3.5. The Characteristic Function Group Analysis of Water-Insoluble Gardenia Blue Pigment
Study on the characteristic functional groups of water-insoluble gardenia blue pigments by FTIR and XPS. The FTIR characteristic peaks were showed in
Figure 4: 3264.47 (OH); 2952.05 (CH); 1738.20 (C=O); 1623.91, 1561.40 (C=C); 1283.61, 1134.53 (COOC); 3025.98, 1439.74, 1401.81 (aromatic); 702.38, 743.26 (mono-substituted benzene ring) cm
−1. The results showed that the characteristic absorption peaks of CH, OCH
3, C=O, COOC, C=C, and aromatic can correspond to the FTIR spectra of genipin and L-Phenylalanine methyl ester hydrochloride [
2]. The characteristic absorption peaks of OH became weak due to the lower proportion of the OH group in the pigment.
The results of the full-spectrum XPS of water-insoluble gardenia blue pigment showed that the major elements of water-insoluble gardenia blue pigment are C, N, and O, and the content of C and O are higher than that of N (
Figure 5a). The method of separating overlapped curves was adopted to analyze C1s, O1s, and N1s peaks (
Figure 5b–d). The contents of the functional groups are in
Table 2,
Table 3 and
Table 4. The C-1s spectrum of water-insoluble gardenia blue pigment presents main peaks at 284.16 eV, 284.68 eV, 286.09 eV related to Sp2 hybrid, C=C, and C-O structures. The O-1s spectrum of water-insoluble gardenia blue pigment presents two peaks at 531.95 eV and 533.47 eV related to O-C and O=C structures. The N-1s spectrum of water-insoluble gardenia blue pigment presents two peaks at 402.24 eV and 400.57 eV related to C-N-C and C-N structures. As a result, XPS shows that water-insoluble gardenia blue pigment has a π-π * resonance system, and functional groups are O-C=O, C=O, COOC, C-N, C=C, and benzene ring.
3.7. Mass Spectrometry Study of Water-Insoluble Gardenia Blue Pigments
The positive-ion mode of ESI mass spectra was performed to investigate the structures of water-insoluble gardenia blue pigments, which were coded as follows, Z-1, Z-2, Z-3, Z-4, Z-5, and Z-6.
The first-order mass spectra of the six different water-insoluble gardenia blue pigments Z-1–Z-6 showed the molecular weights of the pigments. The [M + Na]
+ ion was selected as the precursor ion in the MS/MS experiment to give fragmentation information.
Table 6 shows the mass and composition of the Na
+ adduct ions [M + Na]
+ and main compound ions of the pigments.
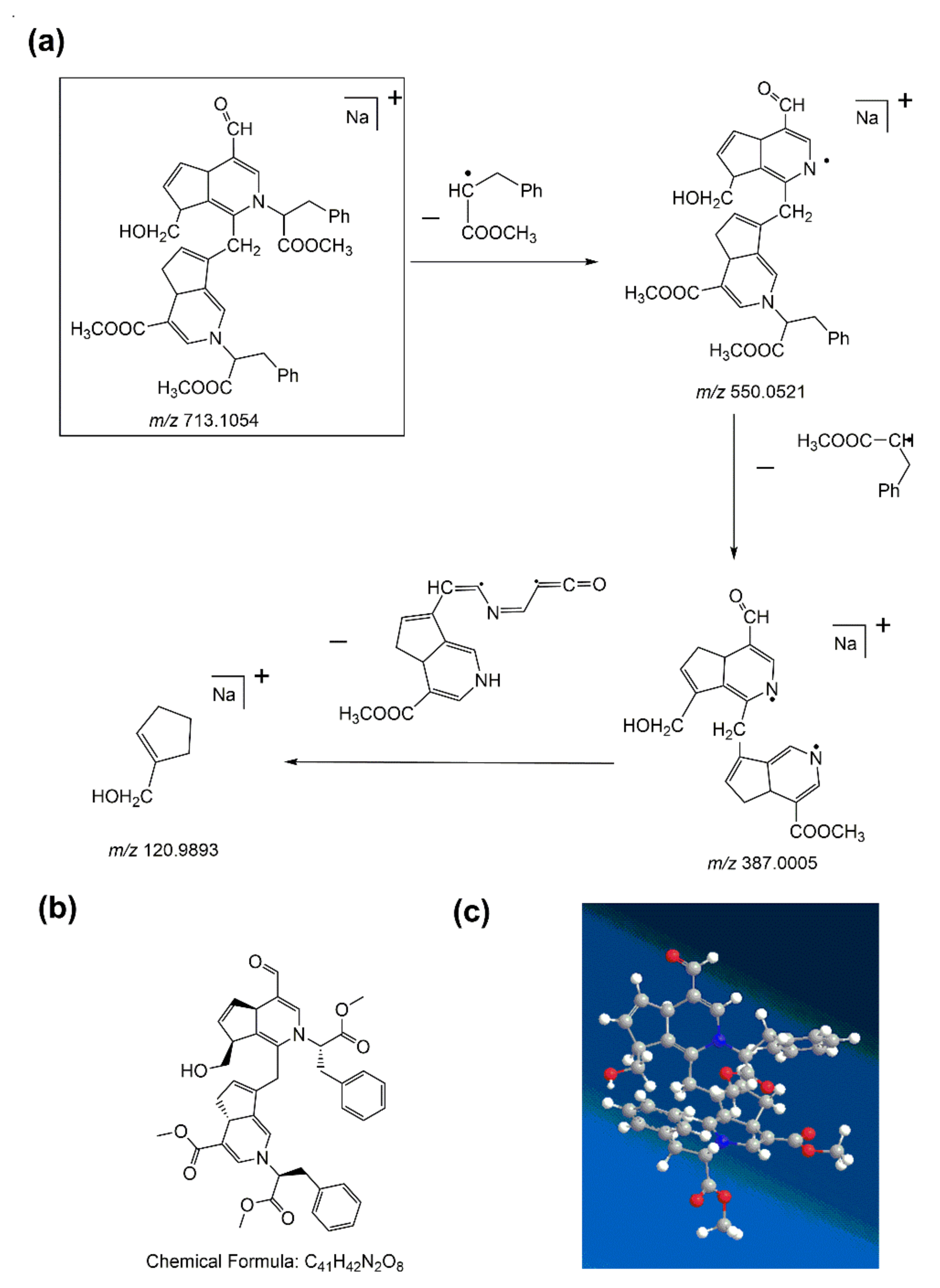
In the first-order MS of water-insoluble gardenia blue pigment Z-1, the [M + Na]
+ ion at
m/z 713.1054 was studied as the base peak, which proved that the formula weight of the water-insoluble gardenia blue pigment Z-1 is 690.1156 Da (the formula weight of sodium (Na) is 22.9898 Da). The precursor ion ([M + Na]
+) in the MS/MS test could offer varying fragmentation information. The product ion (
m/z 550.0521) had the highest abundance and the product (
m/z 387.0005) corresponded to the loss of two Δm = 163 fragments of L-Phenylalanine methyl ester hydrochloride. More peaks simultaneously appeared at
m/z 120.9893 because of ring cleavage, which corresponded to the loss of a Δm = 266 fragment. The fragmentation pattern of water-insoluble gardenia blue pigment Z-1 is shown in
Figure 6a. The structure of water-insoluble gardenia blue pigment Z-1 had significantly better functional substituents and the cleavage of the basic skeleton of the product. Then we can also deduce that the chemical formula of water-insoluble gardenia blue pigment Z-1 is C
41H
42N
2O
8. The planar and 3D molecular structure simulation graphs are shown in
Figure 6b,c.
In the first-order MS of water-insoluble gardenia blue pigment Z-2, the [M + Na]
+ ion at
m/z 743.1124 was researched as the base peak, which proves the formula weight of the water-insoluble gardenia blue pigment Z-2 is 720.1226 Da. The precursor ion ([M + Na]
+) in the MS/MS test could offer more fragmentation information. The product ion is at
m/z 580.0592 with low abundance, and it corresponded to the loss of a Δm = 163 fragment of L-Phenylalanine methyl ester hydrochloride. It is similar to water-insoluble gardenia blue pigment Z-1. With the loss of -OCH
3 (Δm = 31), further peaks simultaneously appeared at
m/z 549.0453, which was the highest abundance. The fragment ion at
m/z 387.0007 was produced by the loss of a Δm = 162 fragment of L-Phenylalanine methyl ester hydrochloride. As water-insoluble gardenia blue pigment Z-1, further peaks simultaneously appeared at
m/z 120.9893 because of ring cleavage, and it corresponded to the loss of a Δm = 266 fragment. The fragmentation pattern of water-insoluble gardenia blue pigment Z-2 is shown in
Figure 7a. The structure of water-insoluble gardenia blue pigment Z-2 can be deduced by the functional substituents and the cleavage of the basic skeleton of the product. In this way, we can deduce that the chemical formula of water-insoluble gardenia blue pigment Z-2 is C
42H
44N
2O
9. The planar and 3D molecular structure simulation graphs are shown in
Figure 7b,c.
In the first-order MS of water-insoluble gardenia blue pigment Z-3, the [M + Na]
+ ion at
m/z 731.1144 was tested as the base peak which proves the formula weight of the water-insoluble gardenia blue pigment Z-3 is 708.1246 Da. The precursor ion ([M + Na]
+) in the MS/MS test could offer more fragmentation information. The product ion is at
m/z 568.0598 with the highest abundance, and it corresponded to the loss of a Δm = 163 fragment of L-Phenylalanine methyl ester hydrochloride, it is similar to water-insoluble gardenia blue pigments Z-1 and Z-2. With the loss of -CH
3 (Δm = 15), further peaks simultaneously appeared at
m/z 553.0389. The fragment ion at
m/z 380.0172 was produced by the loss of a Δm = 173 fragment. Different from water-insoluble gardenia blue pigments Z-1 and Z-2, further peak simultaneously appeared at
m/z 120.9898 because of ring cleavage, and it corresponded to the loss of a Δm = 259 fragment. The fragmentation pattern of water-insoluble gardenia blue pigment Z-3 is shown in
Figure 8a. The structure of water-insoluble gardenia blue pigment Z-3 can be deduced by the functional substituents and the cleavage of the basic skeleton of the product. In this way, we can deduce that the chemical formula of water-insoluble gardenia blue pigment Z-3 is C
41H
44N
2O
9. The planar and 3D molecular structure simulation graphs are shown in
Figure 8b,c.
In the first-order MS of water-insoluble gardenia blue pigments Z-4–Z-6, the [M + Na]+ ions at m/z 699.2772, 1068.4367, and 705.0953, were observed as the base peaks which prove that the formula weights of them are 676.2874, 1045.4469, and 682.1055 Da. The [M + Na]+ ions were voted as the precursor ion in the MS/MS test that could give more fragmentation information. The fragment ions of water-insoluble gardenia blue pigments Z-4–Z-6 were at m/z 536.0379, 373.9934, 120.9894, m/z 1036.1850, 1006.1770, 847.1269, 739.0832, 605.0628, 549.0437, 364.0243, 120.9891, and m/z 616.5550, 542.0380, 364.0250, 120.9898 respectively. Since the decomposition processes of water-insoluble gardenia blue pigments Z-4–Z-6 are different from the water-insoluble gardenia blue pigments Z-1–Z-3, and there are no published papers saying much, the structures, fragmentation pattern, and chemical formulas of water-insoluble gardenia blue pigments Z-4–Z-6 have not been deduced yet.
3.9. Stability Study of Gardenia Blue Pigment
To investigate the light stability of water-soluble and water-insoluble gardenia blue pigments, the experiments were carried out under four different light conditions: sunlight (40,000 lux), lamplight (13,000 lux), room light (300 lux), and dark (control) for 7 days. The residual rate of the pigments is shown in
Figure 9.
It was found that the stability of water-insoluble gardenia blue pigment under lamplight was significantly better than that water-soluble gardenia blue pigment (
p < 0.05), and the residual rate of it was 74.9%. However, there were no obvious differences between the water-soluble gardenia blue pigment and water-insoluble gardenia blue pigment under the light conditions: sunlight, room light, and dark (
p > 0.05). Furthermore, the results of color properties (
Table 8) were consistent with the residual rate. Under the lamplight condition, there was an apparent difference of the
between the two pigments (
p < 0.05), the stability of water-insoluble gardenia blue pigment was significantly better than water-soluble gardenia blue pigment. However, there were no obvious differences of
between the water-soluble gardenia blue pigment and water-insoluble gardenia blue pigment under the light conditions: sunlight, room light, and dark (
p > 0.05).
In previous studies, Zhang (2008a) studied the light stability of the hydrophobic gardenia blue pigment, which was made from the esterification of acetic anhydride and water-soluble gardenia blue pigment. The result showed that the residual rate of the pigment was 52.8% under 12,000 lux for 28 days.
To investigate the temperature stability of water-soluble and water-insoluble gardenia blue pigments, the experiments were carried out under different heat conditions: 4 °C, 40 °C, 60 °C, 80 °C, 100 °C, and 120 °C, and room temperature (control) for 24 h. The residual rates of the pigments are shown in
Figure 10.
It was found that the stability of water-insoluble gardenia blue pigment under 80–120 °C was significantly better than that water-soluble gardenia blue pigment (
p < 0.05), and the residual rates of it were 95.26, 88.27, and 87.72% respectively. However, there were no obvious differences between the water-soluble gardenia blue pigment and water-insoluble gardenia blue pigment under 4–60 °C (
p > 0.05). Furthermore, the results of color properties (
Table 9) were consistent with the residual rate. Under 60–120 °C as there was an apparent difference regarding the
between the two pigments (
p < 0.05), and the stability of water-insoluble gardenia blue pigment was significantly better than water-soluble gardenia blue pigment.