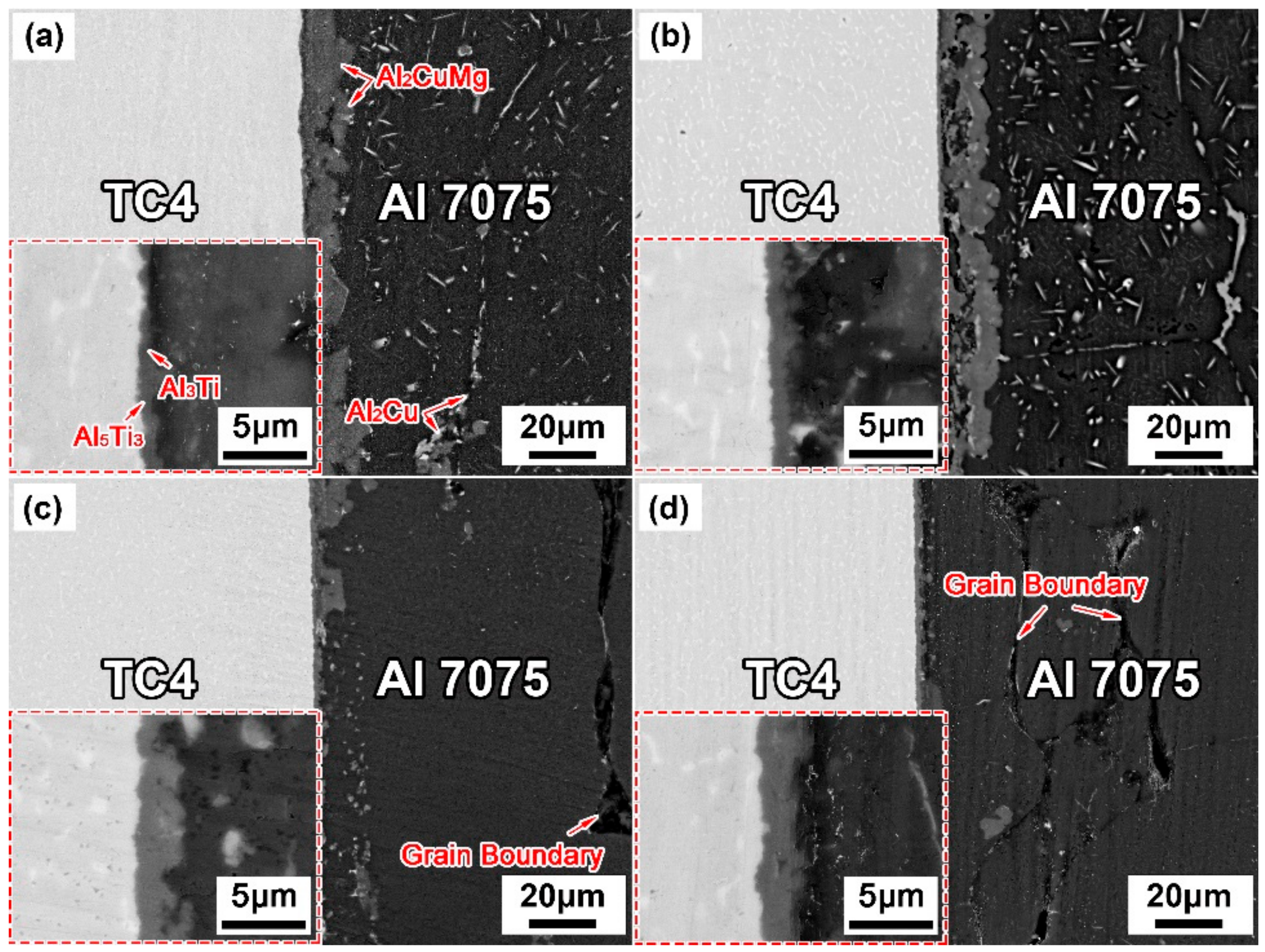
3.2. Effect of Brazing Parameters on the Microstructure of TC4/Cu Layer/Al7075 Brazed Joints
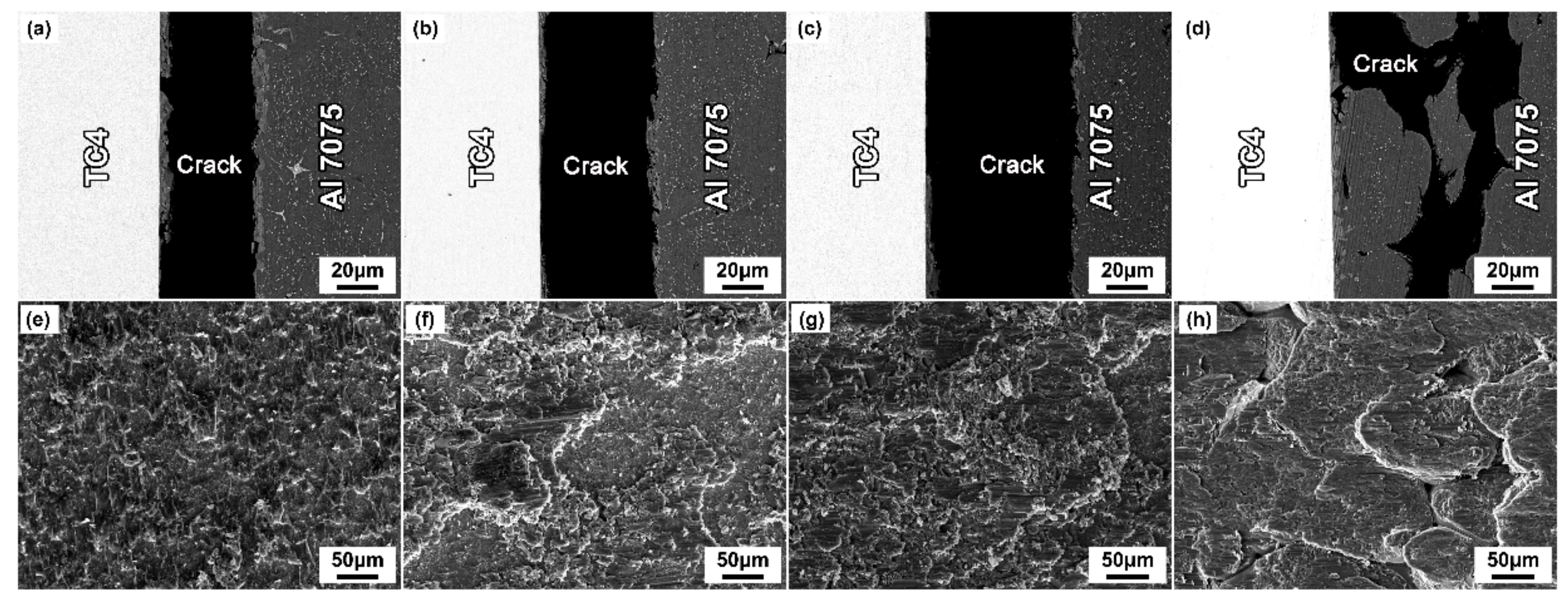
3.3. Effect of Brazing Parameters on the Mechanical Properties of Brazed Joints
4. Conclusions
- The contact reactive brazing of the TC4 alloy to the Al7075 alloy was achieved using deposited Cu as an interlayer. The typical interfacial microstructure of the TC4/Al7075 brazed joint was the TC4 substrate/Al3Ti + Al5Ti3/Al2Cu + Al2CuMg/Al7075 substrate at 600 °C for 30 min.
- With increasing the brazing temperature and holding time, the amount of Al2Cu and Al2CuMg IMCs in the brazed joints decreased and the homogenization of the joint composition improved, while the thickness of the reaction layer (Al3Ti + Al5Ti3) on the TC4 side increased gradually.
- The shear strength improved first and then decreased with increasing brazing parameters, and the maximum shear strength of ~201.45 ± 4.40 MPa was obtained at 600 °C for 30 min. The fracture mode of the joint changed from brittle fracture to transgranular fracture, and the intergranular fracture occurred when the brazing temperature was higher than 600 °C and the holding time exceeded 30 min.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emadinia, O.; Guedes, A.; Tavares, C.; Simões, S. Joining Alumina to Titanium Alloys Using Ag-Cu Sputter-Coated Ti Brazing Filler. Materials 2020, 13, 4802. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Dong, X.; Peng, C.; Ji, B.; Xu, Y.; Li, W. Hot deformation behavior and microstructure evolution of the laser solid formed TC4 titanium alloy. Chin. J. Aeronaut. 2020, 34, 163–182. [Google Scholar] [CrossRef]
- Dong, H.; Yang, Z.; Wang, Z.; Deng, D.; Dong, C. Vacuum Brazing TC4 Titanium Alloy to 304 Stainless Steel with Cu-Ti-Ni-Zr-V Amorphous Alloy Foil. J. Mater. Eng. Perform. 2014, 23, 3770–3777. [Google Scholar] [CrossRef]
- AlHazaa, A.; Alhoweml, I.; Shar, M.A.; Hezam, M.; Abdo, H.S.; AlBrithen, H. Transient Liquid Phase Bonding of Ti-6Al-4V and Mg-AZ31 Alloys Using Zn Coatings. Materials 2019, 12, 769. [Google Scholar] [CrossRef] [Green Version]
- Lan, J.; Chen, Z.; Liu, L.; Zhang, Q.; He, M.; Li, J.; Peng, X.; Fan, T. The Thermal Properties of L12 Phases in Aluminum Enhanced by Alloying Elements. Metals 2021, 11, 1420. [Google Scholar] [CrossRef]
- Alhazaa, A.; Khan, T.; Haq, I. Transient liquid phase (TLP) bonding of Al7075 to Ti–6Al–4V alloy. Mater. Charact. 2010, 61, 312–317. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, C.; Luo, W.; Yang, S.; Zhao, G. Material flow analysis and extrusion die modifications for an irregular and multitooth aluminum alloy radiator. Int. J. Adv. Manuf. Technol. 2016, 85, 1927–1935. [Google Scholar] [CrossRef]
- Chang, S.; Tsao, L.; Lei, Y.; Mao, S.; Huang, C. Brazing of 6061 aluminum alloy/Ti–6Al–4V using Al–Si–Cu–Ge filler metals. J. Mater. Process. Technol. 2012, 212, 8–14. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, J.; Hu, S.; Wang, T.; Bu, X. Investigation of welding crack in laser welding-brazing welded TC4/6061 and TC4/2024 dissimilar butt joints. J. Manuf. Process. 2020, 60, 54–60. [Google Scholar] [CrossRef]
- Gao, M.; Chen, C.; Gu, Y.; Zeng, X. Microstructure and Tensile Behavior of Laser Arc Hybrid Welded Dissimilar Al and Ti Alloys. Materials 2014, 7, 1590–1602. [Google Scholar] [CrossRef]
- Baqer, Y.M.; Ramesh, S.; Yusof, F.; Manladan, S.M. Challenges and advances in laser welding of dissimilar light alloys: Al/Mg, Al/Ti, and Mg/Ti alloys. Int. J. Adv. Manuf. Technol. 2018, 95, 4353–4369. [Google Scholar] [CrossRef]
- Schubert, E.; Klassen, M.; Zerner, I.; Walz, C.; Sepold, G. Light-weight structures produced by laser beam joining for future applications in automobile and aerospace industry. J. Mater. Process. Technol. 2001, 115, 2–8. [Google Scholar] [CrossRef]
- Casalino, G.; D’Ostuni, S.; Guglielmi, P.; Leo, P.; Mortello, M.; Palumbo, G.; Piccininni, A. Mechanical and microstructure analysis of AA6061 and Ti6Al4V fiber laser butt weld. Optik 2017, 148, 151–156. [Google Scholar] [CrossRef]
- Song, Z.; Nakata, K.; Wu, A.; Liao, J. Interfacial microstructure and mechanical property of Ti6Al4V/A6061 dissimilar joint by direct laser brazing without filler metal and groove. Mater. Sci. Eng. A 2012, 560, 111–120. [Google Scholar] [CrossRef]
- Naeimian, H.; Mofid, M.A. TLP bonding of Ti−6Al−4V to Al 2024 using thermal spray Babbitt alloy interlayer. Trans. Nonferrous Met. Soc. China 2020, 30, 1267–1276. [Google Scholar] [CrossRef]
- Takemoto, T.; Okamoto, I. Intermetallic compounds formed during brazing of titanium with aluminium filler metals. J. Mater. Sci. 1988, 23, 1301–1308. [Google Scholar] [CrossRef]
- Assari, A.H.; Eghbali, B. Solid state diffusion bonding characteristics at the interfaces of Ti and Al layers. J. Alloys Compd. 2018, 773, 50–58. [Google Scholar] [CrossRef]
- Chandrappa, K.; Kant, R.; Ali, R.; Vineth, K. Optimization of process parameter of diffusion bonding of Ti-Al and Ti-Cu. Mater. Today Proc. 2020, 27, 1689–1695. [Google Scholar] [CrossRef]
- Lee, T.W.; Kim, I.K.; Lee, C.H.; Kim, J.H. Growth behavior of intermetallic compound layer in sandwich-type Ti/Al diffusion couples inserted with Al-Si-Mg alloy foil. J. Mater. Sci. Lett. 1999, 18, 1599–1602. [Google Scholar] [CrossRef]
- Chang, S.; Tsao, L.; Li, T.; Chuang, T. Joining 6061 aluminum alloy with Al–Si–Cu filler metals. J. Alloys Compd. 2009, 488, 174–180. [Google Scholar] [CrossRef]
- Tan, F.F.; Du, K. Analysis of Organizations of Brazed Seam 5052 Al Alloy Contact Reactive Brazing. Adv. Mater. Res. 2013, 690-693, 2598–2600. [Google Scholar] [CrossRef]
- Wu, M.F.; Yu, C.; Pu, J. Study on microstructures and grain boundary penetration behaviours in contact reactive brazing joints of 6063 Al alloy. Mater. Sci. Technol. 2008, 24, 1422–1426. [Google Scholar] [CrossRef]
- Liu, L.; Tan, J.; Liu, X. Reactive brazing of Al alloy to Mg alloy using zinc-based brazing alloy. Mater. Lett. 2007, 61, 2373–2377. [Google Scholar] [CrossRef]
- Wu, M.-F.; Si, N.-C.; Chen, J. Contact reactive brazing of Al alloy/Cu/stainless steel joints and dissolution behaviors of interlayer. Trans. Nonferrous Met. Soc. China 2011, 21, 1035–1039. [Google Scholar] [CrossRef]
- Schällibaum, J.; Burbach, T.; Münch, C.; Weiler, W.; Wahlen, A. Transient liquid phase bonding of AA 6082 aluminium alloy. Mater. Werkst. 2015, 46, 704–712. [Google Scholar] [CrossRef]
- Niu, C.; Han, J.; Hu, S.; Song, X.; Long, W.; Liu, D.; Wang, G. Surface modification and structure evolution of aluminum under argon ion bombardment. Appl. Surf. Sci. 2020, 536, 147819. [Google Scholar] [CrossRef]
- Park, M.; Baek, S.; Kim, S.; Kim, S.E. Argon plasma treatment on Cu surface for Cu bonding in 3D integration and their characteristics. Appl. Surf. Sci. 2015, 324, 168–173. [Google Scholar] [CrossRef]
- Kim, T.H.; Howlader, M.M.R.; Itoh, T.; Suga, T. Room temperature Cu–Cu direct bonding using surface activated bonding method. J. Vac. Sci. Technol. A 2003, 21, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Song, X.; Hu, S.; Lu, G.; Chen, Z.; Wang, G. Effects of brazing temperature and post weld heat treatment on 7075 alloy brazed joints. J. Mater. Process. Technol. 2018, 266, 363–372. [Google Scholar] [CrossRef]
- Song, X.; Niu, C.; Hu, S.; Liu, D.; Cao, J.; Feng, J. Contact reactive brazing of Al7075 alloy using Cu layer deposited by magnetron sputtering. J. Mater. Process. Technol. 2018, 252, 469–476. [Google Scholar] [CrossRef]
- Niu, C.; Han, J.; Hu, S.; Chao, D.; Song, X.; Howlader, M.; Cao, J. Fast and environmentally friendly fabrication of superhydrophilic-superhydrophobic patterned aluminum surfaces. Surfaces Interfaces 2020, 22, 100830. [Google Scholar] [CrossRef]
- Hu, S.; Niu, C.; Bian, H.; Song, X.; Cao, J.; Tang, D. Surface-activation assisted brazing of Al-Zn-Mg-Cu alloy: Improvement in microstructure and mechanical properties. Mater. Lett. 2018, 218, 86–89. [Google Scholar] [CrossRef]
- Pripanapong, P.; Kariya, S.; Luangvaranunt, T.; Umeda, J.; Tsutsumi, S.; Takahashi, M.; Kondoh, K. Corrosion Behavior and Strength of Dissimilar Bonding Material between Ti and Mg Alloys Fabricated by Spark Plasma Sintering. Materials 2016, 9, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Nakano, T.; Umakoshi, Y. Meta-stable region of Al5Ti3 single-phase in time-temperature-transformation (TTT) diagram of Ti–62.5 at.% Al single crystal. Intermetallics 2002, 10, 771–781. [Google Scholar] [CrossRef]
- Chen, S.-L.; Zuo, Y.; Liang, H.; Chang, Y.A. A thermodynamic description for the ternary Al-Mg-Cu system. Met. Mater. Trans. A 1997, 28, 435–446. [Google Scholar] [CrossRef]
- Okamoto, H. Desk Handbook: Phase Diagrams for Binary Alloys; ASM International: Geauga, OH, USA, 2010; p. 315. [Google Scholar]
- Liu, J.; Su, Y.; Xu, Y.; Luo, L.; Guo, J.; Fu, H. First Phase Selection in Solid Ti/Al Diffusion Couple. Rare Met. Mater. Eng. 2011, 40, 753–756. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Liu, S.; Guan, Z.; Yu, X.; Wu, H.; Yu, S.; Fan, D. Process of welding-brazing and interface analysis of lap joint Ti-6Al-4V and aluminum by plasma arc welding. J. Manuf. Process. 2020, 61, 396–407. [Google Scholar] [CrossRef]


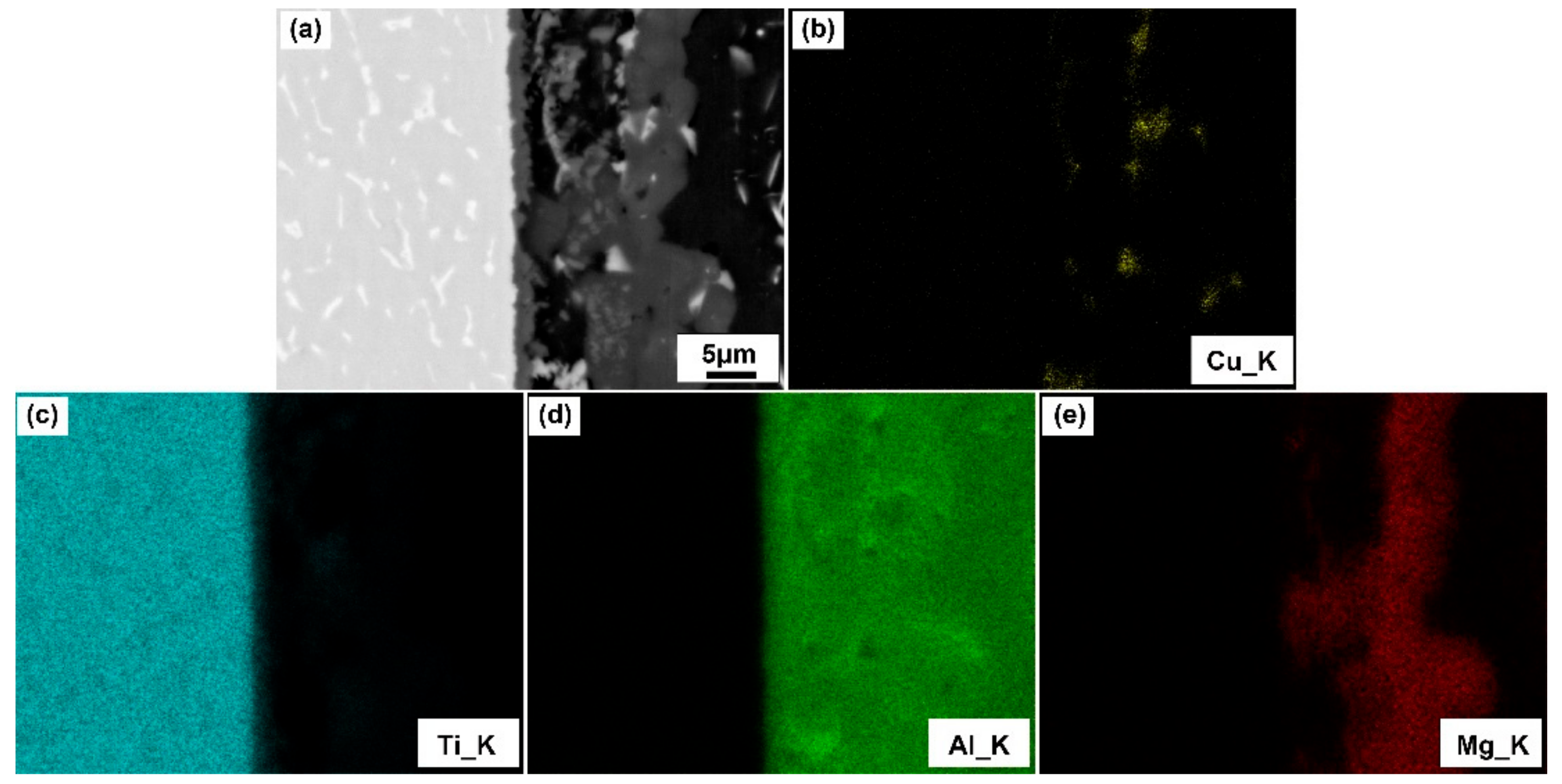



| Spot | Al | Ti | Cu | Mg | Possible Phase |
|---|---|---|---|---|---|
| A | 65.64 | 0.92 | 25.08 | 8.36 | Al2Cu |
| B | 71.95 | 26.45 | 0.54 | 1.06 | Al3Ti |
| C | 60.51 | 36.98 | 1.20 | 1.31 | Al5Ti3 |
| D | 61.84 | 3.80 | 16.24 | 18.12 | Al2CuMg |
| E | 63.60 | 2.30 | 28.57 | 5.53 | Al2Cu |
| F | 96.47 | 1.22 | 1.17 | 1.14 | Al(s, s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Niu, C.; Hu, S.; Song, X.; Pei, Y.; Zhao, J.; Long, W. Contact Reactive Brazing of TC4 Alloy to Al7075 Alloy with Deposited Cu Interlayer. Materials 2021, 14, 6570. https://doi.org/10.3390/ma14216570
Yang M, Niu C, Hu S, Song X, Pei Y, Zhao J, Long W. Contact Reactive Brazing of TC4 Alloy to Al7075 Alloy with Deposited Cu Interlayer. Materials. 2021; 14(21):6570. https://doi.org/10.3390/ma14216570
Chicago/Turabian StyleYang, Mengjuan, Chaonan Niu, Shengpeng Hu, Xiaoguo Song, Yinyin Pei, Jian Zhao, and Weimin Long. 2021. "Contact Reactive Brazing of TC4 Alloy to Al7075 Alloy with Deposited Cu Interlayer" Materials 14, no. 21: 6570. https://doi.org/10.3390/ma14216570






