Influence of Alloy Atoms on Substitution Properties of Hydrogen by Helium in ZrCoH3
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Basic Lattice Parameters and Vacancy Formation Energies
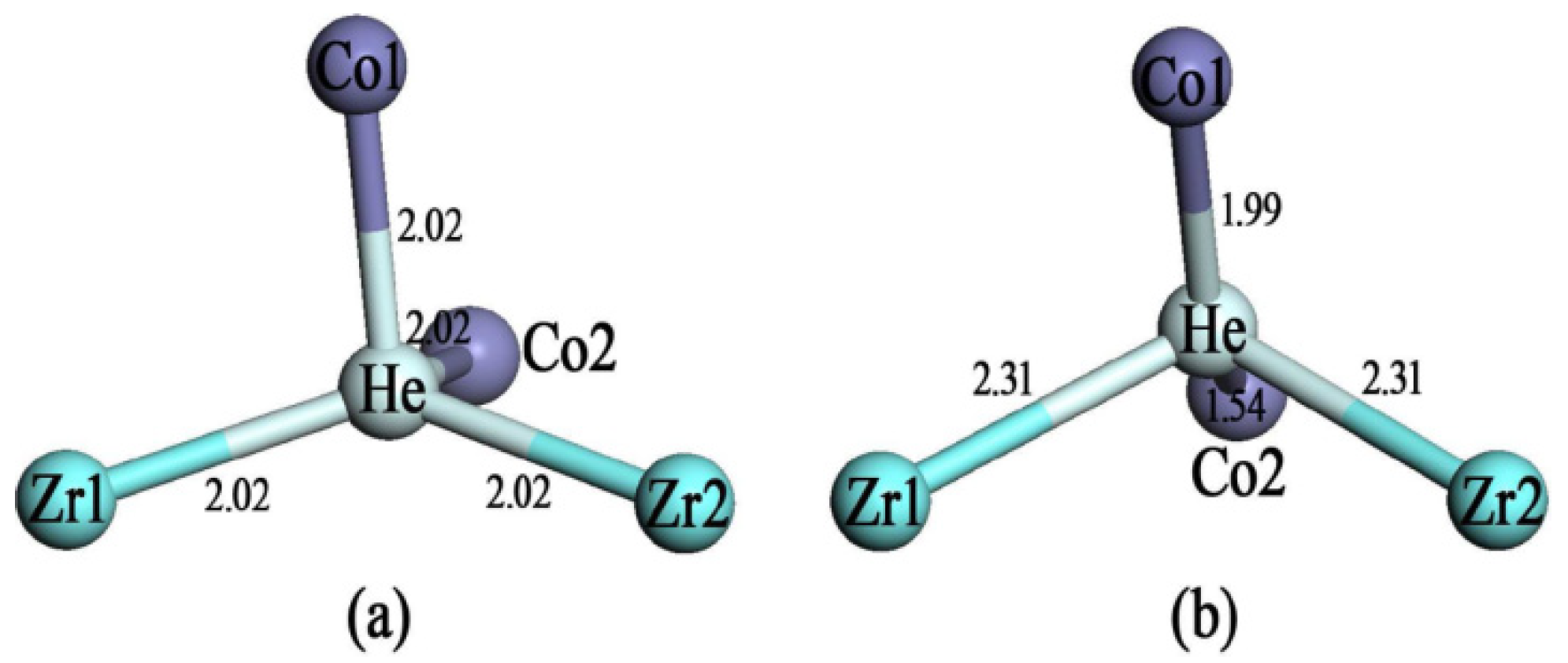
3.2. Stability of Helium at 8e, Zr, Co and H Sites
3.3. Effects of Alloying Elements at Co Site on Stability of Helium
3.4. Effects of Alloying Elements Replacing Zr on Stability of Helium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shmayda, W.T.; Maye, P. Uranium beds for temporary tritium storage. J. Less Common Met. 1984, 104, 239–250. [Google Scholar] [CrossRef]
- Kang, H.G.; Chung, D.Y.; Chang, M.H.; Yun, S.H. Development of depleted uranium bed for tritium fuel cycle and basic absorption/desorption experiments. Fusion Eng. Des. 2018, 132, 86–89. [Google Scholar] [CrossRef]
- Jung, K.; Kim, Y.; Chung, H.; Kang, H.S.; Yun, S.H.; Ahn, D.H. Performance of a depleted uranium bed for a nuclear fusion fuel cycle. Fusion Sci. Tech. 2017, 71, 416–421. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Kang, H.S.; Jung, K.J.; Kim, K.Y.; Yim, S.P.; Kim, S.B.; Ahn, H.J.; Park, C.W.; Lee, S.N.; et al. Tritium research and development status at KAERI. Fusion Sci. Tech. 2020, 76, 267–274. [Google Scholar] [CrossRef]
- Devillers, M.; Sirch, M.; Bredendiek-Kamper, S.; Penzhorn, R.-D. Characterizion of the ZrCo-hydrogen system in view of its use for tritium storage. Chem. Mater. 1990, 2, 255–262. [Google Scholar] [CrossRef]
- Naik, Y.; Rao, G.A.R.; Venugopal, V. Zirconium-cobalt intermetallic compound for storage and recovery of hydrogen isotopes. Intermetallics 2001, 9, 309–312. [Google Scholar] [CrossRef]
- Bekris, N.; Besserer, U.; Sirch, M.; Penzhorn, R.-D. On the thermal stability of the zirconium/cobalt–hydrogen system. Fusion Eng. Des. 2000, 49–50, 781–789. [Google Scholar] [CrossRef]
- Vasileiadis, N.; Tatsios, G.; Misdanitis, S.; Valougeorgis, D. Modeling of complex gas distribution systems operating under any vacuum conditions: Simulations of the ITER divertor pumping system. Fusion Eng. Des. 2016, 103, 125–135. [Google Scholar] [CrossRef]
- Wang, F.; Li, R.F.; Ding, C.P.; Tang, W.K.; Wang, Y.B.; Xu, S.M.; Yu, R.H.; Wu, Y. Recent progress on the hydrogen storage properties of ZrCo-based alloys applied in International Thermonuclear Experimental Reactor (ITER). Prog. Nat. Sci. Mater. Int. 2017, 27, 64–71. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Xiao, X.Z.; Yao, Z.D.; Kou, H.Q.; Luo, W.H.; Chen, C.G.; Chen, L.X. A new strategy for remarkably improving anti-disproportionation performance and cycling stabilities of ZrCo-based hydrogen isotope storage alloys by Cu substitution and controlling cutoff desorption pressure. Int. J. Hydrog. Energy 2019, 44, 28242–28251. [Google Scholar] [CrossRef]
- Luo, L.L.; Ye, X.Q.; Zhao, C.; Zhang, G.H.; Kou, H.Q.; Xiong, R.J.; Sang, G.; Han, T. Effects of Mo substitution on the kinetic and thermodynamic characteristics of ZrCo1-xMox (x=0–0.2) alloys for hydrogen storage. Int. J. Hydrog. Energy 2020, 45, 2989–2998. [Google Scholar] [CrossRef]
- Guo, X.M.; Wang, S.M.; Liu, X.P.; Li, Z.N.; Ye, J.H.; Yuan, H.P.; Jiang, L.J. Structural characteristics and mechanism of hydrogen-induced disproportionation of the ZrCo alloy. Int. J. Metall. Mater. 2012, 19, 1010–1015. [Google Scholar] [CrossRef]
- Yang, G.; Liu, W.G.; Tan, J.; Wu, S.W.; Qiu, J.; Cheng, H.W.; Yin, H.Q.; Qian, N.; Huang, Y.; Wu, X.J.; et al. Theoretical study of ZrCoH3 and the anti-disproportionation ability of alloying elements. Int. J. Hydrog. Energy 2018, 43, 10410–10419. [Google Scholar] [CrossRef]
- Yang, G.; Liu, W.G.; Wu, S.W.; Qian, N.; Cheng, H.W.; Gao, J.; Zeng, Y.S.; Liu, W.; Li, Y. Theoretical investigation of Ti and Ni co-doping on the anti-disproportionation ability of ZrCo alloy. Mater. Res. Express 2018, 6, 105501. [Google Scholar] [CrossRef]
- Devillers, M.; Sirch, M.; Penzhorn, R.D. Hydrogen-induced disproportionation of the intermetallic compound ZrCo. Chem. Mater. 1992, 4, 631–639. [Google Scholar] [CrossRef]
- Penzhorn, R.D.; Devillers, M.; Sirch, M. Evaluation of ZrCo and other getters for tritium handling and storage. J. Nucl. Mater. 1990, 170, 217–231. [Google Scholar] [CrossRef]
- Hara, M.; Hayakawa, R.; Kaneko, Y.; Watanabe, K. Hydrogen-induced disproportionation of Zr2M (M=Fe, Co, Ni) and reproportionation. J. Alloys Compd. 2003, 352, 218–225. [Google Scholar] [CrossRef]
- Jat, R.A.; Parida, S.C.; Agarwal, R.; Kulkarni, S.G. Effect of Ni content on the hydrogen storage behavior of ZrCo1-xNix alloys. Int. J. Hydrog. Energy 2013, 38, 1490–1500. [Google Scholar] [CrossRef]
- Zhang, G.H.; Sang, G.; Xiong, R.J.; Kou, H.Q.; Liu, K.Z.; Luo, W.H. Effects and mechanism of Ti, Ni, Sc, Fe substitution on the thermal stability of zirconium cobalt-hydrogen system. Int. J. Hydrog. Energy 2015, 40, 6582–6593. [Google Scholar] [CrossRef]
- Jat, R.A.; Singh, R.; Parida, S.C.; Agarwal, R.; Mukerjee, S.K. Structural and hydrogen isotope storage properties of Zr-Co-Fe alloy. Int. J. Hydrog. Energy 2015, 40, 5135–5143. [Google Scholar] [CrossRef]
- Weng, C.C.; Xiao, X.Z.; Huang, X.; Jiang, F.L.; Yao, Z.D.; Li, S.Q.; Ge, H.W.; Chen, L.X. Effect of Mn substitution for Co on the structural, kinetic, and thermodynamic characteristics of ZrCo1−xMnx (x = 0–0.1) alloys for tritium storage. Int. J. Hydrog. Energy 2017, 42, 28498–28506. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Kong, X.G.; Yu, Y.; Han, H.; Sang, G.; Zhang, G.H.; Yi, Y.G.; Gao, T. Effect of multiple Ti doping on helium behavior in ZrCo. J. Nucl. Mater. 2021, 551, 152955. [Google Scholar] [CrossRef]
- Wang, F.; Li, R.F.; Ding, C.P.; Wan, J.; Yu, R.H.; Wang, Z.M. Effect of catalytic Ni coating with different depositing time on the hydrogen storage properties of ZrCo alloy. Int. J. Hydrog. Energy 2016, 41, 17421–17432. [Google Scholar] [CrossRef]
- Irvine, S.J.C.; Harris, I.R. An investigation of the systems ZrCo-H2 and ZrCo0.84Ni0.16H2. J. Less Common Met. 1980, 74, 33–43. [Google Scholar] [CrossRef]
- Jat, R.V.; Singh, R.; Parida, S.C.; Das, A.; Agarwal, R.; Ramakumar, K.L. Determination of deuterium site occupancy in ZrCoD3 and its role in improved durability of Zr-Co-Ni deuterides against disproportionation. Int. J. Hydrog. Energy 2014, 39, 15665–15669. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, C.; Xu, Q.; Wu, X. Hydrogen-induced disproportionation characteristics of Zr(1−x)Hf(x)Co(x = 0, 0.1, 0.2 and 0.3) alloys. Fusion Eng. Des. 2013, 88, 299–303. [Google Scholar] [CrossRef]
- Konishi, S.; Nagasaki, T.; Yokokawa, N.; Naruse, Y. Development of zirconium-cobalt beds for recovery, storage and supply of tritium. Fusion Eng. Des. 1989, 10, 355–358. [Google Scholar] [CrossRef]
- Konishi, S.; Nagasaki, T.; Okuno, K. Reversible disproportionation of ZrCo under high temperature and hydrogen pressure. J. Nucl. Mater. 1995, 223, 294–299. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.P.; Jiang, L.J.; Wang, S.M. Hydrogen storage properties of Zr1-xTixCo intermetallic compound. Rare Met. 2006, 25, 200–203. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Li, R.F.; Tang, R.H.; Li, B.Y.; Yu, R.H.; Liu, W.; Kou, H.Q.; Meng, J.B. Effect of Ti substitution on hydrogen storage properties of Zr1-xTixCo (x = 0, 0.1, 0.2, 0.3) alloys. J. Energy Chem. 2014, 23, 9–14. [Google Scholar] [CrossRef]
- Qi, Y.; Ju, X.; Wan, C.; Qiu, J.; Xin, Y.; Wang, S.; Liu, X.; Jiang, L. EXAFS and SAXS studies of ZrCo alloy doped with Hf, Sc and Ti atoms. Int. J. Hydrog. Energy 2010, 35, 2931–2935. [Google Scholar] [CrossRef]
- You, Y.W.; Yu, J.Y.; Yuan, H.; Xu, Y.C.; Wu, X.B.; Sun, J.J.; Wang, J.H.; Fang, Q.F.; Liu, C.S. Systematical study on the roles of transition alloying substitutions on anti-disproportionation reaction of ZrCo during charging and releasing hydrogen. Int. J. Hydrog. Energy 2020, 45, 14028–14037. [Google Scholar] [CrossRef]
- Jung, P.K.; Chang, M.H.; Chung, D.Y.; Kang, H.G.; Lee, J.U. Helium blanketing and gas circulation effect of hydriding reaction with ZrCo. Fusion Eng. Des. 2020, 157, 111626. [Google Scholar] [CrossRef]
- Daghbouj, N.; Li, B.S.; Callisti, M.; Sen, H.S.; Karlik, M.; Polcar, T. Microstructural evolution of helium-irradiated 6H–SiC subjected to different irradiation conditions and annealing temperatures: A multiple characterization study. Acta Mater. 2019, 181, 160–172. [Google Scholar] [CrossRef]
- Daghbouj, N.; Li, B.S.; Callisti, M.; Sen, H.S.; Lin, J.; Ou, X.; Karlik, M.; Polcar, T. The structural evolution of light-ion implanted 6H-SiC single crystal: Comparison of the effect of helium and hydrogen. Acta Mater. 2020, 188, 609–622. [Google Scholar] [CrossRef]
- Daghbouj, N.; Li, B.S.; Karlik, M.; Declemy, A. 6H-SiC blistering efficiency as a function of the hydrogen implantation fluence. Appl. Surf. Sci. 2019, 466, 141–150. [Google Scholar] [CrossRef]
- Hayashi, T.; Suzuki, T.; Okuno, K. Long-term measurement of helium-3 release behavior from zirconium-cobalt tritide. J. Nucl. Mater. 1994, 212–215, 1431–1435. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Kong, X.G.; Han, H.L.; Sang, G.; Zhang, G.H.; Yi, Y.G.; Gao, T. Influence of Ti on the dissolution and migration of He in ZrCo based on first principles investigation. Phys. Chem. Chem. Phys. 2019, 21, 14692–14700. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Chattaraj, D.; Parida, S.C.; Dash, S.; Majumder, C. Density functional study of vibrational, thermodynamic and elastic properties of ZrCo and ZrCoX3 (X = H, D and T) compounds. J. Alloys Compd. 2015, 629, 297–304. [Google Scholar] [CrossRef]
- Jat, R.A.; Parida, S.C.; Nuwad, J.; Agarwal, R.; Kulkarni, S.G. Hydrogen sorption–desorption studies on ZrCo–hydrogen system. Therm. Anal. Calorim. 2013, 112, 37–43. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.G.; Han, X.B.; Han, H.; Qian, Y.; Zeng, T.S.; Wu, X.L.; Qiu, J.; Yin, H.Q.; Liu, W.; et al. Effects of alloying substitutions on the anti-disproportionation behavior of ZrCo alloy. Int. J. Hydrog. Energy 2017, 42, 15782–15789. [Google Scholar]
- Chattaraj, D.; Parida, S.C.; Dash, S.; Majumder, C. Structural, electronic and thermodynamic properties of ZrCo and ZrCoH3: A first-principles study. Int. J. Hydrog. Energy 2012, 37, 18952–18958. [Google Scholar] [CrossRef]






| Object | Present | Reference |
|---|---|---|
| Latt(a) (Å) | 3.54 | 3.51–3.54 [43,44,45] |
| Latt(b) (Å) | 10.41 | 10.40–10.48 [43,44,45] |
| Latt(c) (Å) | 4.32 | 4.30–4.33 [43,44,45] |
| EZrVacf (eV) | 3.38 | - |
| ECoVacf (eV) | 0.97 | - |
| EHVac1f (eV) | 0.55 | - |
| EHVac2f (eV) | 0.45 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Cao, Q.; You, Y.; Kong, X.; Wu, X.; Liu, C. Influence of Alloy Atoms on Substitution Properties of Hydrogen by Helium in ZrCoH3. Materials 2021, 14, 6704. https://doi.org/10.3390/ma14216704
Wang P, Cao Q, You Y, Kong X, Wu X, Liu C. Influence of Alloy Atoms on Substitution Properties of Hydrogen by Helium in ZrCoH3. Materials. 2021; 14(21):6704. https://doi.org/10.3390/ma14216704
Chicago/Turabian StyleWang, Panpan, Qilong Cao, Yuwei You, Xiangshan Kong, Xuebang Wu, and Changsong Liu. 2021. "Influence of Alloy Atoms on Substitution Properties of Hydrogen by Helium in ZrCoH3" Materials 14, no. 21: 6704. https://doi.org/10.3390/ma14216704
APA StyleWang, P., Cao, Q., You, Y., Kong, X., Wu, X., & Liu, C. (2021). Influence of Alloy Atoms on Substitution Properties of Hydrogen by Helium in ZrCoH3. Materials, 14(21), 6704. https://doi.org/10.3390/ma14216704







