Plant Material as a Novel Tool in Designing and Formulating Modern Biostimulants—Analysis of Botanical Extract from Linum usitatissimum L.
Abstract
1. Introduction
2. Materials and Methods
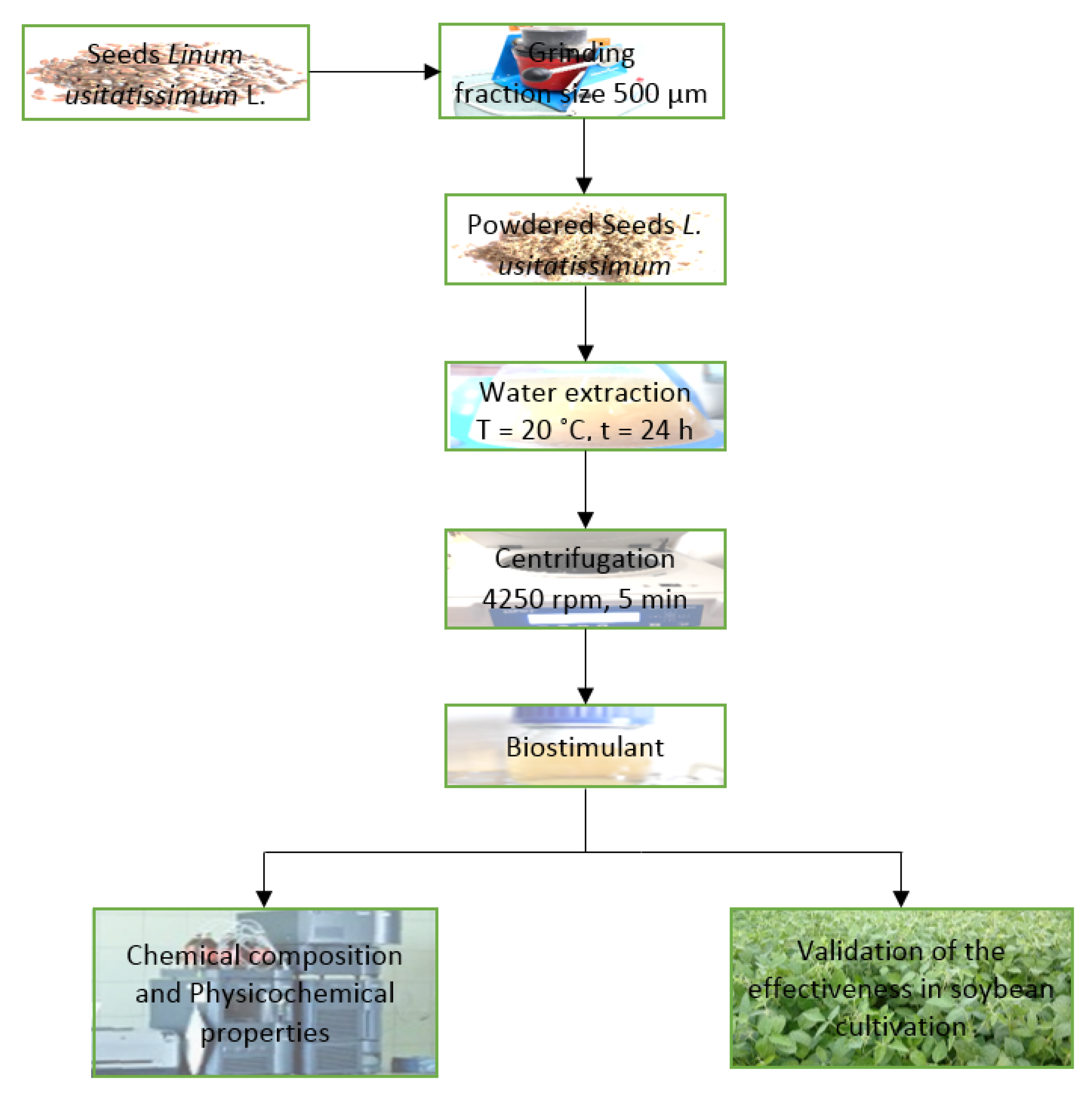
2.1. Extract Production
2.2. Multielemental Composition and Carbohydrate Content in Extracts from L. Usitatissimum Seeds
2.3. Protein Amino Acids Composition in Extracts from L. Usitatissimum Seeds
2.4. Determination of Fatty Acids in Extracts from L. Usitatissimum Seeds
2.5. Microbiological Analyses of Non-Microbial Biostimulant
2.6. Physical Properties of Extracts from L. Usitatissimum Seeds
2.7. Plant Material and Growth Conditions
2.8. Evaluation of the Economic Effect
2.9. Statistical Analysis
3. Results
3.1. Chemical Composition of Extracts
3.2. Effect of the Application of the Extract on the Morphological Characteristics and Yields of Soybean
3.3. Physical Properties of the Biostimulant from L. Usitatissimum Seeds
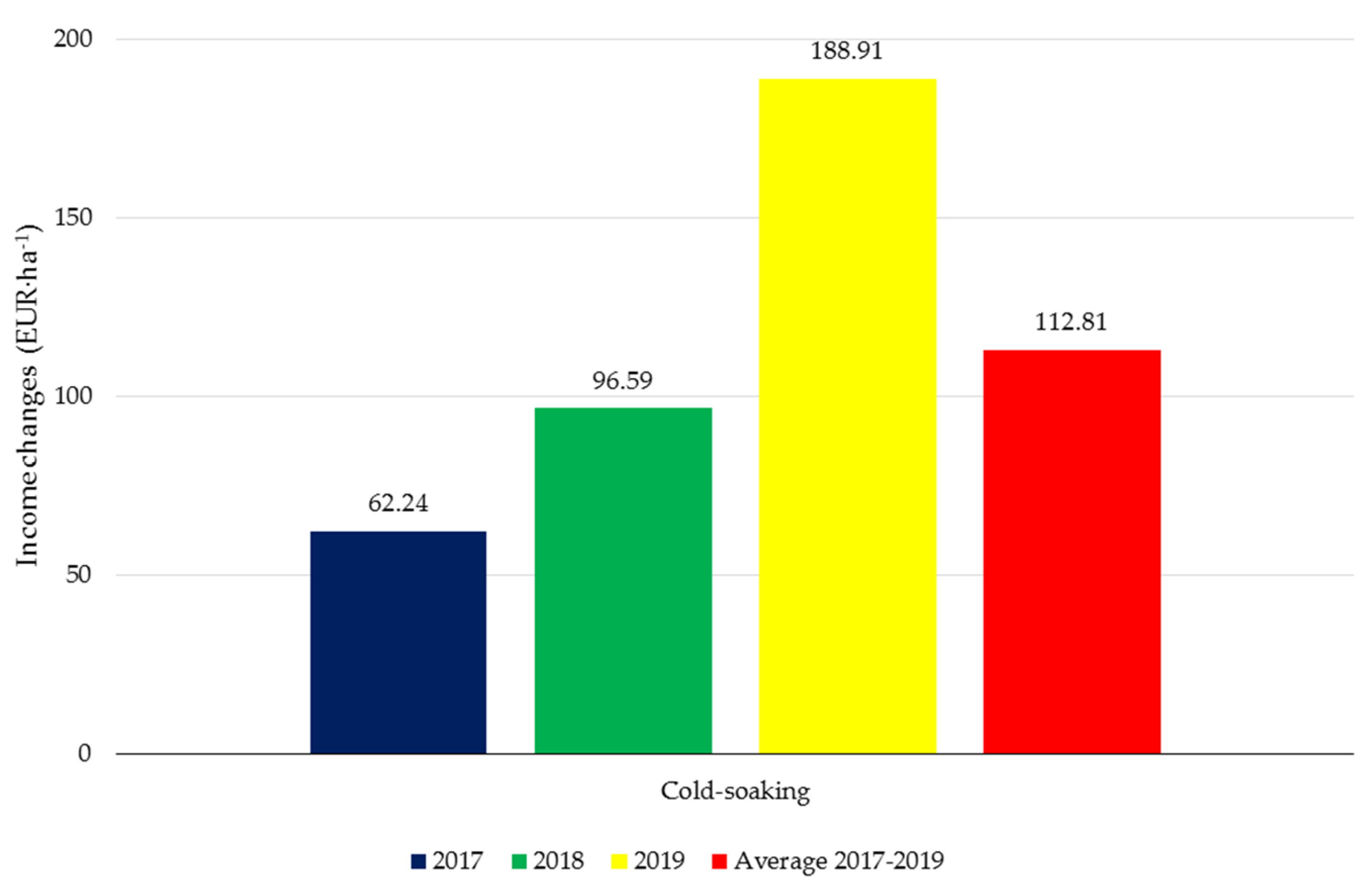
3.4. Botanical Biostimulants versus Profits from Soybean Cultivation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the Biostimulant Effects of a Novel Plant-Based Formulation on Tomato Crop. Sustainability 2020, 12, 8432. [Google Scholar] [CrossRef]
- Francesca, S.; Cirillo, V.; Raimondi, G.; Maggio, A.; Barone, A.; Rigano, M.M. A Novel Protein Hydrolysate-Based Biostimulant Improves Tomato Performances under Drought Stress. Plants 2021, 10, 783. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kapusta, I. Identification of a Biostimulating Potential of an Organic Biomaterial Based on the Botanical Extract from Arctium lappa L. Roots. Materials 2021, 14, 4920. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kapusta, I.; Zaguła, G. Prototyping extracts from Artemisia absinthium L. for their biostimulating properties yield-enhancing, and farmer income-increasing properties. Ind. Crop. Prod. 2021, 160, 113125. [Google Scholar] [CrossRef]
- Jakubowski, T. Effects of microwave radiation on the germination of Solanum tuberosum L. tubers. Bangladesh J. Bot. 2016, 45, 1255–1257. [Google Scholar]
- Jakubowski, T. The reaction of garden cress (Lepidium sativum L.) to microwave radiation. In Proceedings of the 2018 Applications of Electromagnetics in Modern Techniques and Medicine (PTZE), Racławice, Poland, 9–12 September 2018; pp. 81–84. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A new trend towards solving an old problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar] [CrossRef]
- Koo, A.J. Metabolism of the plant hormone jasmonate: A sentinel for tissue damage and master regulator of stress response. Phytochem. Rev. 2018, 17, 51–80. [Google Scholar] [CrossRef]
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural Functions and Action Mechanisms of Plant Biostimulants (PBs): An Introduction. In The Chemical Biology of Plant Biostimulants, 1st ed.; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 3–30. [Google Scholar]
- Michałek, W.; Kocira, A.; Findura, P.; Szparaga, A.; Kocira, S. The influence of biostimulant Asahi SL on the photosynthetic activity of selected cultivars of Phaseolus vulgaris L. Rocz. Ochr. Sr. 2018, 20, 1286–1301. [Google Scholar]
- Szparaga, A.; Kuboń, M.; Kocira, S.; Czerwińska, E.; Pawłowska, A.; Hara, P.; Kobus, Z.; Kwaśniewski, D. Towards sustainable agriculture—Agronomic and economic effects of biostimulant use in common bean cultivation. Sustainability 2019, 11, 4575. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 6 September 2021).
- Szparaga, A.; Kocira, S. Generalized logistic functions in modelling emergence of Brassica napus L. PLoS ONE 2018, 13, e0201980. [Google Scholar] [CrossRef] [PubMed]
- Szparaga, A.; Kocira, S.; Findura, P.; Kapusta, I.; Zaguła, G.; Świeca, M. Uncovering the multi-level response of Glycine max L. to the application of allelopathic biostimulant from Levisticum officinale Koch. Sci. Rep. 2021, 11, 15360. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plantbased natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Shale, T.; Stirk, W.; van Staden, J. Screening of medicinal plants used in Lesotho for antibacterial and anti-inflammatory activity. J. Ethnopharmacol. 1999, 67, 347–354. [Google Scholar] [CrossRef]
- Al-Bayati, F.A. Antibacterial Activity of Linum usitatissimum L. Seeds and Active Compound Detection. Rafidain J. Sci. 2007, 18, 27–36. [Google Scholar] [CrossRef]
- Rady, M.M.; Varma, B.C.; Howladar, S.M. Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci. Hortic. 2013, 162, 63–70. [Google Scholar] [CrossRef]
- Semida, W.M.; Rady, M.M. Presoaking application of propolis and maize grain extracts alleviates salinity stress in common bean (Phaseolus vulgaris L.). Sci. Hortic. 2014, 68, 210–217. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of salt stress effects on the growth, physiochemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.S.M.; Elrys, A.S.; Boghdady, M.S. Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants? S. Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Desoky, E.M.; Merwad, A.M.; Rady, M.M. Natural biostimulants improve saline soil characteristics and salt stressed-sorghum performance. Commun. Soil Sci. Plant Anal. 2018, 49, 967–983. [Google Scholar] [CrossRef]
- Rehman, H.U.; Alharby, H.F.; Alzahrani, Y.; Rady, M.M. Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiol. Biochem. 2018, 126, 97–105. [Google Scholar] [CrossRef]
- Taha, R.S.; Alharby, H.F.; Bamagoos, A.A.; Medani, R.A.; Rady, M.M. Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Hussein, N.M.; Hussein, M.I.; Gadel, H.S.H.; Hammad, M.A. Effect of two plant extracts and four aromatic oils on Tuta absoluta population and productivity of tomato cultivar gold stone. J. Plant Prot. Pathol. 2015, 6, 969–985. [Google Scholar] [CrossRef][Green Version]
- Hayat, S.; Ahmad, H.; Ali, M.; Hayat, K.; Khan, M.A.; Cheng, Z. Cheng Aqueous garlic extract as a plant biostimulant enhances physiology, improves crop quality and metabolite abundance, and primes the defense responses of receiver plants. Appl. Sci. 2018, 8, 1505. [Google Scholar] [CrossRef]
- Cheema, Z.A.; Farooq, M.; Khaliq, A. Application of allelopathy in crop production: Success story from Pakistan. In Allelopathy; Cheema, Z., Farooq, M., Wahid, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 113–143. [Google Scholar] [CrossRef]
- Farooq, M.; Bajwa, A.A.; Cheema, S.A.; Cheema, Z.A. Application of allelopathy in crop production. Int. J. Agric. Biol. 2013, 15, 1367–1378. [Google Scholar]
- Kamran, M.; Ali, Q.; Cheema, Z.A. Foliar application of crop water extract improves the performance of maize. Plant Environ. 2021, 1, 138–151. [Google Scholar]
- Vasey-Genser, M.; Morris, D.H. Introduction: History of the cultivation and uses of flaxseed. In Flax, the Genus Linum, 1st ed.; Muir, A.D., Westcott, N.D., Eds.; CRC Press: London, UK, 2015. [Google Scholar] [CrossRef]
- Anjum, F.M.; Hussain, S. Flaxseed (Linseed), a valuable grain: A review. Food Aust. 2007, 59, 597–601. [Google Scholar]
- Goyal, A.; Sharma, V.; Upadhay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef]
- Oomah, B.D.; Kenaschuk, E.O.; Mazza, G. Tocopherols in flaxseed. J. Agric. Food Chem. 1997, 45, 2076–2080. [Google Scholar] [CrossRef]
- Makkar, C.; Singh, J.; Parkash, C. Modulatory role of vermicompost and vermiwash on growth, yield and nutritional profiling of Linum usitatissimum L. (Linseed): A field study. Environ. Sci. Pollut. Res. 2019, 26, 3006–3018. [Google Scholar] [CrossRef]
- Jhala, J.A.; Hall, L.M. Flax (Linum usitatissimum L.): Current Uses and Future Applications. Aust. J. Basic Appl. Sci. 2010, 4, 4304–4312. [Google Scholar]
- Czerwińska, E.; Szparaga, A. The vitality and healthiness of oil seeds treated by plant extracts. Acta Sci. Pol. Tech. Agrar. 2015, 14, 47–59. [Google Scholar]
- Czerwińska, E.; Szparaga, A.; Deszcz, E. Estimation of effect of dressing in plant extracts on germination capacity of beetroots seeds. Zesz. Nauk. Uniw. Przyr. Wroc.-Rol. 2015, 611, 7–20. [Google Scholar]
- Kocira, S.; Hara, P.; Szparaga, A.; Czerwińska, E.; Beloev, H.; Findura, P.; Bajus, P. Evaluation of the Effectiveness of the Use of Biopreparations as Seed Dressings. Agriculture 2020, 10, 90. [Google Scholar] [CrossRef]
- Szparaga, A.; Czerwińska, E.; Piskier, T. The effect of treating the seeds of Brassica oleracea L. with aqueous extracts on the germination capacity and seed healthiness. J. Res. Appl. Agric. Eng. 2017, 62, 162–167. [Google Scholar]
- Kocira, S.; Czerwińska, E.; Szparaga, A. Analysis of the Ecological Method of Treatment in the Aspect of Increasing the Vitality and Healthiness of Spring Barley Grains Hordeum vulgare L. Rocz. Ochr. Sr. 2018, 20, 1746–1763. [Google Scholar]
- Macias, F.A.; Marin, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M.G. Allelopathy as a new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Han, X.; Cheng, Z.H.; Meng, H.W.; Yang, X.L.; Ahmad, I. Allelopathic effect of decomposed garlic (Allium Sativum L.) stalk on lettuce (L. Sativa Var. Crispa L.). Pak. J. Bot. 2013, 45, 225–233. [Google Scholar]
- Bhadoria, P.B.S. Allelopathy: A natural way towards weed management. Am. J. Exp. Agric. 2011, 1, 7–20. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Pedrol, N.; González, L.; Reigosa, M.J. Allelopathy and abiotic stress. In Allelopathy: A Physiological Process with Ecological Implications; Reigosa, M.J., Pedrol, N., González, L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 171–209. [Google Scholar]
- Hara, P.; Szparaga, A.; Czerwinska, E. Ecological Methods Used to Control Fungi that Cause Diseases of the Crop Plant. Rocz. Ochr. Sr. 2018, 20, 1764–1775. [Google Scholar]
- Le Mire, G.; Nguyen, M.L.; Fassotte, B.; du Jardin, P.; Verheggen, F.; Delaplace, P.; Jijakli, H. Implementing plant biostimulants and biocontrol strategies in the agroecological management of cultivated ecosystems, A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 299–313. [Google Scholar]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and economical effect of application biostimulants containing seaweed extracts and amino acids as an element of agroecological management of bean cultivation. Sci. Rep. 2020, 10, 17759. [Google Scholar] [CrossRef] [PubMed]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of growth, yield, and the nutraceutical and antioxidative potential of soybean through the use of synthetic biostimulants. Front. Plant Sci. 2018, 9, 1401. [Google Scholar] [CrossRef]
- Biegański, J. Herbal Medicine-Our Herbs and Treatment; Jamiołkowski i Evert Sp. z o.o.: Łódź, Poland, 1950. [Google Scholar]
- Zaguła, G.; Bajcar, M.; Saletnik, B.; Czernicka, M.; Puchalski, C.; Kapusta, I.; Oszmiański, J. Comparison of the Effectiveness of Water-Based Extraction of Substances from Dry Tea Leaves with the Use of Magnetic Field Assisted Extraction Techniques. Molecules 2017, 22, 1656. [Google Scholar] [CrossRef] [PubMed]
- Pereira da Costa, M.; Conte-Junior, C.A. Chromatographic Methods for the Determination of Carbohydrates and Organic Acids in Foods of Animal Origin. Compr. Rev. Food Sci. Food Saf. 2015, 14, 586–600. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, O. Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acids. J. Pharm. Anal. 2015, 5, 223–230. [Google Scholar] [CrossRef]
- European Committee for Standardization. Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.; ISO 6579-1:2017; European Committee for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- European Committee for Standardization. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl beta-D-Glucuronide; ISO 16649-2:2001; European Committee for Standardization: Geneva, Switzerland, 2001. [Google Scholar]
- Stauffer, C.D. The Measurements of Surface Tension by the Pendant Drop Technique. J. Phys. Chem. 1965, 69, 1933–1938. [Google Scholar] [CrossRef]
- Hansen, F.K.; Rødsrud, G. Surface tension by pendant drop: I. A fast standard instrument using computer image analysis. J. Colloid Interface Sci. 1991, 141, 1–9. [Google Scholar] [CrossRef]
- Song, B.; Springer, J. Determination of Interfacial Tension from the Profile of a Pendant Drop Using Computer-Aided Image Processing: 1. Theoretical. J. Colloid Interface Sci. 1996, 184, 64–76. [Google Scholar] [PubMed]
- Kalantarian, A.; Saad, S.M.I.; Neumann, A.W. Accuracy of surface tension measurement from drop shapes: The role of image analysis. Adv. Colloid Interface Sci. 2013, 199–200, 15–22. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Kuboń, M.; Czerwińska, E.; Piskier, T. Morphological and Biochemical Responses of Glycine max (L.) Merr. to the Use of Seaweed Extract. Agronomy 2019, 9, 93. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1009 (accessed on 6 September 2021).
- Paleckiene, R.; Sviklas, A.; Šlinkšiene, R. Physicochemical properties of a microelement fertilizer with amino acids. Russ. J. Appl. Chem. 2007, 80, 352–357. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the New Plant Growth Biostimulants Based on Amino Acids on Yield and Grain Quality of Winter Wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef]
- Jakiene, E. The effect of the microelement fertilizers and biological preparation Terra Sorb Foliar on spring rape crop. Žemes Ukio Moksl. 2013, 20, 75–83. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Tegeder, M. Transporters for amino acids in plant cells: Some functions and many unknowns. Curr. Opin. Plant Biol. 2012, 15, 315–321. [Google Scholar] [CrossRef]
- Tegeder, M.; Hammes, U.Z. The way out and in: Phloem loading and unloading of amino acids. Curr. Opin. Plant Biol. 2018, 43, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Ward, J.M. Molecular evolution of plant AAP and LHT amino acid transporters. Front. Plant Sci. 2012, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, M.M.; Mogor, A.F.; Pauletti, V.; Goicoechea, N.; Aranjuelo, I.; Garmendia, I. Nutritional quality and yield of onion as affected by different application methods and doses of humic substances. J. Food Comp. Anal. 2016, 51, 37–44. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tugnoli, V.; Righi, V.; Nardi, S. Effect of commercial lignosulfonate-humate on Zea mays L. metabolism. J. Agric. Food Chem. 2011, 59, 11940–11948. [Google Scholar] [CrossRef] [PubMed]
- Shahabivand, S.; Padash, A.; Aghaee, A.; Nasiri, Y.; Rezaei, P.F. Plant biostimulants (Funneliformis mosseae and humic substances) rather than chemical fertilizer improved biochemical responses in peppermint. Plant Physiol. 2018, 8, 2333–2344. [Google Scholar] [CrossRef]
- Briglia, N.; Petrozza, A.; Hoeberichts, F.A.; Verhoef, N.; Povero, G. Investigating the Impact of Biostimulants on the Row Crops Corn and Soybean Using High-Efficiency Phenotyping and Next Generation Sequencing. Agronomy 2019, 9, 761. [Google Scholar] [CrossRef]
- Barone, V.; Bertoldo, G.; Magro, F.; Broccanello, C.; Puglisi, I.; Baglieri, A.; Cagnin, M.; Concheri, G.; Squartini, A.; Pizzeghello, D.; et al. Molecular and Morphological Changes Induced by Leonardite-based Biostimulant in Beta vulgaris L. Plants 2019, 8, 181. [Google Scholar] [CrossRef]
- Godlewska, A.; and Ciepiela, G. Carbohydrate and lignin contents in perennial ryegrass (Lolium perenne L.) treated with sea bamboo (Ecklonia maxima) extract against the background of nitrogen fertilisation regime. Appl. Ecol. Environ. Res. 2020, 18, 6087–6097. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Michalak, I.; Biesiada, A.; Szumny, A.; Pachura, N.; Piszcz, U. Field-Scale Evaluation of Botanical Extracts Effect on the Yield, Chemical Composition and Antioxidant Activity of Celeriac (Apium graveolens L. Var. rapaceum). Molecules 2020, 25, 4212. [Google Scholar] [CrossRef]
- Godlewska, K.; Pacyga, P.; Michalak, I.; Biesiada, A.; Szumny, A.; Pachura, N.; Piszcz, U. Effect of Botanical Extracts on the Growth and Nutritional Quality of Field-Grown White Head Cabbage (Brassica oleracea var. capitata). Molecules 2021, 26, 1992. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. in response to an Alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2018, 8, 1159. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth and N uptake in tomato coupled with N-dependent gene expression involved in N assimilation. Front Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Seyed Sharifi, R. Application of biofertilizers and zinc increases yield, nodulation and unsaturated fatty acids of soybean. Zemdirbyste 2016, 103, 251–258. [Google Scholar] [CrossRef]
- Thenua, O.V.S.; Kuldeep, S.; Vivek, R.; Jasbir, S. Effect of sulphur and zinc application on growth and productivity of soybean [Glycine max. (L.) Merrill] in northern plain zone of India. Ann. Agric. Res. New Ser. 2014, 35, 183–187. [Google Scholar]
- Sarkar, D.; Mandal, B.; Kundu, M.C. Increasing use efficiency of boron fertilisers by rescheduling the time and methods of application for crops in India. Plant Soil 2007, 301, 77–85. [Google Scholar] [CrossRef]
- He, M.; Qin, C.X.; Wang, X.; and Ding, N.Z. Plant unsaturated fatty acids: Biosynthesis and regulation. Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, A.; Ciepiela, G.A. Italian Ryegrass (Lolium multiflorum Lam.) Fiber Fraction Content and Dry Matter Digestibility Following Biostimulant Application against the Background of Varied Nitrogen Regime. Agronomy 2021, 11, 39. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, H.; Ozkan, H.E.; Derksen, R.C.; Krause, C.R. Evaporation and deposition coverage area of droplets containing insecticides and spray additives on hydrophilic, hydrophobic, and crabapple leaf surfaces. Trans. ASAE 2009, 52, 39–49. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, H.; Ozkan, H.E.; Bagley, W.E.; Krause, C.R. Droplet evaporation and spread on waxy and hairy leaves associated with type and concentration of adjuvants. Pest Manag. Sci. 2011, 67, 842–851. [Google Scholar] [CrossRef]
- He, Y.; Xiao, S.; Wu, J.; Fang, H. Influence of multiple factors on the wettability and surface free energy of leaf surface. Appl. Sci. 2019, 9, 593. [Google Scholar] [CrossRef]
- Subr, A.; Parafiniuk, S.; Milanowski, M.; Krawczuk, A.; Kachel, M. Study of deposited spray quality of spraying agents with different physical properties. Plant Arch. 2020, 20, 6109–6114. [Google Scholar]
| Element | Extract Obtained by Cold-Soaking |
|---|---|
| Macroelements (mg·L−1) | |
| Ca | 68.737 ± 0.217 |
| K | 182.800 ± 1.135 |
| Mg | 32.863 ± 0.442 |
| Na | 28.380 ± 0.101 |
| P | 25.70 ± 0.011 |
| S | 33.387 ± 0.061 |
| Microelements (mg·L−1) | |
| Cu | 0.239 ± 0.001 |
| Mn | 0.069 ± 0.002 |
| Sr | 1.185 ± 0.005 |
| Zn | 0.399 ± 0.002 |
| Fe | 0.033 ± 0.003 |
| Al | 0.004 ± 0.003 |
| Toxic metals (mg·L−1) | |
| As | <LLD |
| Cd | <LLD |
| Pb | <LLD |
| Compound | Extract Obtained by Cold-Soaking |
|---|---|
| Carbohydrates (mg·mL−1) | |
| Sucrose | 1.070 ± 0.021 |
| Glucose | 0.540 ± 0.018 |
| Fructose | 0.374 ± 0.014 |
| Amino acids (mg·L−1) | |
| Aspartic acid (Asp) | 0.661 ± 0.018 |
| Threonine (Thr) | 0.214 ± 0.016 |
| Serine (Ser) | 0.309 ± 0.019 |
| Glutamic acid (Glu) | 1.490 ± 0.015 |
| Proline (Pro) | 0.119 ± 0.007 |
| Glycine (Gly) | 0.402 ± 0.012 |
| Alanine (Ala) | 0.321 ± 0.009 |
| Valine (Val) | 0.284 ± 0.010 |
| Isoleucine (Ile) | 0.239 ± 0.011 |
| Leucine (Leu) | 0.354 ± 0.010 |
| Tyrosine (Tyr) | 0.135 ± 0.009 |
| Phenylalanine (Phe) | 0.291 ± 0.007 |
| Histidine (His) | 0.129 ± 0.012 |
| Lysine (Lys) | 0.271 ± 0.014 |
| Arginine (Arg) | 0.650 ± 0.015 |
| Compound | Extract Obtained by Cold-Soaking |
|---|---|
| Fatty acids (g·100 g−1) | |
| C6:0 | 0.0001 ± 0.0000 |
| C8:0 | 0.0006 ± 0.0001 |
| C10:0 | 0.0007 ± 0.0001 |
| C12:0 | 0.0011 ± 0.0001 |
| C14:0 | 0.0032 ± 0.0001 |
| C14:1n5 | 0.0001 ± 0.0000 |
| C15:0 | 0.0003 ± 0.0000 |
| C16:0 | 0.0180 ± 0.0009 |
| C16:1n7 | 0.0003 ± 0.0000 |
| C17:0 | 0.0003 ± 0.0000 |
| C18:0 | 0.0086 ± 0.0005 |
| C18:1n9c + C18:1n9t | 0.0217 ± 0.0011 |
| C18:2n6c + C18:2n6t | 0.0031 ± 0.0002 |
| C18:3n3 (alpha) | 0.0026 ± 0.0002 |
| C20:0 | 0.0003 ± 0.0000 |
| C22:0 | 0.0004 ± 0.0000 |
| C23:0 | 0.0001 ± 0.0000 |
| C24:0 | 0.0002 ± 0.0000 |
| SFA | 0.0342 ± 0.0012 |
| MUFA | 0.0221 ± 0.0012 |
| PUFA | 0.0057 ± 0.0006 |
| OMEGA 3 | 0.0026 ± 0.0002 |
| OMEGA 6 | 0.0031 ± 0.0002 |
| OMEGA 9 | 0.0217 ± 0.0011 |
| Year | Treatment | Plant Height (cm) | Height of the Location of the First Pod (cm) | Number of Pods (per Plant) | Seed Yield (g m−2) | 1000 Seed Weight (g) |
|---|---|---|---|---|---|---|
| 2017 | Control | 110.1 ± 4.2 a | 10.8 ± 2.4 a | 19.4 ± 1.1 a | 246.2 ± 13.0 a | 177.47 ± 0.89 a |
| Cold-soaking | 115.3 ± 3.6 a | 11.0 ± 1.9 a | 22.0 ± 2.2 a | 284.9 ± 55.3 a | 175.01 ± 2.03 a | |
| 2018 | Control | 107.3 ± 2.6 b | 13.4 ± 1.4 b | 17.5 ± 1.3 b | 236.3 ± 21.5 b | 177.58 ± 0.93 a |
| Cold-soaking | 113.3 ± 2.7 a | 11.7 ± 1.2 b | 21.9 ± 2.4 a | 285.8 ± 24.9 a | 174.12 ± 0.86 b | |
| 2019 | Control | 113.1 ± 2.7 a | 13.9 ± 2.1 a | 17.2 ± 1.5 b | 232.1 ± 31.8 b | 178.92 ± 0.82 a |
| Cold-soaking | 118.3 ± 5.8 a | 7.5 ± 1.4 b | 22.6 ± 3.6 a | 302.3 ± 35.7 a | 174.82 ± 1.34 b | |
| Average 2017–2019 | Control | 106.8 ± 3.8 b | 12.7 ± 2.3 a | 18.0 ± 1.4 b | 238.2 ± 23.3 b | 177.99 ± 1.05 a |
| Cold-soaking | 115.6 ± 4.4 a | 10.1 ± 2.4 b | 22.2 ± 2.6 a | 291.0 ± 38.5 a | 174.65 ± 1.41 b |
| Preparation | Surface Tension (mN m−1) | Area (mm2) | Volume (µL) |
|---|---|---|---|
| CS | 50.82 ± 0.97b | 33.69 ± 1.31b | 20.15 ± 0.81b |
| W | 71.56 ± 0.71a | 43.54 ± 0.69a | 28.91 ± 0.54a |
| SE | 71.23 ± 0.74a | 43.97 ± 0.86a | 29.09 ± 0.66a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocira, S.; Szparaga, A.; Krawczuk, A.; Bartoš, P.; Zaguła, G.; Plawgo, M.; Černý, P. Plant Material as a Novel Tool in Designing and Formulating Modern Biostimulants—Analysis of Botanical Extract from Linum usitatissimum L. Materials 2021, 14, 6661. https://doi.org/10.3390/ma14216661
Kocira S, Szparaga A, Krawczuk A, Bartoš P, Zaguła G, Plawgo M, Černý P. Plant Material as a Novel Tool in Designing and Formulating Modern Biostimulants—Analysis of Botanical Extract from Linum usitatissimum L. Materials. 2021; 14(21):6661. https://doi.org/10.3390/ma14216661
Chicago/Turabian StyleKocira, Sławomir, Agnieszka Szparaga, Anna Krawczuk, Petr Bartoš, Grzegorz Zaguła, Michał Plawgo, and Pavel Černý. 2021. "Plant Material as a Novel Tool in Designing and Formulating Modern Biostimulants—Analysis of Botanical Extract from Linum usitatissimum L." Materials 14, no. 21: 6661. https://doi.org/10.3390/ma14216661
APA StyleKocira, S., Szparaga, A., Krawczuk, A., Bartoš, P., Zaguła, G., Plawgo, M., & Černý, P. (2021). Plant Material as a Novel Tool in Designing and Formulating Modern Biostimulants—Analysis of Botanical Extract from Linum usitatissimum L. Materials, 14(21), 6661. https://doi.org/10.3390/ma14216661











