Fabrication of a Selective Sensor Amplification Probe Modified with Multi-Component Zn2SnO4/SnO2 Heterostructured Microparticles as a Robust Electrocatalyst for Electrochemical Detection of Antibacterial Drug Secnidazole
Abstract
1. Introduction
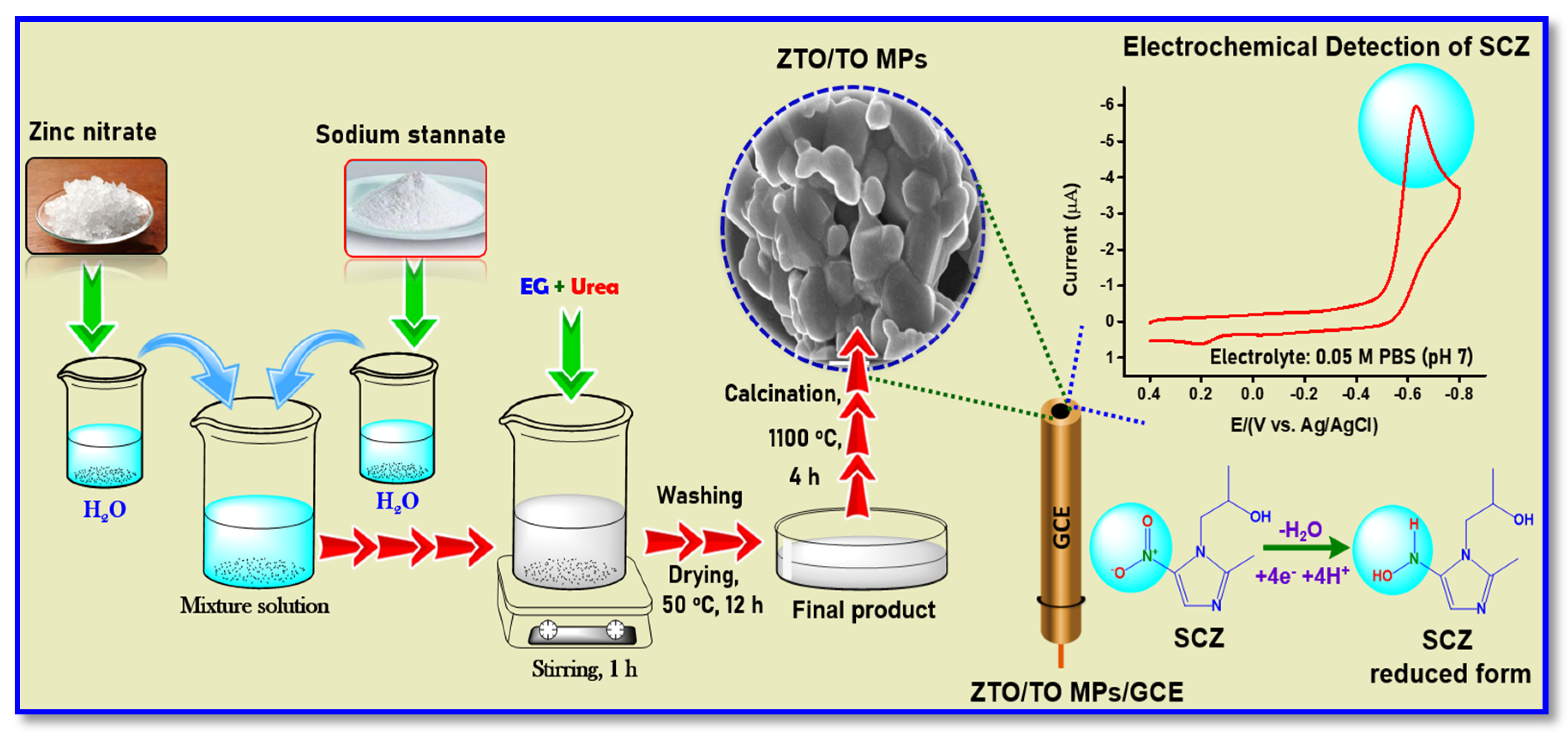
2. Materials and Methods
3. Result and Discussion
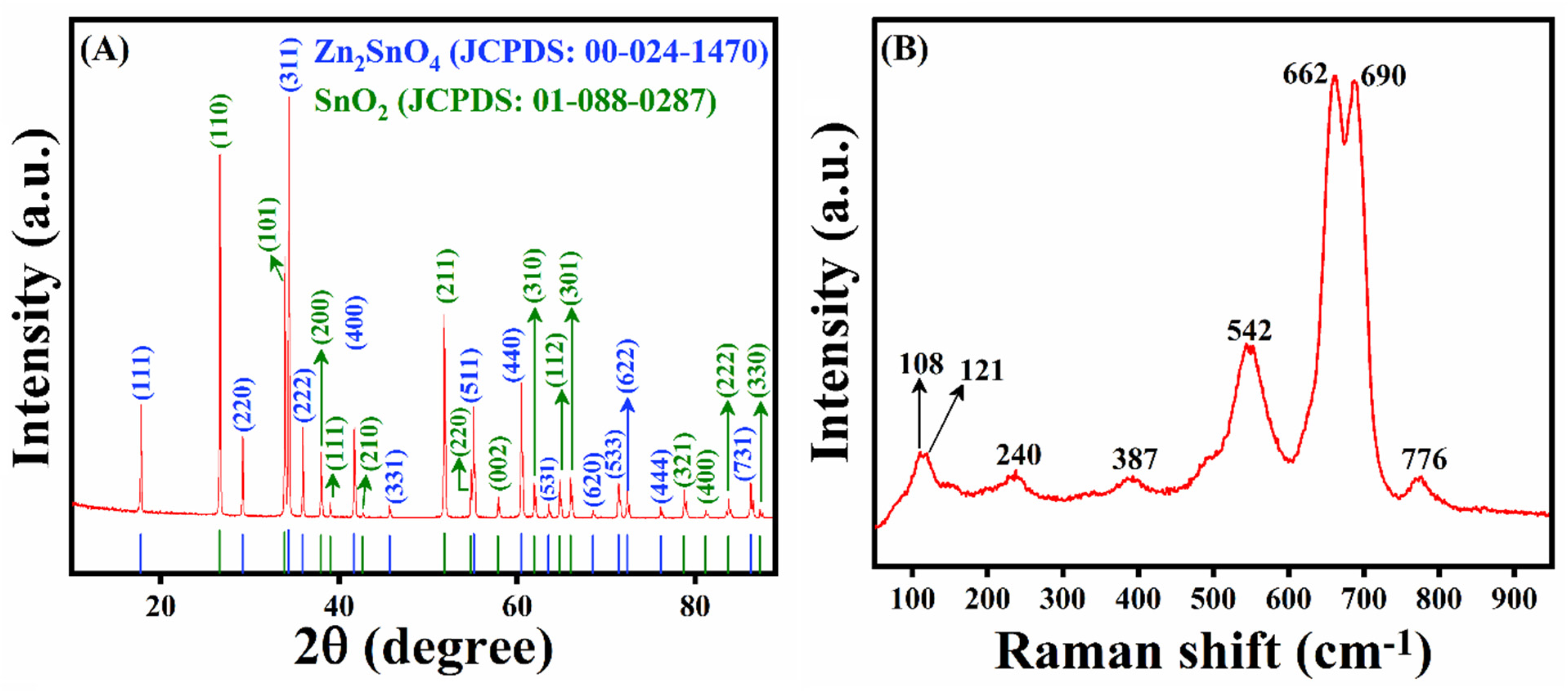
3.1. X-ray Diffraction and Raman Studies
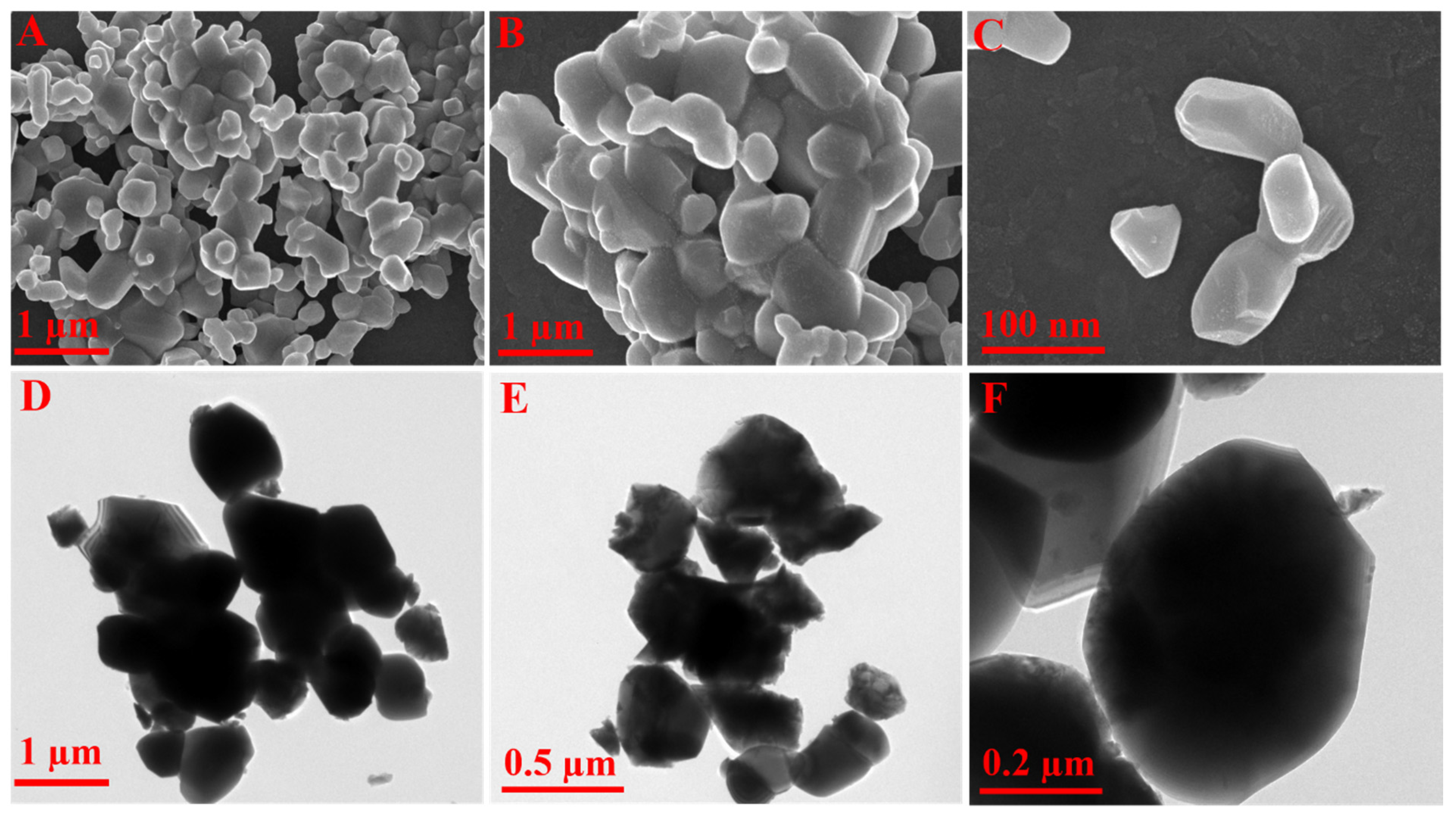
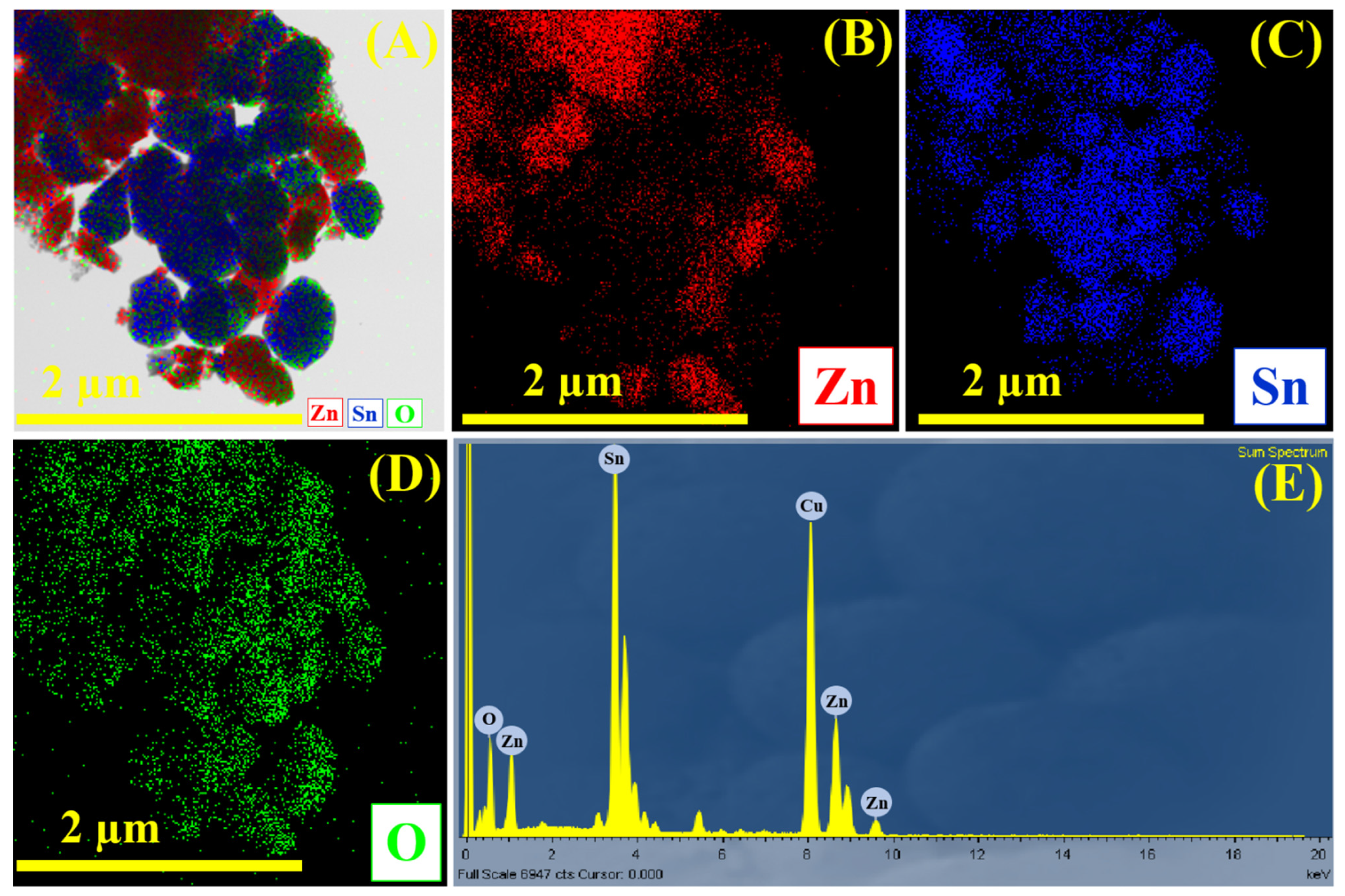
3.2. Morphological Investigation Using FE-SEM and HR-TEM Analysis
3.3. X-ray Photoelectron Spectroscopy Analysis
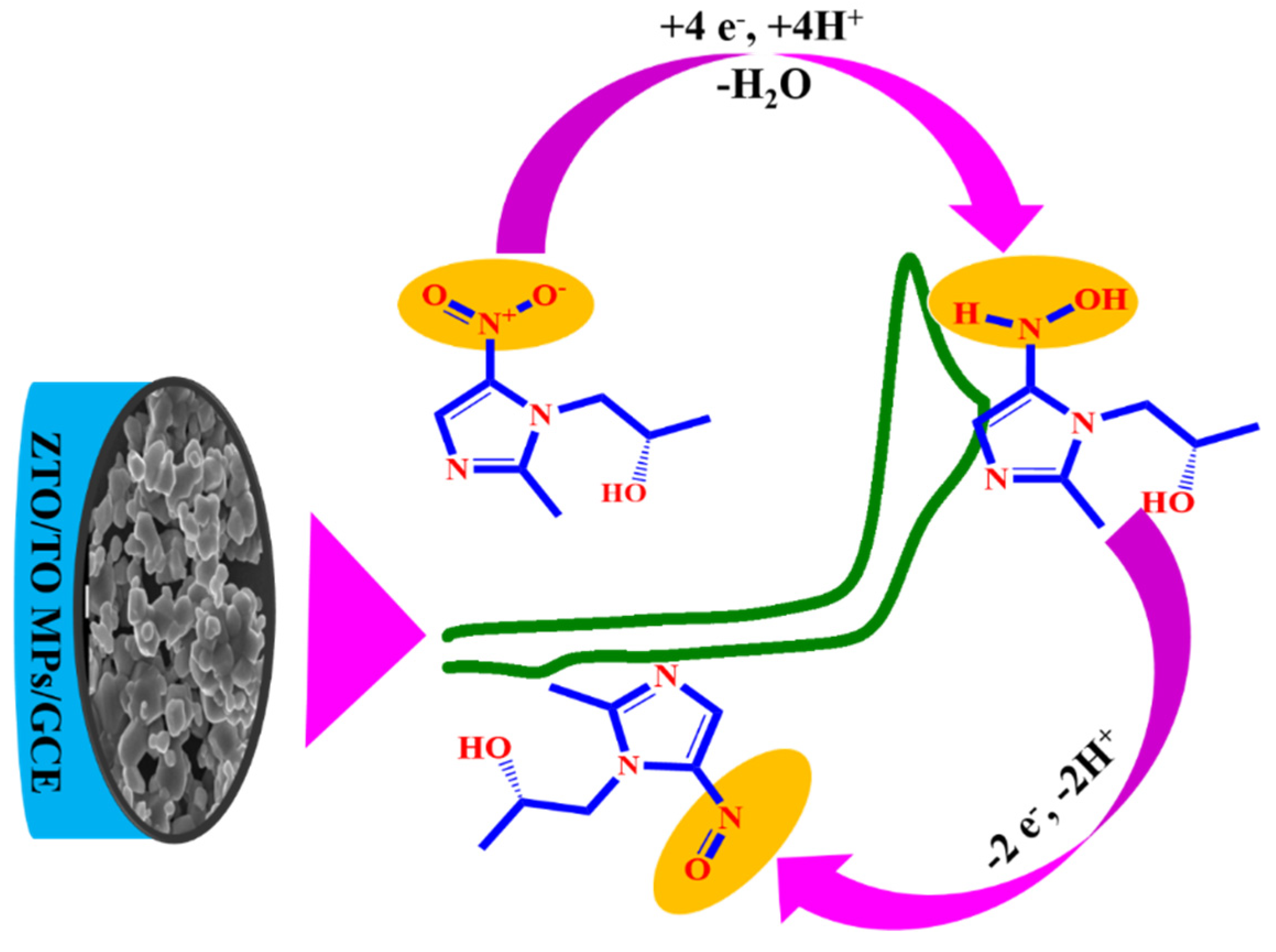
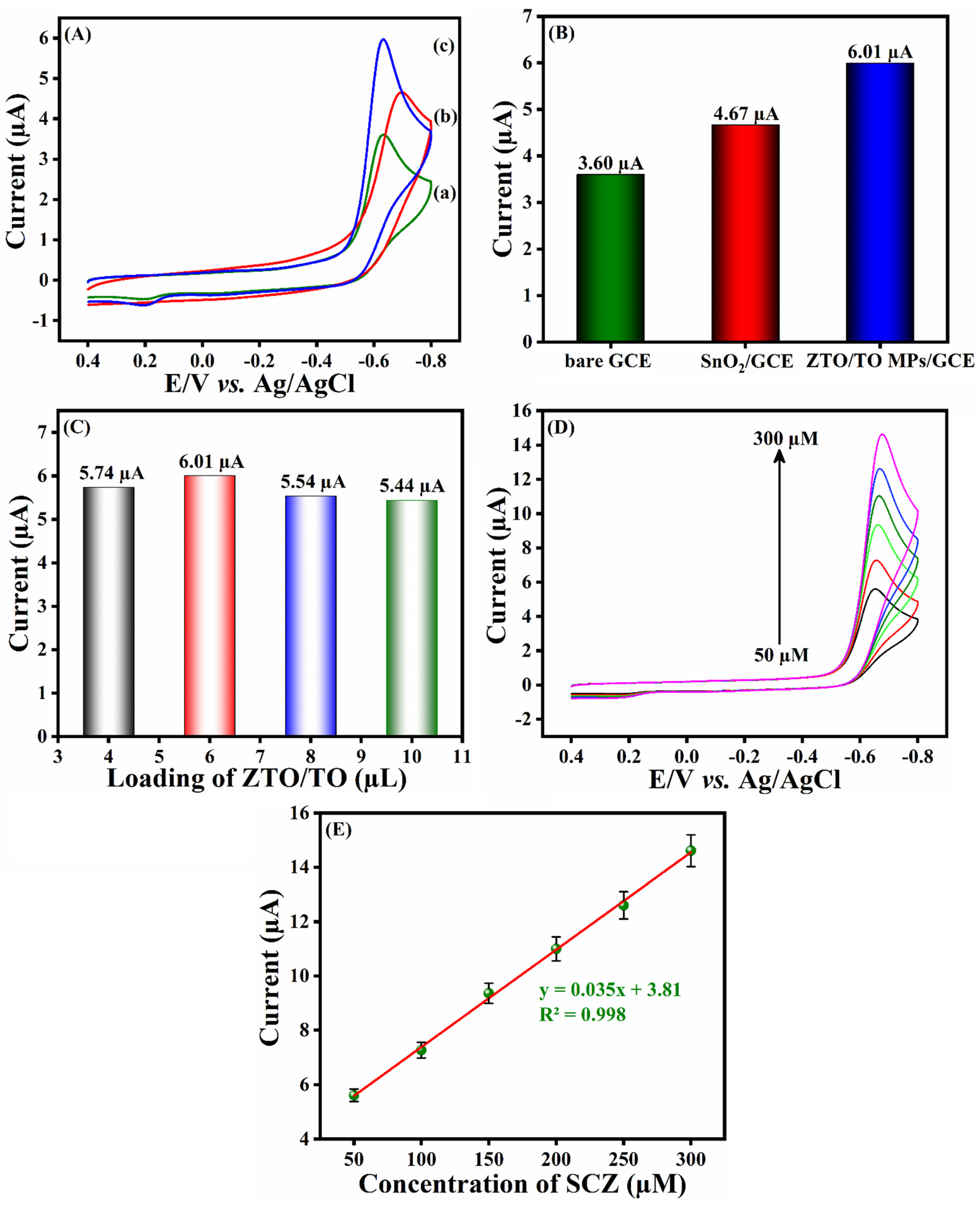
3.4. Electrochemical Reduction of SCZ
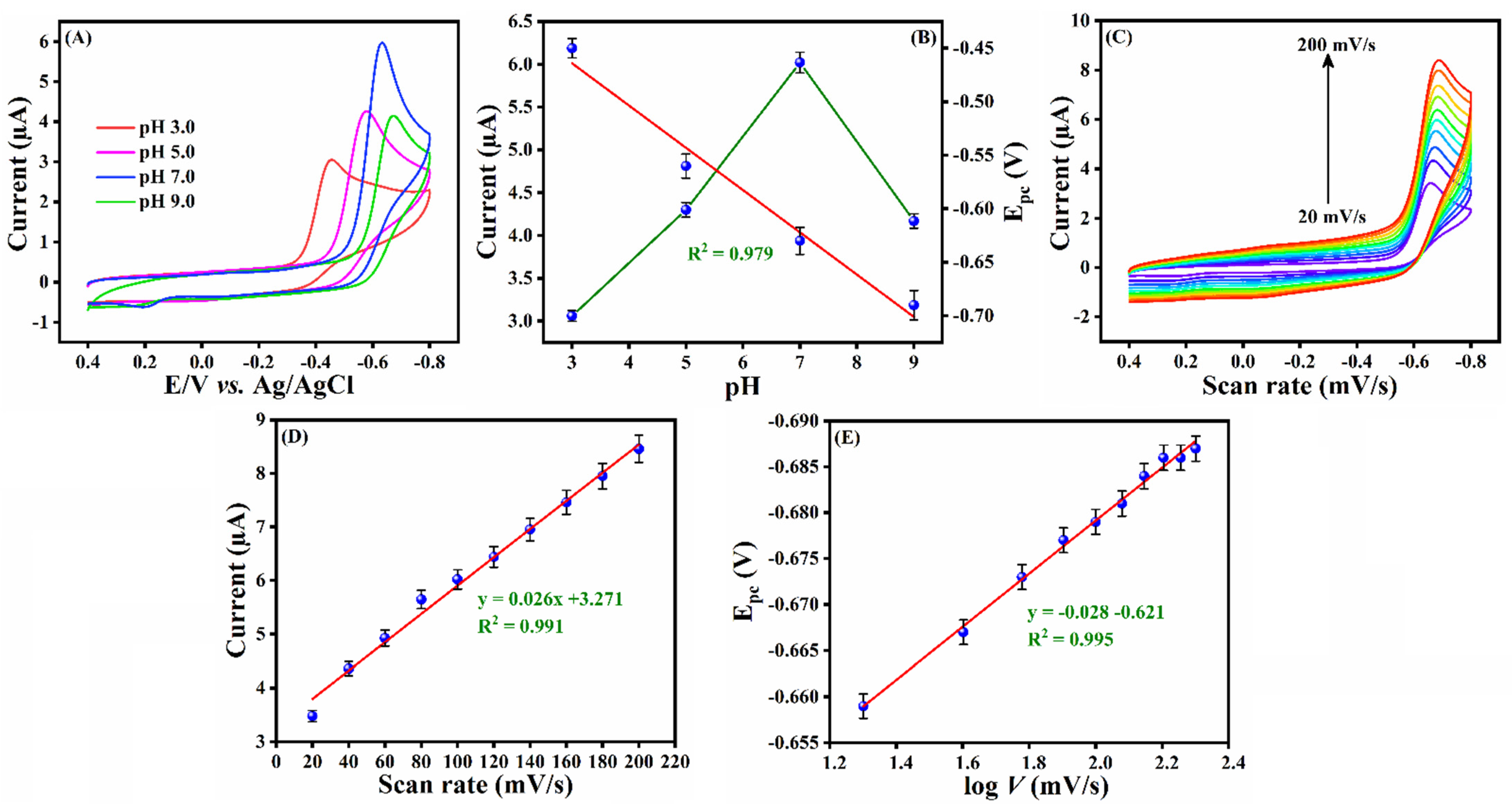
3.5. Optimization of the pH and Scan Rate Studies
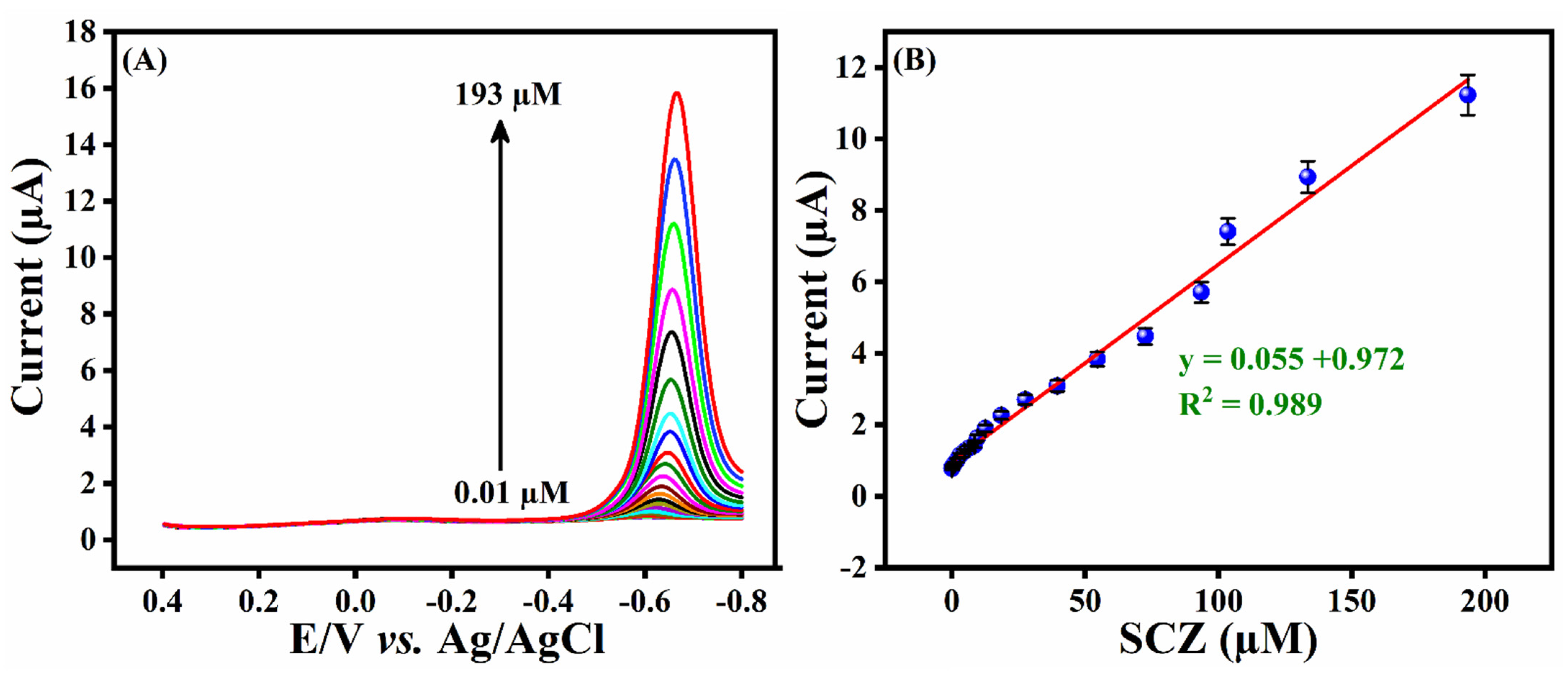
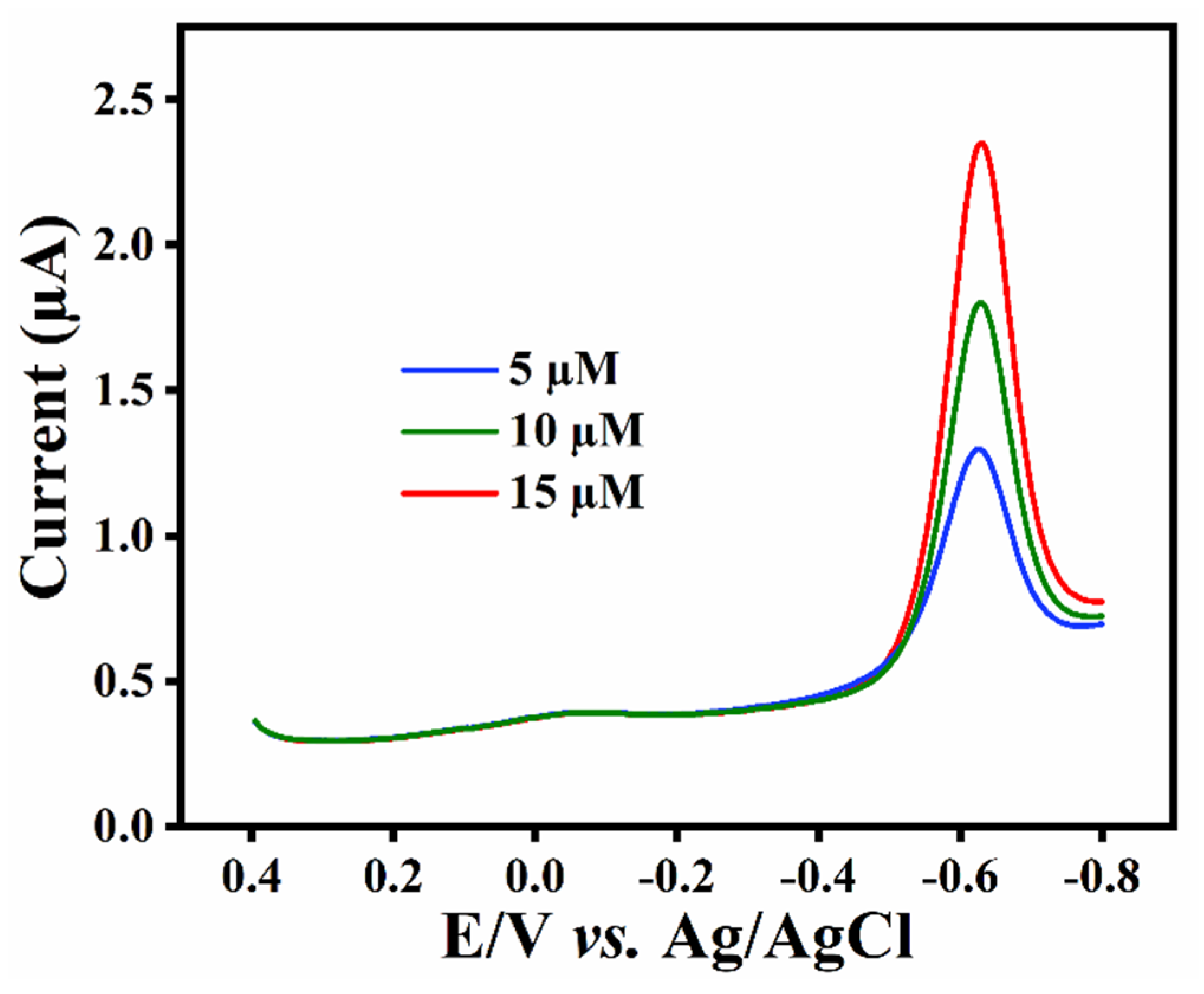
3.6. Analytical Performance of ZTO/TO MPs/GCE towards SCZ
3.7. Practical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habibi, M.H.; Mardani, M. Synthesis and characterization of bi-component ZnSnO3/Zn2SnO4 (perovskite/spinel) nano-composites for photocatalytic degradation of Intracron Blue: Structural, opto-electronic and morphology study. J. Mol. Liq. 2017, 238, 397–401. [Google Scholar] [CrossRef]
- Jain, S.; Shah, A.P.; Shimpi, N.G. An efficient photocatalytic degradation of organic dyes under visible light using zinc stannate (Zn2SnO4) nanorods prepared by microwave irradiation. Nano Struct. Nano Objects 2020, 21, 100410. [Google Scholar] [CrossRef]
- Li, Z.; Bi, D.; Zhao, Y.; Liu, R.; Ye, J.; Zhou, Y. In situ growth of zinc oxide nanoribbons within the interstices of a zinc stannate nanoplates network on compacted woven metal wires and their enhanced solar energy application. Electrochim. Acta 2018, 262, 124–134. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Z.; Mentbayeva, A.; Yuan, G.; Wang, B.; Wang, H.; Wang, G. Enhanced electrochemical performance of hollow heterostructured carbon encapsulated znic metastanate microcube composite for lithium-ion and sodium-ion batteries. Electrochim. Acta 2019, 312, 31–44. [Google Scholar] [CrossRef]
- Santhoshkumar, P.; Prasanna, K.; Jo, Y.N.; Kang, S.H.; Joe, Y.C.; Lee, C.W. Synthesis of highly crystalline octahedron 3D-Zn2SnO4 as an advanced high-performance anode material for lithium ion batteries. Appl. Surf. Sci. 2018, 449, 514–520. [Google Scholar] [CrossRef]
- Masjedi-Arani, M.; Salavati-Niasari, M. Metal (Mn, Co, Ni and Cu) doped ZnO-Zn2SnO4-SnO2 nanocomposites: Green sol-gel synthesis, characterization and photocatalytic activity. J. Mol. Liq. 2017, 248, 197–204. [Google Scholar] [CrossRef]
- Sumithra, S.; Victor Jaya, N. Band gap tuning and room temperature ferromagnetism in Co doped Zinc stannate nanostructures. Phys. B Condens. Matter 2016, 493, 35–42. [Google Scholar] [CrossRef]
- Hanh, N.H.; Van Duy, L.; Hung, C.M.; Van Duy, N.; Heo, Y.W.; Van Hieu, N.; Hoa, N.D. VOC gas sensor based on hollow cubic assembled nanocrystal Zn2SnO4 for breath analysis. Sens. Actuators A Phys. 2020, 302, 111834. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Q.; Li, Y.; Liu, R.; Yang, H. Flexible woven metal wires supported nanosheets and nanoparticles double-layered nitrogen-doped zinc stannate toward enhanced solar energy utilization. Ceram. Int. 2018, 44, 905–914. [Google Scholar] [CrossRef]
- Yang, X.; Gao, H.; Zhao, L.; Wang, T.; Sun, P.; Liu, F.; Lu, G. Enhanced gas sensing properties of monodisperse Zn2SnO4 octahedron functionalized by PdO nanoparticals. Sens. Actuators B Chem. 2018, 266, 302–310. [Google Scholar] [CrossRef]
- Keles, E.; Yildirim, M.; Öztürk, T.; Yildirim, O.A. Hydrothermally synthesized UV light active zinc stannate:tin oxide (ZTO:SnO2) nanocomposite photocatalysts for photocatalytic applications. Mater. Sci. Semicond. Process. 2020, 110. [Google Scholar] [CrossRef]
- Mrabet, C.; Dridi, R.; Mahdhi, N.; Amlouk, M. Mechanism of wettability conversion on sprayed Zn2SnO4 thin films surfaces modified by thermal annealing in air. J. Alloys Compd. 2017, 725, 765–772. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Zhang, L.; Liu, R.; Zhou, Y. Stainless steel mesh-supported three-dimensional hierarchical SnO2/Zn2SnO4 composite for the applications in solar cell, gas sensor, and photocatalysis. Appl. Surf. Sci. 2020, 502, 144113. [Google Scholar] [CrossRef]
- Wang, K.; Liu, D.; Deng, P.; Liu, L.; Lu, S.; Sun, Z.; Ma, Y.; Wang, Y.; Li, M.; Xia, B.Y.; et al. Band alignment in Zn2SnO4/SnO2 heterostructure enabling efficient CO2 electrochemical reduction. Nano Energy 2019, 64, 103954. [Google Scholar] [CrossRef]
- Kim, T.G.; Samuel, E.; Park, C.W.; Joshi, B.; Kim, M.W.; Swihart, M.T.; Yoon, S.S. Supersonically sprayed Zn2SnO4/SnO2/carbon nanotube films for high-efficiency water splitting photoanodes. J. Alloys Compd. 2020, 828, 154374. [Google Scholar] [CrossRef]
- Khalile, S.M.; EIQudaby, H.M.; Ali, F.A.; Eid, S.M. Spectrophotometric determination of ornidazole, secnidazole and tinidazole in pharmaceutical preparations based on formation of dyes. Egypt. J. Chem. 2010, 53, 233–241. [Google Scholar] [CrossRef][Green Version]
- Radi, A.; El-Laban, S.; El-Kourashy, A. Electrochemical reduction of secnidazole and its determination in tablets. Electroanalysis 1997, 9, 625–628. [Google Scholar] [CrossRef]
- Peng, P.; Liao, L.; Yu, Z.; Jiang, M.; Deng, J.; Xiao, X. A novel sensor based on multi-walled carbon nanotubes and boron-doped double-layer molecularly imprinted membrane for the analysis of SCZ in pharmaceutical and biological samples. Int. J. Environ. Anal. Chem. 2019, 99, 1495–1514. [Google Scholar] [CrossRef]
- Saffaj, T.; Charrouf, M.; Abourriche, A.; Abboud, Y.; Bennamara, A.; Berrada, M. Spectrophotometric determination of metronidazole and secnidazole in pharmaceutical preparations. Farmaco 2004, 59, 843–846. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Wang, G.; Zheng, Y.; Yan, B.; Xie, H.; Gu, Y.; Ren, H. Determination of secnidazole in human plasma by high-performance liquid chromatography with UV detection and its application to the bioequivalence studies. Biomed. Chromatogr. 2007, 21, 304–309. [Google Scholar] [CrossRef]
- Bhatia, S.C.; Shanbhag, V.D. Electron-capture gas chromatographic assays of 5-nitroimidazole class of antimicrobials in blood. J. Chromatogr. B Biomed. Sci. Appl. 1984, 305, 325–334. [Google Scholar] [CrossRef]
- Mitrowska, K.; Posyniak, A.; Zmudzki, J. Selective determination of fourteen nitroimidazoles in honey by high-performance liquid chromatography-tandem mass spectrometry. Anal. Lett. 2014, 47, 1634–1649. [Google Scholar] [CrossRef]
- El-Sayed, G.O.; Yasin, S.A.; El Badawy, A.A. Determination of secnidazole in tablets and human serum by cathodic adsorptive stripping voltammetry. Arab. J. Chem. 2010, 3, 167–172. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.Y.; Zhang, Q.X.; Zhu, K.M.; Tie, Y.; Pei, S.T.; Wang, B.J.; Zhang, J.L. Highly sensitive formaldehyde gas sensors based on Ag doped Zn2SnO4/SnO2 hollow nanospheres. Mater. Lett. 2019, 254, 178–181. [Google Scholar] [CrossRef]
- Saafi, I.; Dridi, R.; Mimouni, R.; Amlouk, A.; Yumak, A.; Boubaker, K.; Petkova, P.; Amlouk, M. Microstructural and optical properties of SnO2-ZnSnO3 ceramics. Ceram. Int. 2016, 42, 6273–6281. [Google Scholar] [CrossRef]
- Montenegro, J.E.; Ochoa-Muñoz, Y.; Rodríguez-Páez, J.E. Nanoparticles of zinc stannates (ZTO): Synthesis, characterization and electrical behavior in oxygen and acetone vapors. Ceram. Int. 2020, 46, 2016–2032. [Google Scholar] [CrossRef]
- Mehraj, S.; Ansari, M.S. Alimuddin Annealed SnO2 thin films: Structural, electrical and their magnetic properties. Thin Solid Films 2015, 589, 57–65. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Lee, W.J.; Lee, C.W.; Selvan, R.K. In situ and ex situ carbon coated Zn2SnO4 nanoparticles as promising negative electrodes for Li-ion batteries. RSC Adv. 2015, 5, 67210–67219. [Google Scholar] [CrossRef]
- Mali, S.S.; Shim, C.S.; Kim, H.; Hong, C.K. Reduced graphene oxide (rGO) grafted zinc stannate (Zn2SnO4) nanofiber scaffolds for highly efficient mixed-halide perovskite solar cells. J. Mater. Chem. A 2016, 4, 12158–12169. [Google Scholar] [CrossRef]
- Durai, L.; Badhulika, S. One pot hydrothermal synthesis of large area nano cube like ZnSnO3 perovskite for simultaneous sensing of uric acid and dopamine using differential pulse voltammetry. IEEE Sens. J. 2020, 20, 13212–13219. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L. Rational design and fabrication of multifunctional catalyzer Co2SnO4-SnO2/GC for catalysis applications: Photocatalytic degradation/catalytic reduction of organic pollutants. Appl. Catal. B Environ. 2018, 231, 34–42. [Google Scholar] [CrossRef]
- Vinoth Kumar, J.; Karthik, R.; Chen, S.M.; Chen, K.H.; Sakthinathan, S.; Muthuraj, V.; Chiu, T.W. Design of novel 3D flower-like neodymium molybdate: An efficient and challenging catalyst for sensing and destroying pulmonary toxicity antibiotic drug nitrofurantoin. Chem. Eng. J. 2018, 346, 11–23. [Google Scholar] [CrossRef]
- Venkatesh, K.; Rajakumaran, R.; Chen, S.M.; Karuppiah, C.; Yang, C.C.; Ramaraj, S.K.; Ali, M.A.; Al-Hemaid, F.M.A.; El-Shikh, M.S.; Almunqedhi, B.M.A. A Novel hybrid construction of MnMoO4 nanorods anchored graphene nanosheets; an efficient electrocatalyst for the picomolar detection of ecological pollutant ornidazole in water and urine samples. Chemosphere 2021, 273, 129665. [Google Scholar] [CrossRef]
- Rivera, A.B.; Hernández, R.G.; De Armas, H.N.; Cuéllar Elizástegi, D.M.; Losada, M.V. Physico-chemical and solid-state characterization of secnidazole. Farmaco 2000, 55, 700–707. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Montovani, P.A.B.; Pinto, A.M.P.; Dos Santos, M.B.; Vieira, D.L.; Do Prado, A.W.; Manfio, J.L. Bioavailability of two oral formulas of secnidazole in healthy volunteers. Braz. J. Pharm. Sci. 2009, 45, 687–692. [Google Scholar] [CrossRef]
- Bibi, S.; Nawaz, R.; Shahid, M.; Kiani, S.Q. Analysis of blood for secnidazole in female volunteers by HPLC method. J. Biol. Sci. 2002, 2, 769–770. [Google Scholar] [CrossRef][Green Version]


| Material/Technique | Linear Range/µM | LOD/nM | Ref. |
|---|---|---|---|
| GCE/stripping voltammetry | 4.0–120 | 1200 | [23] |
| Hg/Polarography/CV | 10–400 | 1000 | [17] |
| ZTO/TO MPs/GCE/DPV | 0.01–193 | 5.4 | This work |
| Added (µM) | Found (µM) | Recoveries (%) |
|---|---|---|
| 5.0 | 5.1 | 103 |
| 10.0 15.0 | 9.7 14.6 | 97 97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukanya, R.; Balamurugan, K.; Chen, S.-M.; Rajakumaran, R.; Muthupandi, K.; Shim, J.-J.; Breslin, C.B. Fabrication of a Selective Sensor Amplification Probe Modified with Multi-Component Zn2SnO4/SnO2 Heterostructured Microparticles as a Robust Electrocatalyst for Electrochemical Detection of Antibacterial Drug Secnidazole. Materials 2021, 14, 6700. https://doi.org/10.3390/ma14216700
Sukanya R, Balamurugan K, Chen S-M, Rajakumaran R, Muthupandi K, Shim J-J, Breslin CB. Fabrication of a Selective Sensor Amplification Probe Modified with Multi-Component Zn2SnO4/SnO2 Heterostructured Microparticles as a Robust Electrocatalyst for Electrochemical Detection of Antibacterial Drug Secnidazole. Materials. 2021; 14(21):6700. https://doi.org/10.3390/ma14216700
Chicago/Turabian StyleSukanya, Ramaraj, Karuppaiah Balamurugan, Shen-Ming Chen, Ramachandran Rajakumaran, K. Muthupandi, Jae-Jin Shim, and Carmel B. Breslin. 2021. "Fabrication of a Selective Sensor Amplification Probe Modified with Multi-Component Zn2SnO4/SnO2 Heterostructured Microparticles as a Robust Electrocatalyst for Electrochemical Detection of Antibacterial Drug Secnidazole" Materials 14, no. 21: 6700. https://doi.org/10.3390/ma14216700
APA StyleSukanya, R., Balamurugan, K., Chen, S.-M., Rajakumaran, R., Muthupandi, K., Shim, J.-J., & Breslin, C. B. (2021). Fabrication of a Selective Sensor Amplification Probe Modified with Multi-Component Zn2SnO4/SnO2 Heterostructured Microparticles as a Robust Electrocatalyst for Electrochemical Detection of Antibacterial Drug Secnidazole. Materials, 14(21), 6700. https://doi.org/10.3390/ma14216700










