The Role of the Activator Additives Introduction Method in the Cold Sintering Process of ZnO Ceramics: CSP/SPS Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composites Preparation
- injecting the activator solution using a syringe directly into the die with pre-pressed ZnO powder, similar to [14];
- preliminary application by impregnation method;
- TVT of ZnO powder previously impregnated by Zn(Ac)2.
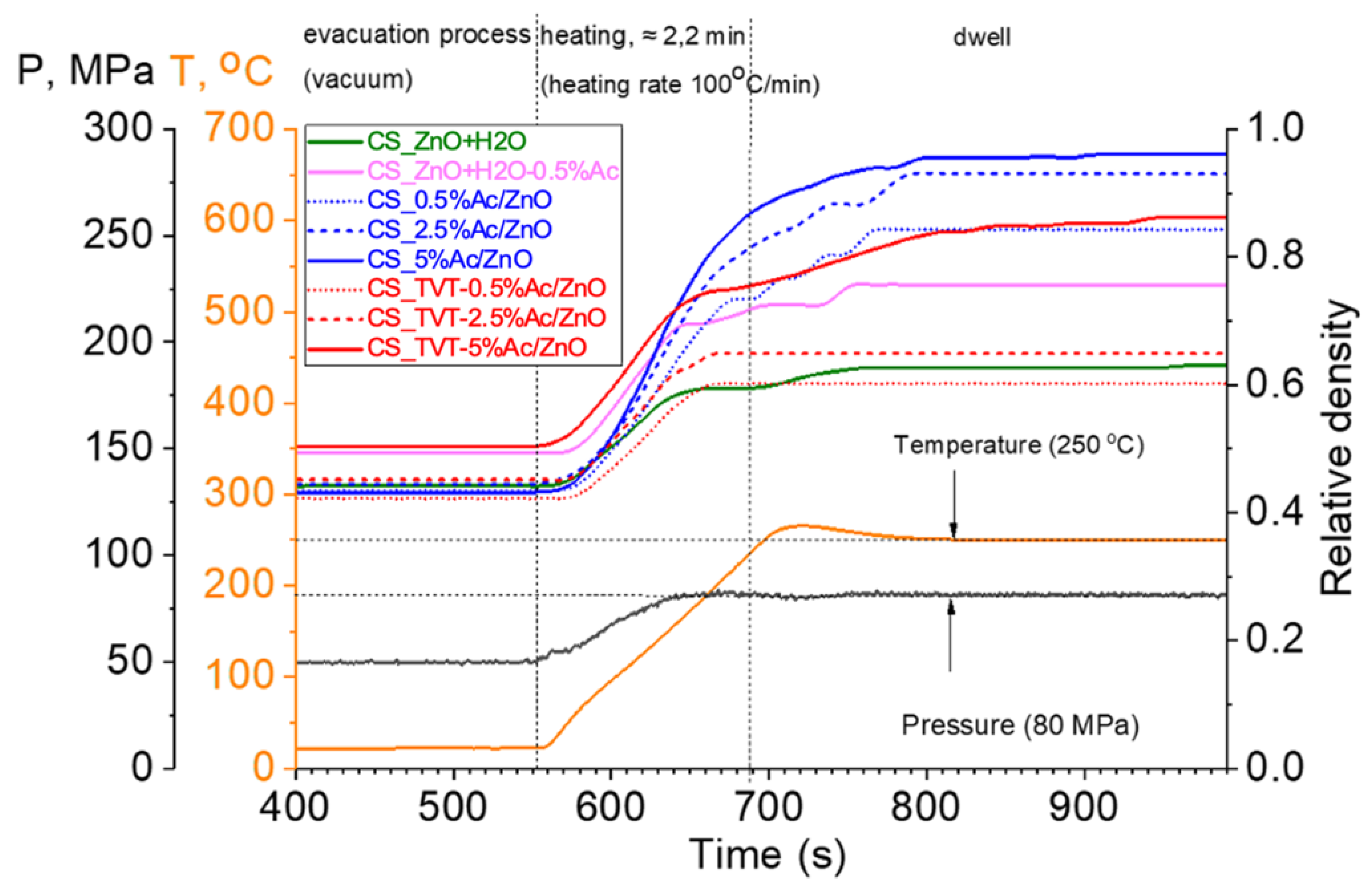
2.2. Cold Sintering Process
- Pressing up to 15 MPa.
- Evacuation process (0.23 mbar vacuum).
- Pressing up to 50 MPa.
- Heating rate 100 °C/min.
- Pressing up to 80 MPa.
- Dwell time for 5 min at 80 MPa and a temperature of 250 °C.
- Cooling at a pressure of 15 MPa.
- Venting, extracting the mold.
2.3. Density Measurements
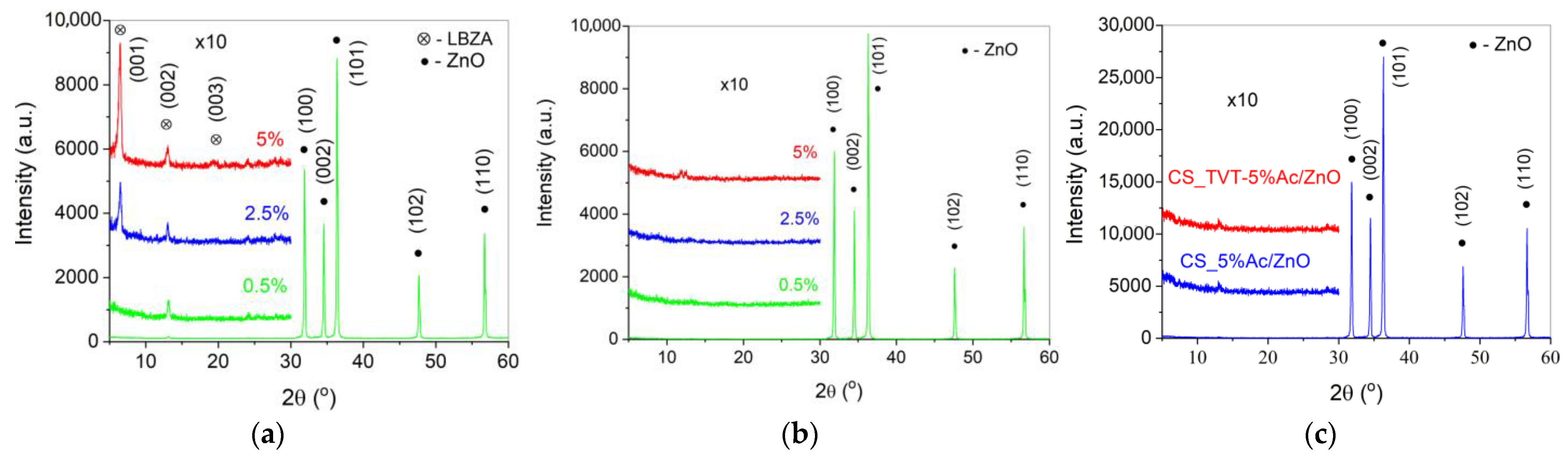
2.4. XRD Characterization
2.5. Microstructural Characterization
3. Results
3.1. The Effect of Activator Introduction Method on the Initial ZnO Powder Characteristics
3.2. The Effect of Activator Introduction Method on the ZnO CSP/SPS Process
4. Discussion
- The impregnation of ZnO powder by Zn(Ac)2 allows reducing the mechanical pressure from 150 MPa to at least 80 MPa while maintaining the same CSP/SPS process short time (about 7 min) and achieving the high relative density of 0.97 as in work [14], without using a special expensive high-strength mold. As a result, it becomes possible to effectively use standard SPS tools for CSP research under controlled conditions and with the possibility to vary the heating speed, holding temperature, and cooling conditions within a wide range, which is difficult or completely inaccessible when using specially assembled laboratory setups for CSP based on hand presses.
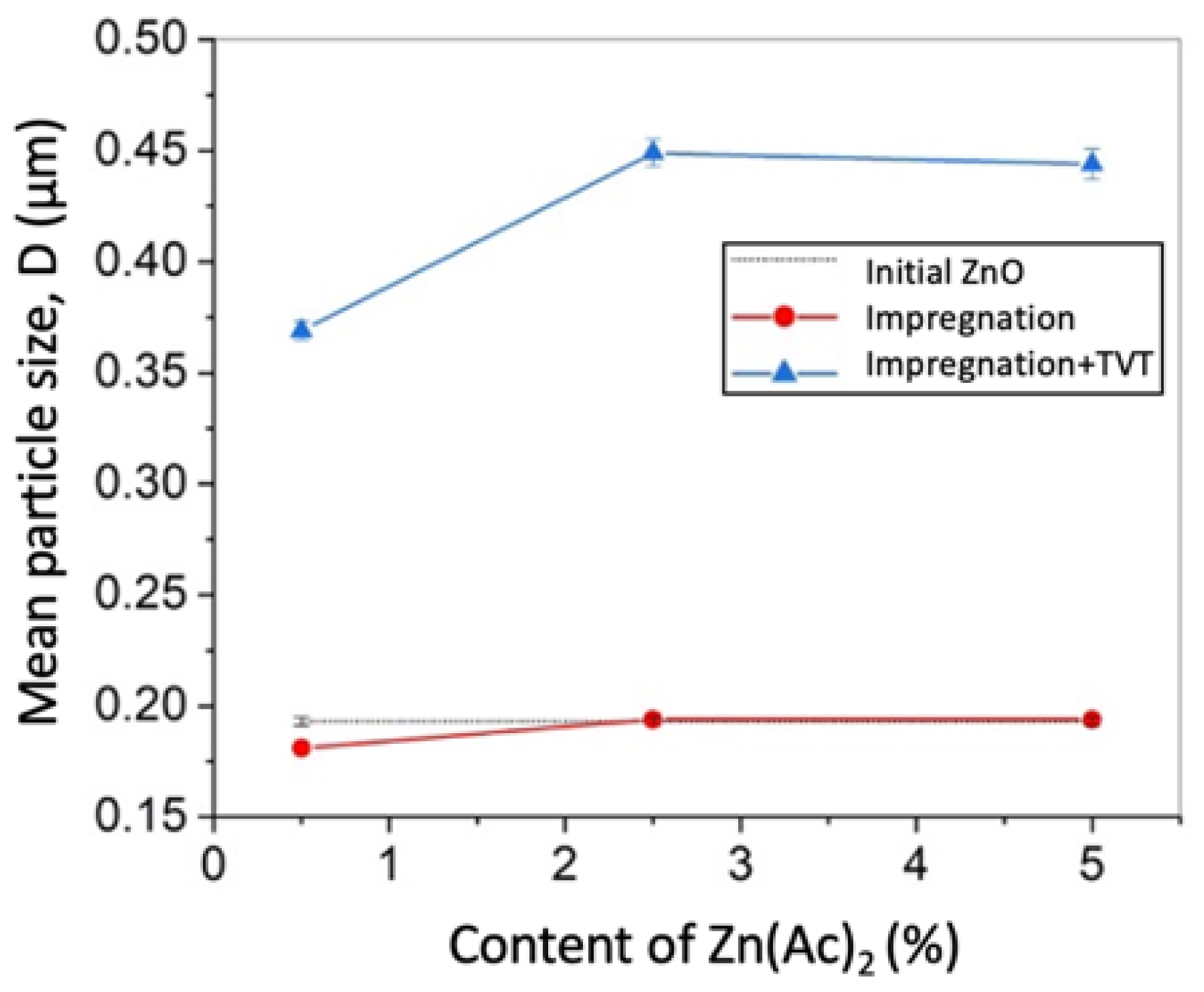
- An increase in the content of Zn(Ac)2 introduced by the impregnation method in the range of 0.5, 2.5, 5 wt.% leads to an increase in the relative density of CSP samples and a simultaneous decrease in grain growth (Table 2 and Table 3). This result shows the possibility to control the microstructure of ceramics due to the selection method of additive introduction even in a short time CSP/SPS approach.
- The experimentally established possibility to reduce the pressure and the CSP time while maintaining a high relative density of ceramics due to the selection of additives introduction method have a substantial practical impact for further applied research aimed at industrial applicability of CSP.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cologna, M.; Rashkova, B.; Raj, R. Flash sintering of nanograin zirconia in <5 s at 850 °C. J. Am. Ceram. Soc. 2010, 93, 3556–3559. [Google Scholar] [CrossRef]
- Grigoriev, S.; Peretyagin, P.; Smirnov, A.; Solís, W.; Díaz, L.A.; Fernandez, A.; Torrecillas, R. Effect of graphene addition on the mechanical and electrical properties of Al2O3-SiCw ceramics. J. Eur. Ceram. Soc. 2017, 37, 2473–2479. [Google Scholar] [CrossRef]
- Smirnov, A.; Peretyagin, P.; Bartolomé, J.F. Processing and mechanical properties of new hierarchical metal-graphene flakes reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 3491–3497. [Google Scholar] [CrossRef]
- Smirnov, A.; Seleznev, A.; Solís Pinargote, N.W.; Pristinskiy, Y.; Peretyagin, P.; Bartolomé, J.F. The Influence of Wire Electrical Discharge Machining Cutting Parameters on the Surface Roughness and Flexural Strength of ZrO2/TiN Ceramic Nanocomposites Obtained by Spark Plasma Sintering. Nanomaterials 2019, 9, 1391. [Google Scholar] [CrossRef] [Green Version]
- Muche, D.N.F.; Drazin, J.W.; Mardinly, J.; Dey, S.; Castro, R.H.R. Colossal grain boundary strengthening in ultrafine nanocrystalline oxides. Mater. Lett. 2017, 186, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Kupp, E.R.; Messing, G.L.; Randall, C.A. Cold Sintering: A Paradigm Shift for Processing and Integration of Ceramics. Angew. Chem. 2016, 55, 11457–11461. [Google Scholar] [CrossRef] [PubMed]
- European Comission. Ceramic Manufacturing Industry. Eur. Commun. 2007, 210–211. Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2019-11/cer_bref_0807.pdf (accessed on 2 November 2021).
- Ibn-Mohammed, T.; Randall, C.A.; Mustapha, K.B.; Guo, J.; Walker, J.; Berbano, S.; Reaney, I.M. Decarbonising ceramic manufacturing: A techno-economic analysis of energy efficient sintering technologies in the functional materials sector. J. Eur. Ceram. Soc. 2019, 39, 5213–5235. [Google Scholar] [CrossRef]
- Biesuz, M.; Grasso, S.; Sglavo, V.M. What’s new in ceramics sintering? A short report on the latest trends and future prospects. Curr. Opin. Solid State Mater. Sci. 2020, 24, 100868. [Google Scholar] [CrossRef]
- Sohrabi Baba Heidary, D.; Lanagan, M.; Randall, C.A. Contrasting energy efficiency in various ceramic sintering processes. J. Eur. Ceram. Soc. 2018, 38, 1018–1029. [Google Scholar] [CrossRef]
- Maria, J.P.; Kang, X.; Floyd, R.D.; Dickey, E.C.; Guo, H.; Guo, J.; Baker, A.; Funihashi, S.; Randall, C.A. Cold sintering: Current status and prospects. J. Mater. Res. 2017, 32, 3205–3218. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, N.; Yanagisawa, K.; Nishioka, M.; Kanahara, S. A hydrothermal hot-pressing method: Apparatus and application. J. Mater. Sci. Lett. 1986, 5, 355–356. [Google Scholar] [CrossRef]
- Guo, J.; Floyd, R.; Lowum, S.; Maria, J.-P.; Herisson de Beauvoir, T.; Seo, J.-H.; Randall, C.A. Cold Sintering: Progress, Challenges, and Future Opportunities. Annu. Rev. Mater. Res. 2019, 49, 275–295. [Google Scholar] [CrossRef]
- Gonzalez-Julian, J.; Neuhaus, K.; Bernemann, M.; da Silva, J.P.; Laptev, A.; Bram, M.; Guillon, O. Unveiling the mechanisms of cold sintering of ZnO at 250 °C by varying applied stress and characterizing grain boundaries by Kelvin Probe Force Microscopy. Acta Mater. 2018, 144, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.H.; Tsuji, K.; Ndayishimiye, A.; Dursun, S.; Seo, J.H.; Otieno, S.; Randall, C.A. Toward a size scale-up cold sintering process at reduced uniaxial pressure. J. Am. Ceram. Soc. 2020, 103, 2322–2327. [Google Scholar] [CrossRef]
- Ndayishimiye, A.; Sengul, M.Y.; Bang, S.H.; Tsuji, K.; Takashima, K.; de Beauvoir, T.H.; Denux, D.; Thibaud, J.-M.; van Duin, A.C.; Elissalde, C.; et al. Comparing hydrothermal sintering and cold sintering process: Mechanisms, microstructure, kinetics and chemistry. J. Eur. Ceram. Soc. 2020, 40, 1312–1324. [Google Scholar] [CrossRef]
- Serrano, A.; Caballero-Calero, O.; García, M.Á.; Lazić, S.; Carmona, N.; Castro, G.R.; MarisolMartín-González, M.; Fernández, J.F. Cold sintering process of ZnO ceramics: Effect of the nanoparticle/microparticle ratio. J. Eur. Ceram. Soc. 2020, 40, 5535–5542. [Google Scholar] [CrossRef]
- Nur, K.; Mishra, T.P.; da Silva, J.G.P.; Gonzalez-Julian, J.; Bram, M.; Guillon, O. Influence of powder characteristics on cold sintering of nano-sized ZnO with density above 99%. J. Eur. Ceram. Soc. 2021, 41, 2648–2662. [Google Scholar] [CrossRef]
- Kang, X.; Floyd, R.; Lowum, S.; Long, D.; Dickey, E.; Maria, J.P. Cold sintering with dimethyl sulfoxide solutions for metal oxides. J. Mater. Sci. 2019, 54, 7438–7446. [Google Scholar] [CrossRef]
- Ivakin, Y.D.; Smirnov, A.V.; Tarasovskii, V.P.; Rybal’Chenko, V.V.; Vasin, A.A.; Kholodkova, A.A.; Kormilitsin, M.N. Cold sintering of ZnO ceramic in water medium: Test demonstration. Glass Ceram. 2019, 76, 210–215. [Google Scholar] [CrossRef]
- Ivakin, Y.; Smirnov, A.; Kholodkova, A.; Vasin, A.; Kormilicin, M.; Kornyushin, M.; Stolyarov, V. Comparative Study of Cold Sintering Process and Autoclave Thermo-Vapor Treatment on a ZnO Sample. Crystals 2021, 11, 71. [Google Scholar] [CrossRef]
- Funahashi, S.; Guo, J.; Guo, H.; Wang, K.; Baker, A.L.; Shiratsuyu, K.; Randall, C.A. Demonstration of the cold sintering process study for the densification and grain growth of ZnO ceramics. J. Am. Ceram. Soc. 2017, 100, 546–553. [Google Scholar] [CrossRef]
- Ivakin, Y.; Smirnov, A.; Kormilitsin, M.; Kholodkova, A.; Vasin, A.; Kornyushin, M.; Tarasovskii, V.; Rybalchenko, V. Effect of mechanical pressure on zinc oxide recrystallization in aqueous medium during cold sintering. Russ. J. Phys. Chem. B 2022, in press. [Google Scholar]
- Kuzin, V.V.; Grigoriev, S.N.; Volosova, M.A. The role of the thermal factor in the wear mechanism of ceramic tools: Part 1. Macrolevel. J. Frict. Wear 2014, 35, 505–510. [Google Scholar] [CrossRef]
- Ndayishimiye, A.; Fan, Z.; Funahashi, S.; Randall, C.A. Assessment of the Role of Speciation during Cold Sintering of ZnO Using Chelates. Inorg. Chem. 2021, 60, 13453–13460. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.; Biesuz, M.; Zoli, L.; Taveri, G.; Duff, A.I.; Ke, D.; Jiang, A.; Reece, M.J. A review of cold sintering processes. Adv. Appl. Ceram. 2020, 119, 115–143. [Google Scholar] [CrossRef] [Green Version]
- Floyd, R.D.; Lowum, S.; Maria, J.P. Cold sintering zinc oxide with a crystalline zinc acetate dihydrate mass transport phase. J. Mater. Sci. 2020, 55, 15117–15129. [Google Scholar] [CrossRef]
- Dargatz, B.; Gonzalez-Julian, J.; Bram, M.; Jakes, P.; Besmehn, A.; Schade, L.; Röder, R.; Ronning, C.; Guillon, O. FAST/SPS sintering of nanocrystalline zinc oxide-Part I: Enhanced densification and formation of hydrogen-related defects in presence of adsorbed water. J. Eur. Ceram. Soc. 2016, 36, 1207–1220. [Google Scholar] [CrossRef]
- Dargatz, B.; Gonzalez-Julian, J.; Bram, M.; Shinoda, Y.; Wakai, F.; Guillon, O. FAST/SPS sintering of nanocrystalline zinc oxide-Part II: Abnormal grain growth, texture and grain anisotropy. J. Eur. Ceram. Soc. 2016, 36, 1221–1232. [Google Scholar] [CrossRef]
- Sengul, M.Y.; Guo, J.; Randall, C.A.; van Duin, A.C. Water-Mediated Surface Diffusion Mechanism Enables the Cold Sintering Process: A Combined Computational and Experimental Study. Angew. Chem. 2019, 58, 12420–12424. [Google Scholar] [CrossRef]
- Tarat, A.; Nettle, C.J.; Bryant, D.T.J.; Jones, D.R.; Penny, M.W.; Brown, R.A.; Majitha, R.; Meissner, K.E.; Maffeis, T.G.G. Microwave-assisted synthesis of layered basic zinc acetate nanosheets and their thermal decomposition into nanocrystalline ZnO. Nanoscale Res. Lett. 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivakin, Y.D.; Danchevskaya, M.N. Analysis of Recrystallization of Fine-Crystalline Corundum in a Supercritical Water Medium Using the Lognormal Particle Size Distribution Function. Russ. J. Phys. Chem. B. 2018, 12, 1205–1211. [Google Scholar] [CrossRef]
- German, R.M. Prediction of sintered density for bimodal powder mixtures. Metall. Trans. A 1992, 23, 1455–1465. Available online: https://link.springer.com/content/pdf/10.1007/BF02647329.pdf (accessed on 2 November 2021). [CrossRef]
- Shi, Y.; Zhang, Y. Simulation of random packing of spherical particles with different size distributions. Appl. Phys. A 2008, 92, 621–626. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, F.; Zhang, J.; Xia, L. Converting layered zinc acetate nanobelts to one-dimensional structured ZnO nanoparticle aggregates and their photocatalytic activity. Nanoscale Res. Lett. 2008, 3, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.C. The Carbonyl Group, Part V: Carboxylates—Coming Clean. Spectroscopy 2018, 33, 20–23. Available online: https://www.spectroscopyonline.com/view/carbonyl-group-part-v-carboxylates-coming-clean (accessed on 2 November 2021).
- Ivakin, Y.D.; Danchevskaya, M.N.; Muravieva, G.P. Recrystallization of Zinc Oxide in a Sub-and Supercritical Water Medium. Russ. J Phys. Chem. B 2019, 13, 1189–1200. [Google Scholar] [CrossRef]
- Danchevskaya, M.N.; Torbin, S.N.; Muravieva, G.P.; Ovchinnikova, O.G.; Ivakin, Y.D. Synthesis and investigation of crystalline modifications of silicon dioxide. React. Solids 1988, 5, 293–303. [Google Scholar] [CrossRef]
- Danchevskaya, M.N.; Ivakin, Y.D.; Muravieva, G.P.; Luchkov, I.V. Synthesis and doping of fine-crystalline corundum in sub- and supercritical conditions. J. Phys. Conf. Ser. 2008, 121, 082001. [Google Scholar] [CrossRef]
- Maryashkin, A.V.; Ivakin, Y.u.D.; Danchevskaya, M.N.; Murav’eva, G.P.; Kirikova, M.N. Synthesis of Corundum Doped with Cerium in Supercritical Water Fluid. Mosc. Univ. Chem. Bull. 2011, 66, 290–298. [Google Scholar] [CrossRef]
- Danchevskaya, M.N.; Ivakin, Y.D.; Torbin, S.N.; Panasyuk, G.P.; Belan, V.N.; Voroshilov, I.L. Scientific basis of technology of finecrystalline quartz and corundum. High Press. Res. Int. J. 2001, 20, 229–239. [Google Scholar] [CrossRef]
- Ivakin, Y.D.; Danchevskaya, M.N.; Muravieva, G.P. Kinetic model and mechanism of Y3Al5O12 Formation in hydrothermal and thermovaporous synthesis. High Press. Res. 2001, 20, 87–98. [Google Scholar] [CrossRef]
- Danchevskaya, M.N.; Ivakin, Y.D.; Torbin, S.N.; Muravieva, G.P. The role of water fluid in the formation of fine-crystalline oxide structure. J. Supercrit. Fluids 2007, 42, 419–424. [Google Scholar] [CrossRef]

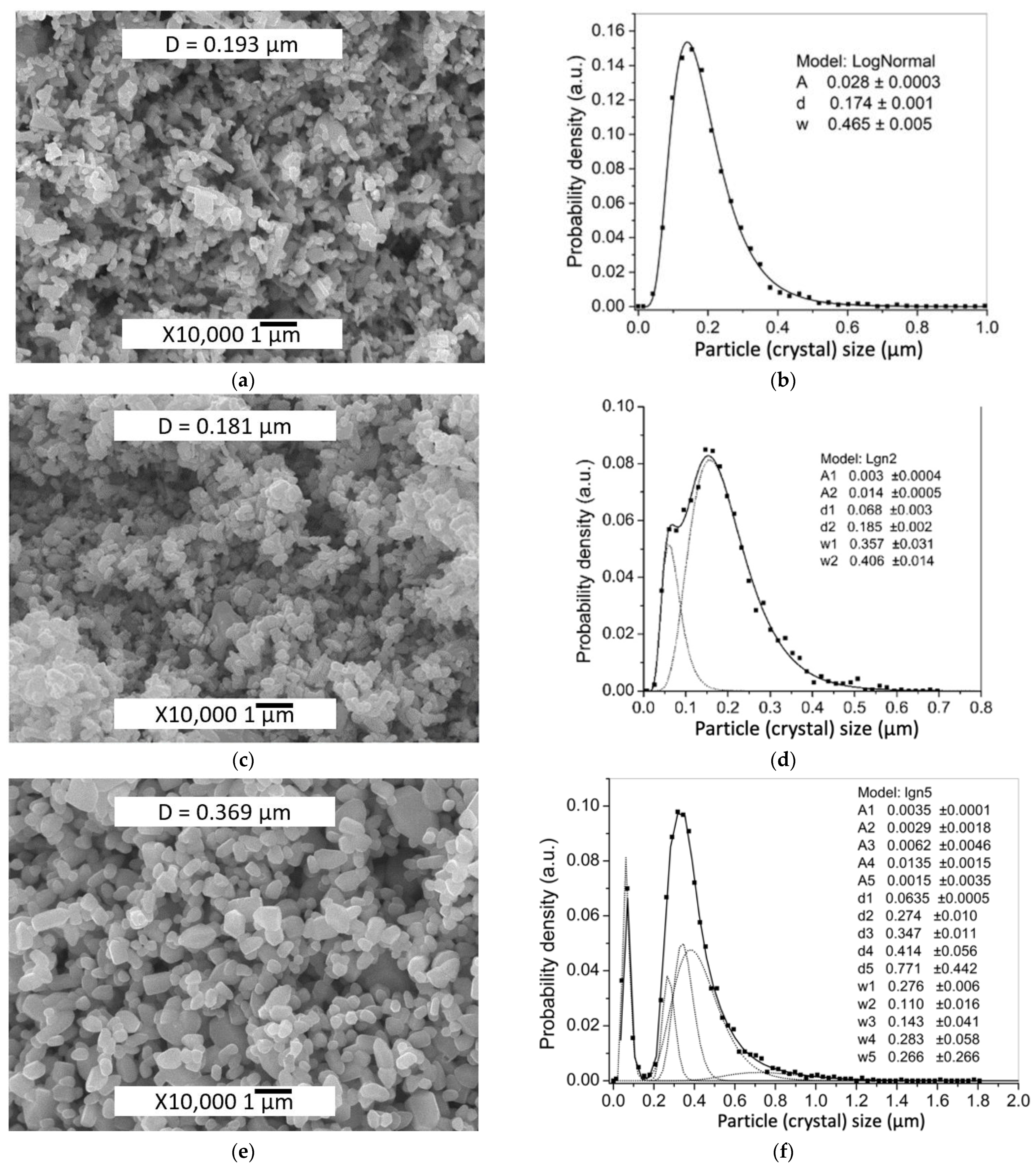
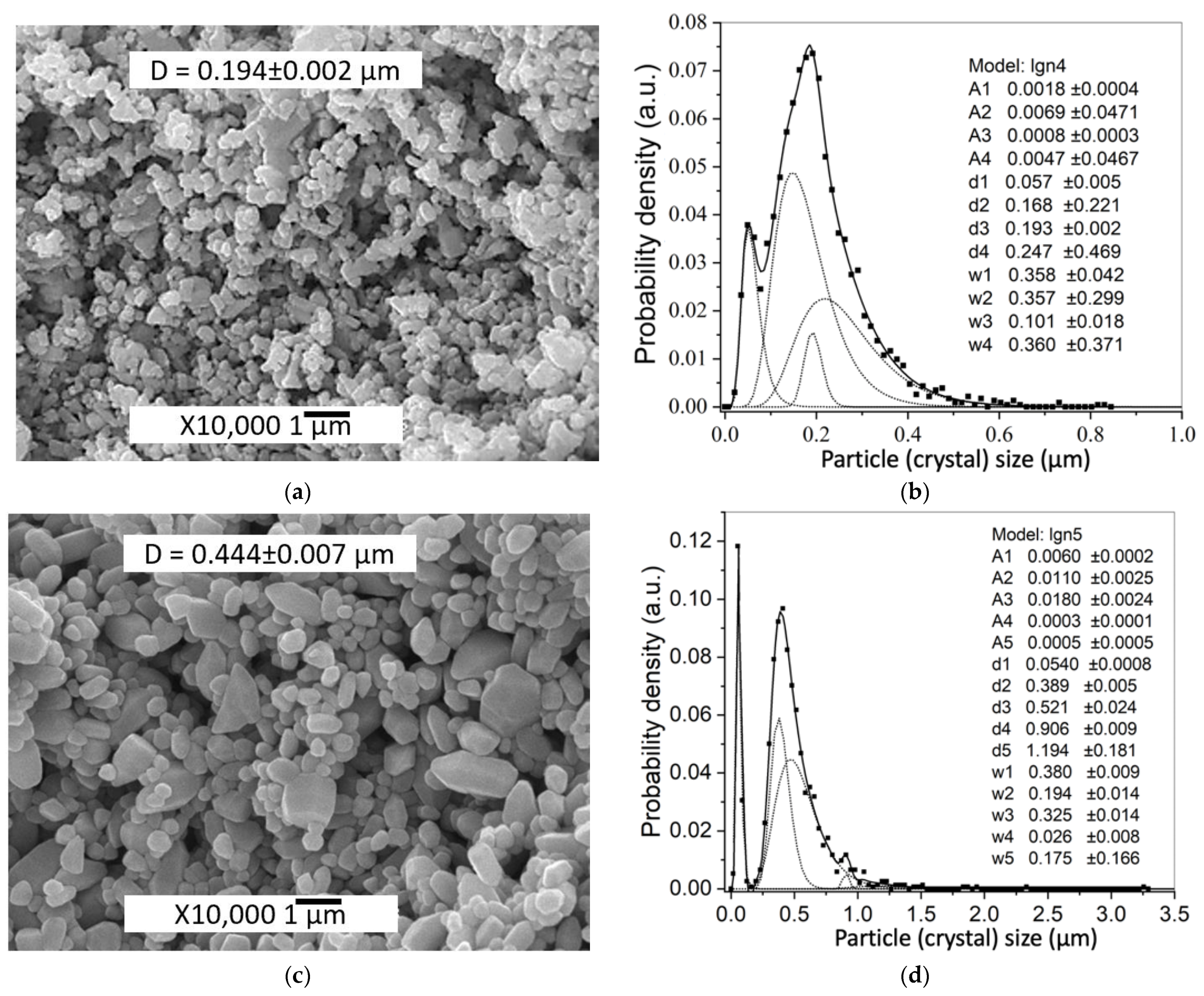

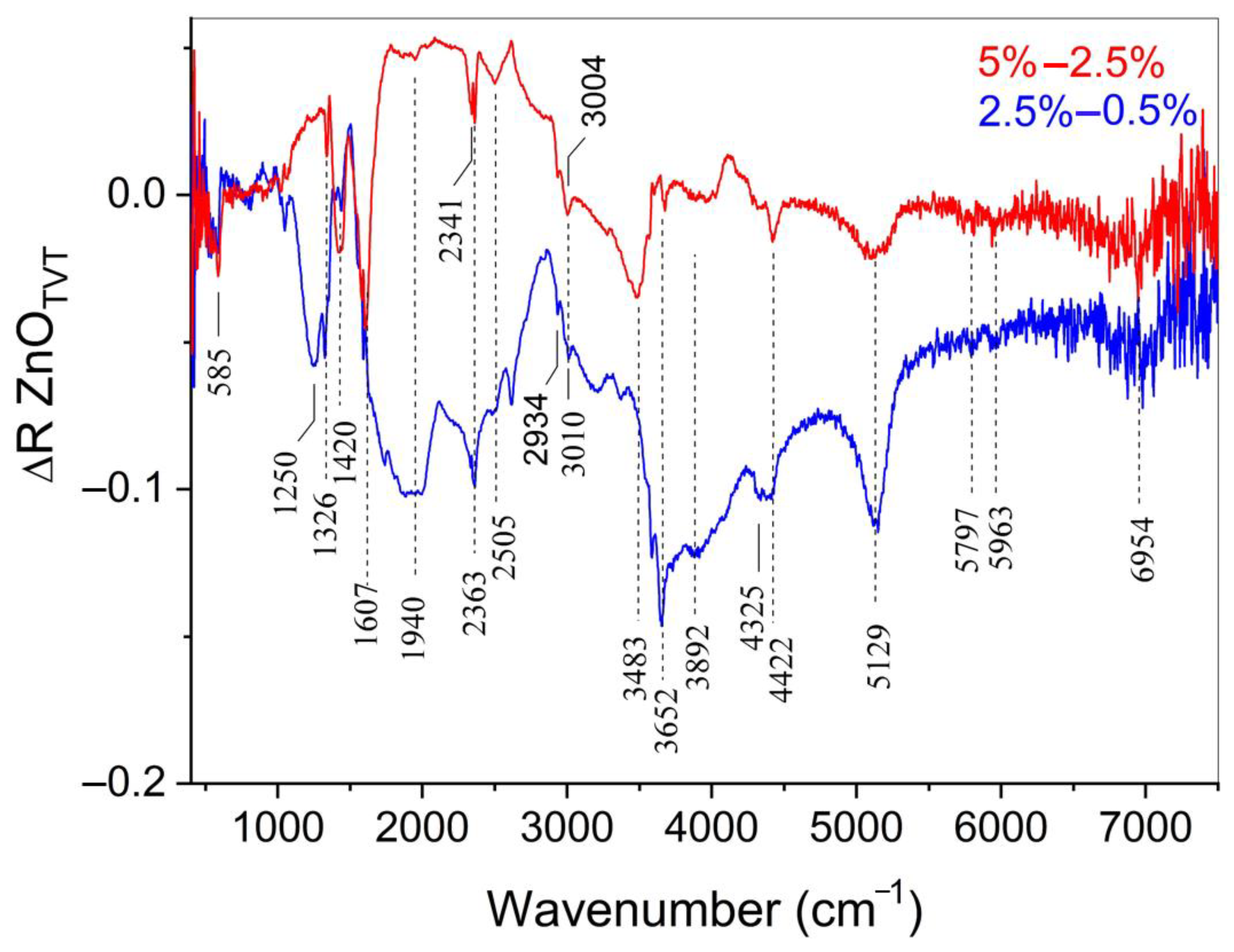
| Sample | Zn(Ac)2 Content, wt% | Zn(Ac)2 Introduction Method | Mean Particle Size, μm | |
|---|---|---|---|---|
| DLS, d50 | SEM Image Analysis, D | |||
| ZnO initial | 0 | - | 0.210 ± 0.004 | 0.193 ± 0.002 |
| 0.5%Ac/ZnO | 0.5 | Impregnation | 0.228 ± 0.004 | 0.181 ± 0.002 |
| 2.5%Ac/ZnO | 2.5 | 0.234 ± 0.005 | 0.194 ± 0.003 | |
| 5%Ac/ZnO | 5 | 0.245 ± 0.005 | 0.194 ± 0.002 | |
| TVT_0.5%Ac/ZnO | 0.5 | Impregnation + TVT | 0.268 ± 0.005 | 0.369 ± 0.004 |
| TVT_2.5%Ac/ZnO | 2.5 | 0.291 ± 0.006 | 0.449 ± 0.006 | |
| TVT_5%Ac/ZnO | 5 | 0.310 ± 0.006 | 0.449 ± 0.006 | |
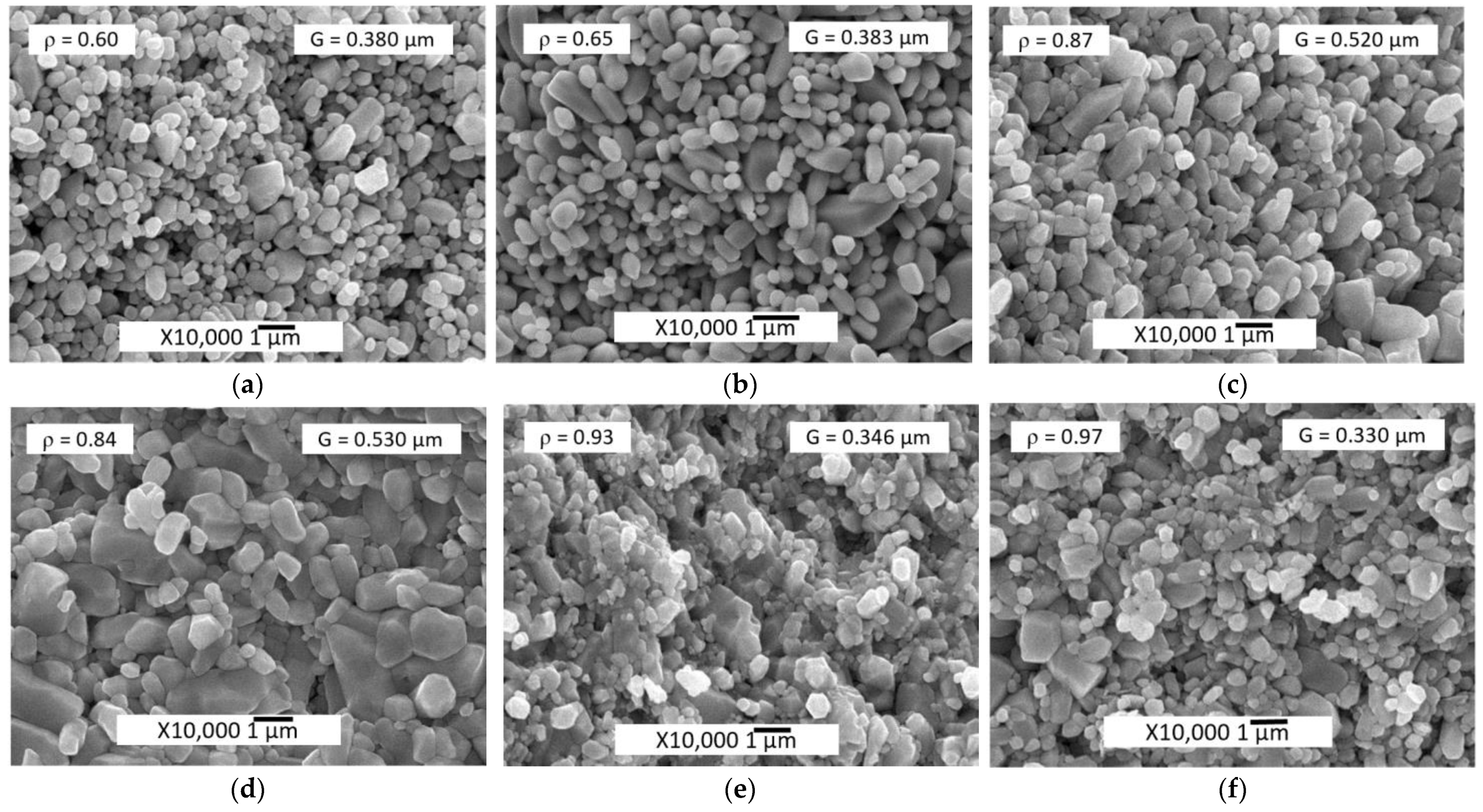
| Sample | Zn(Ac)2 Introduction Method | Mean Powder Particle Size D, μm | Mean Grain Size G, μm | Relative Density ρ |
|---|---|---|---|---|
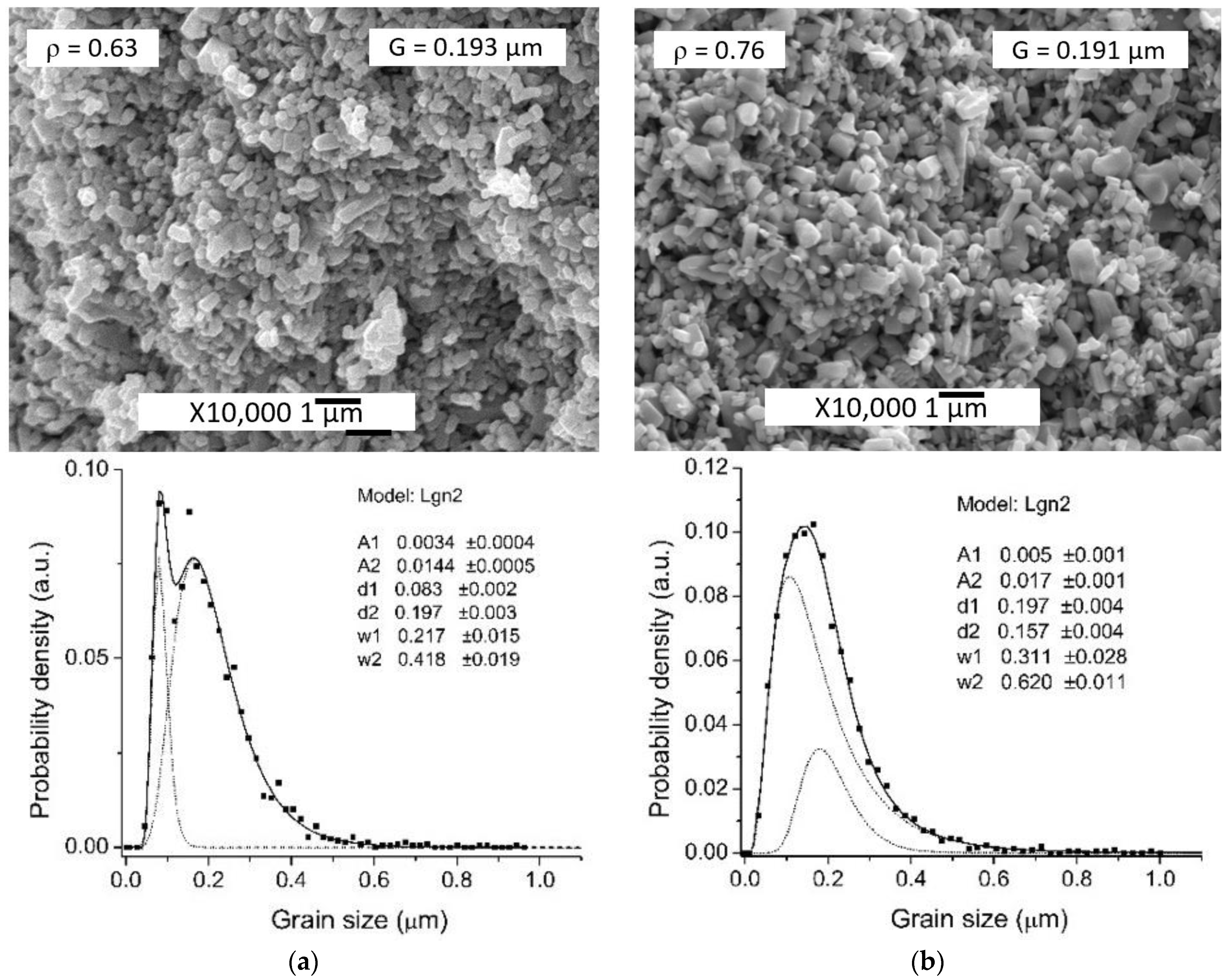
| CS_ZnO + H2O | injecting the DI water | 0.193 ± 0.002 | 0.193 ± 0.002 | 0.63 |
| CS_0.5%Ac/ZnO | impregnation | 0.181 ± 0.002 | 0.530 ± 0.014 | 0.84 |
| CS_2.5%Ac/ZnO | 0.194 ± 0.003 | 0.346 ± 0.004 | 0.93 | |
| CS_5%Ac/ZnO | 0.194 ± 0.002 | 0.330 ± 0.004 | 0.97 | |
| CS_TVT-0.5%Ac/ZnO | impregnation + TVT | 0.369 ± 0.004 | 0.380 ± 0.004 | 0.60 |
| CS_TVT-2.5%Ac/ZnO | 0.449 ± 0.006 | 0.383 ± 0.01 | 0.65 | |
| CS_TVT-5%Ac/ZnO | 0.444 ± 0.007 | 0.520 ± 0.007 | 0.87 | |
| CS_ZnO + H2O-0.5%Ac | injecting the solution | 0.193 ± 0.002 | 0.191 ± 0.002 | 0.76 |
| Heating Rate, °C/min | Dwell Time, min | Pressure, MPa | Powder Particle Size | Zn(Ac)2 Content, wt.% | DI Water Content, wt.% | Zn(Ac)2 Introduction Method | Mean Grain Size, μm | Relative Density | References |
|---|---|---|---|---|---|---|---|---|---|
| 100 | 5 | 80 | 0.212 μm | 0.5 | 1.6 | impregnation (CS_0.5%Ac/ZnO) | 0.530 | 0.84 | This work |
| 100 | 5 | 80 | 0.243 μm | 5 | 1.6 | impregnation (CS_5%Ac/ZnO) | 0.330 | 0.97 | This work |
| 100 | 5 | 80 | 0.193 μm | 0.5 | 1.6 | injecting the solution (CS_ZnO + H2O-0.5%Ac) | 0.191 | 0.76 | This work |
| 100 | 5 | 150 | 20–50 nm | 0.5 | 1.6 | injecting the solution | >0.100 | 0.97 | [14] |
| 20 | 60 | 300 | 40–100 nm | 0 | 3.2 | - | 0.138 | 0.99 | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivakin, Y.D.; Smirnov, A.V.; Kurmysheva, A.Y.; Kharlanov, A.N.; Solís Pinargote, N.W.; Smirnov, A.; Grigoriev, S.N. The Role of the Activator Additives Introduction Method in the Cold Sintering Process of ZnO Ceramics: CSP/SPS Approach. Materials 2021, 14, 6680. https://doi.org/10.3390/ma14216680
Ivakin YD, Smirnov AV, Kurmysheva AY, Kharlanov AN, Solís Pinargote NW, Smirnov A, Grigoriev SN. The Role of the Activator Additives Introduction Method in the Cold Sintering Process of ZnO Ceramics: CSP/SPS Approach. Materials. 2021; 14(21):6680. https://doi.org/10.3390/ma14216680
Chicago/Turabian StyleIvakin, Yurii D., Andrey V. Smirnov, Alexandra Yu. Kurmysheva, Andrey N. Kharlanov, Nestor Washington Solís Pinargote, Anton Smirnov, and Sergey N. Grigoriev. 2021. "The Role of the Activator Additives Introduction Method in the Cold Sintering Process of ZnO Ceramics: CSP/SPS Approach" Materials 14, no. 21: 6680. https://doi.org/10.3390/ma14216680







