Antibacterial Efficacy of Cold-Sprayed Copper Coatings against Gram-Positive Staphylococcus aureus and Gram-Negative Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
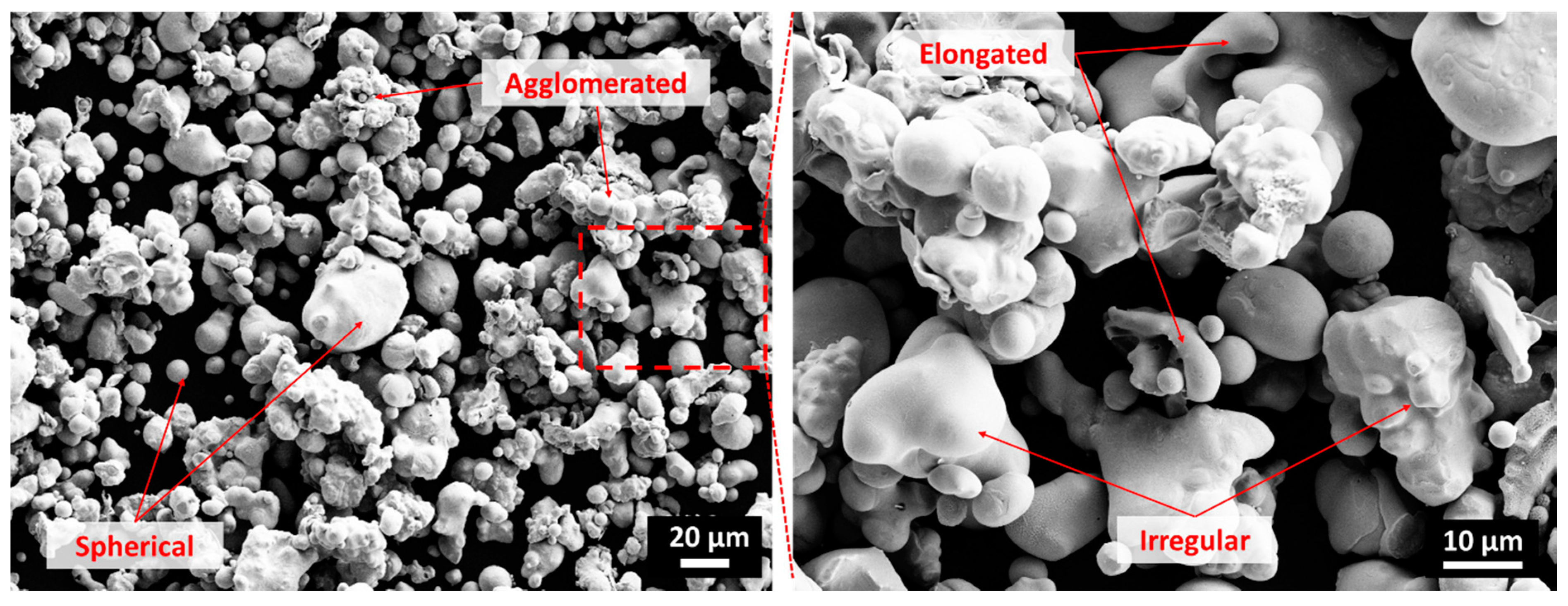
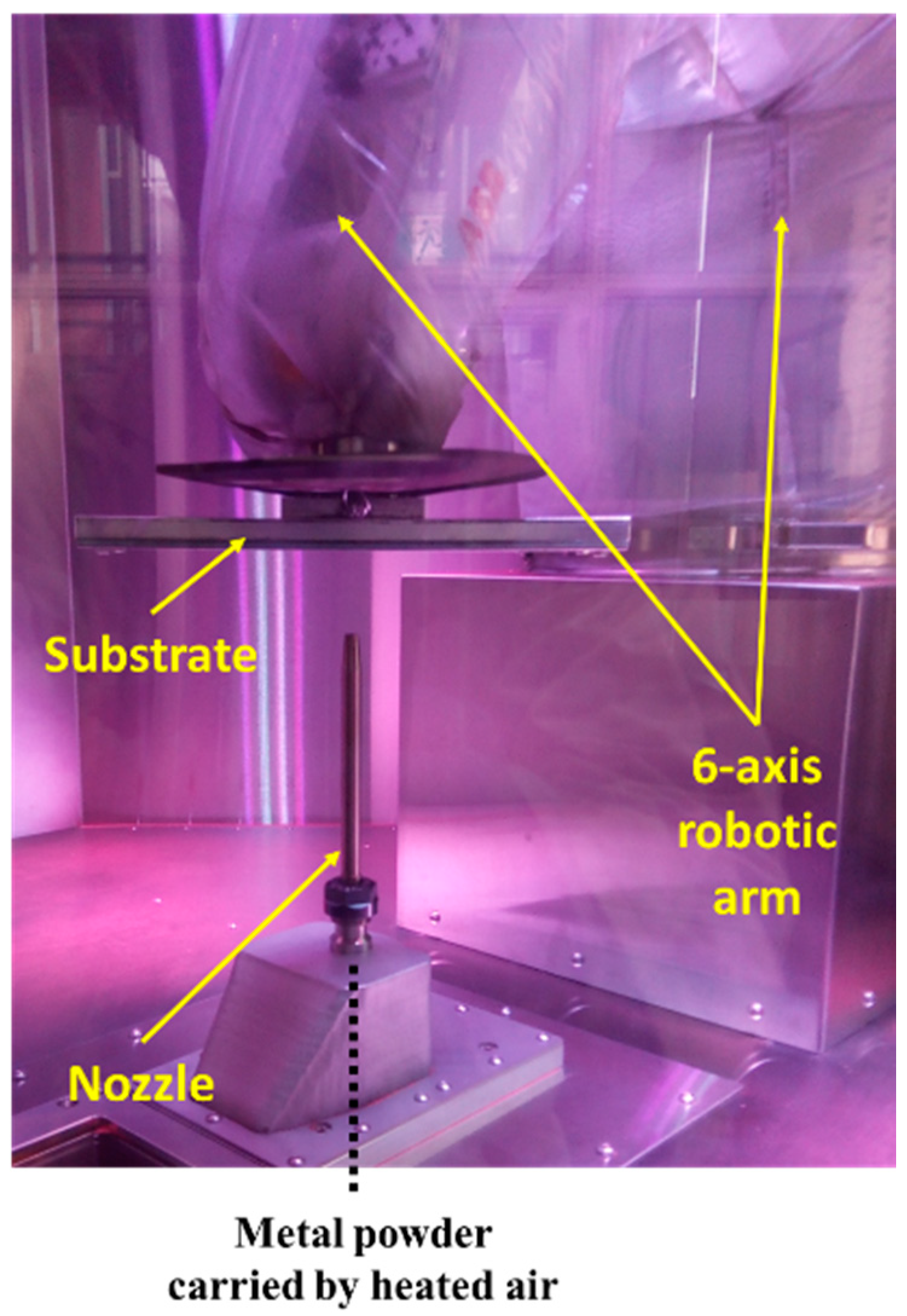
2.2. Cold Spray Coatings
2.3. Material Characterization
2.4. Antibacterial Tests
2.5. Bacteria Attachment Tests
3. Results
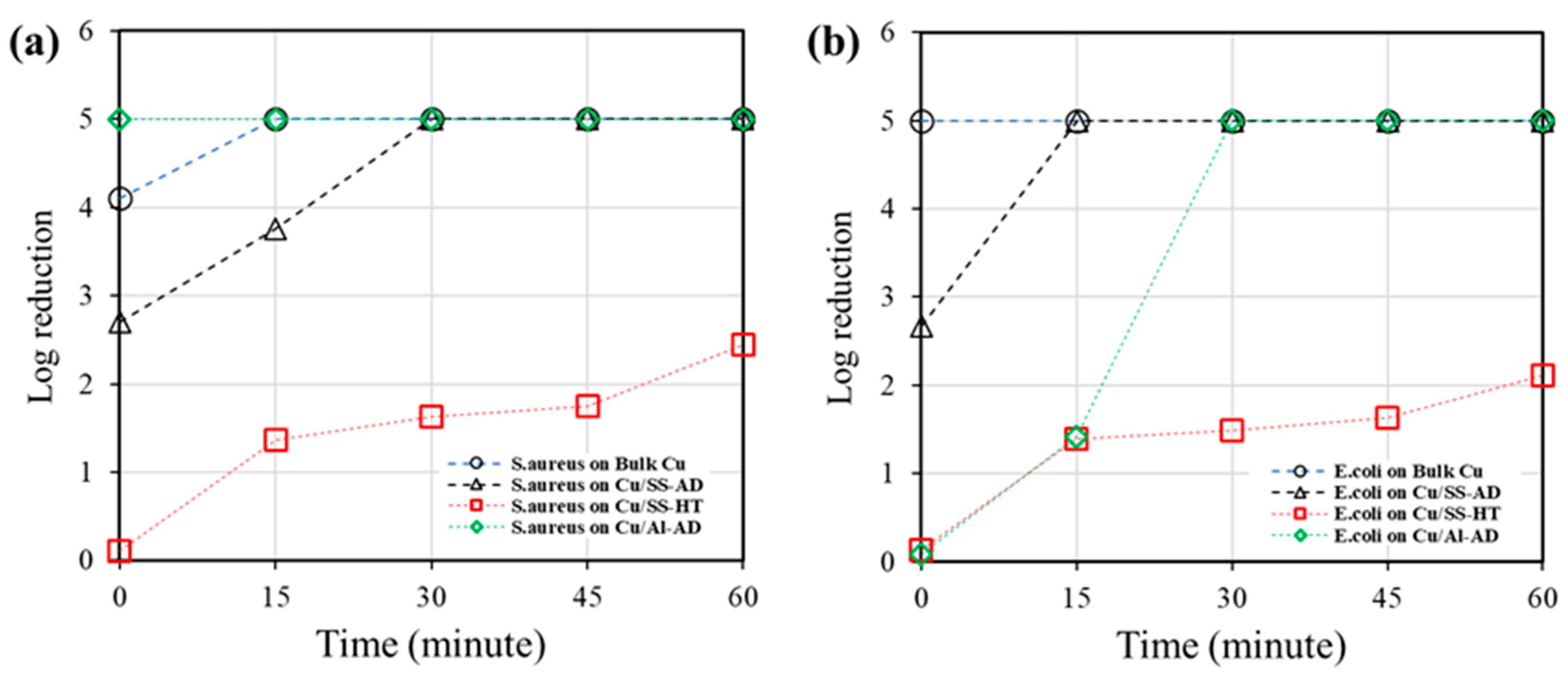
3.1. Antibacterial Testing
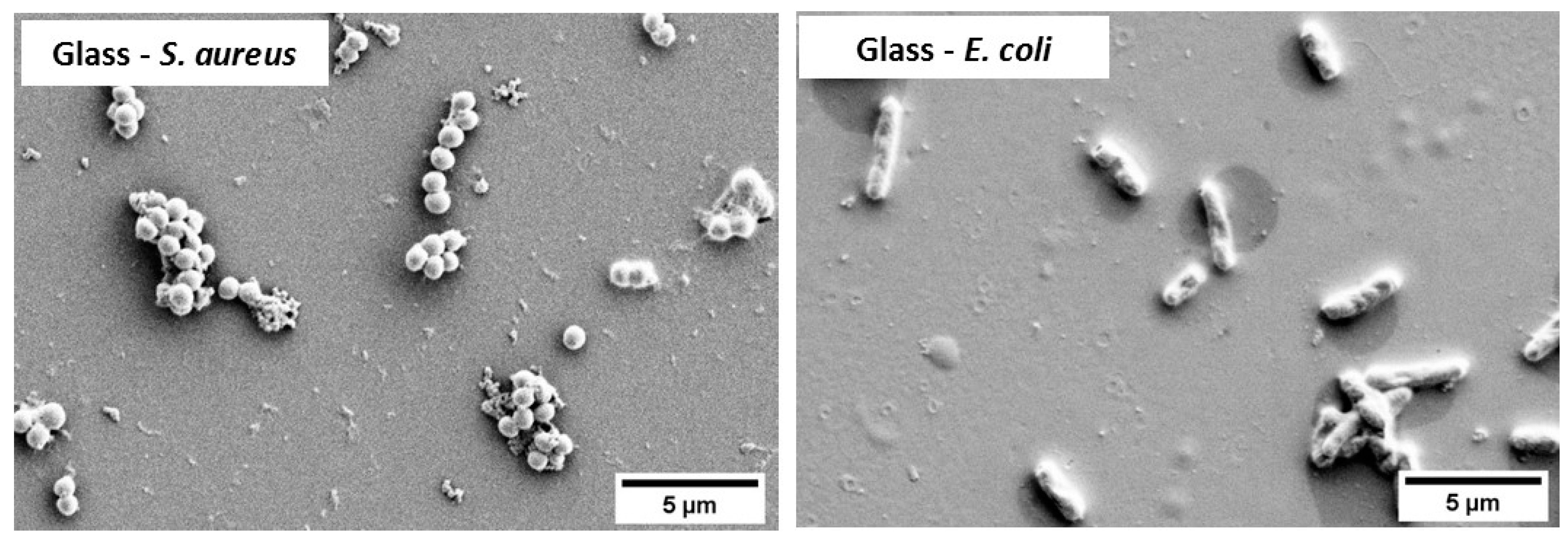
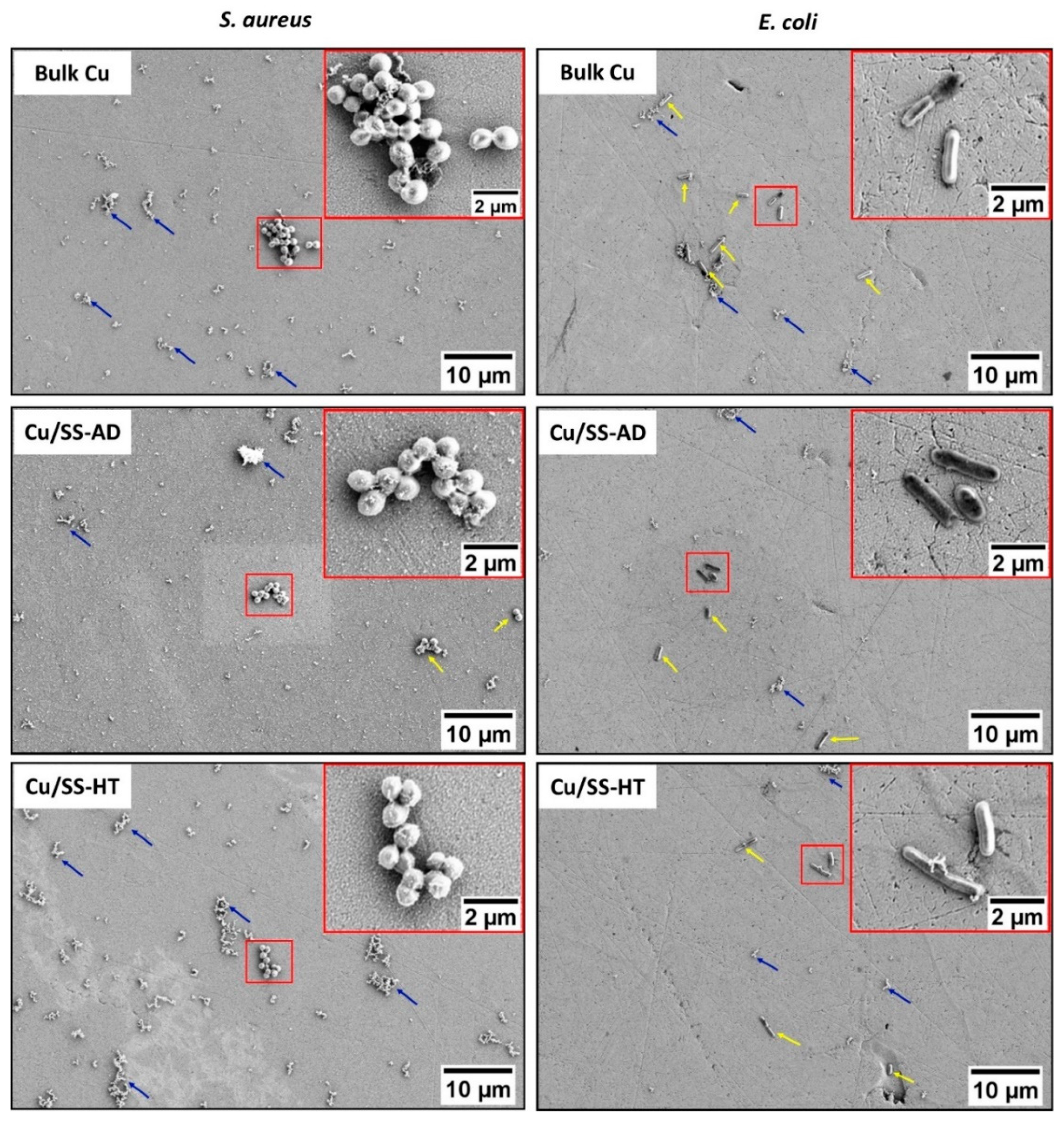
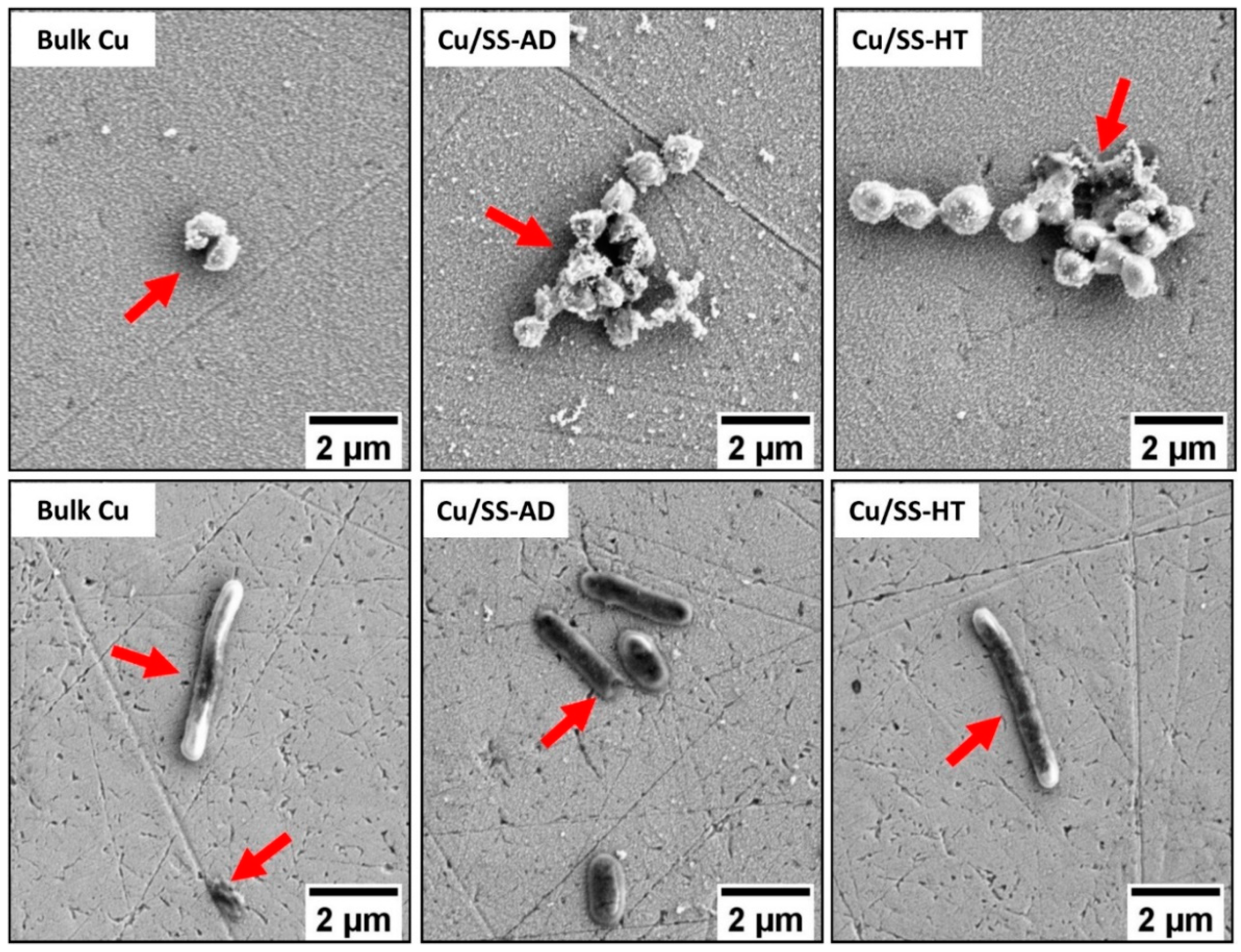
3.2. Bacterial Attachment Testing
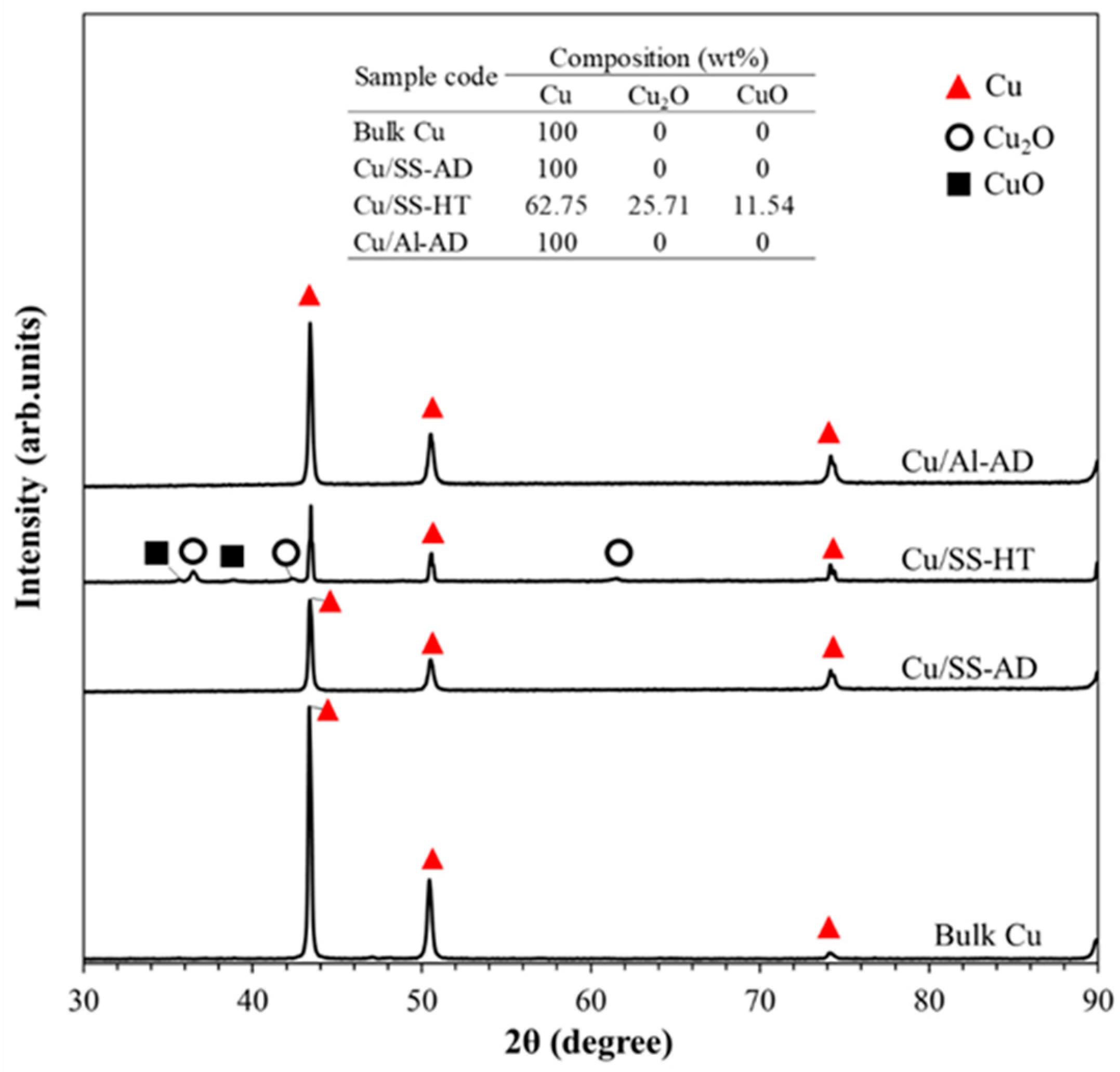
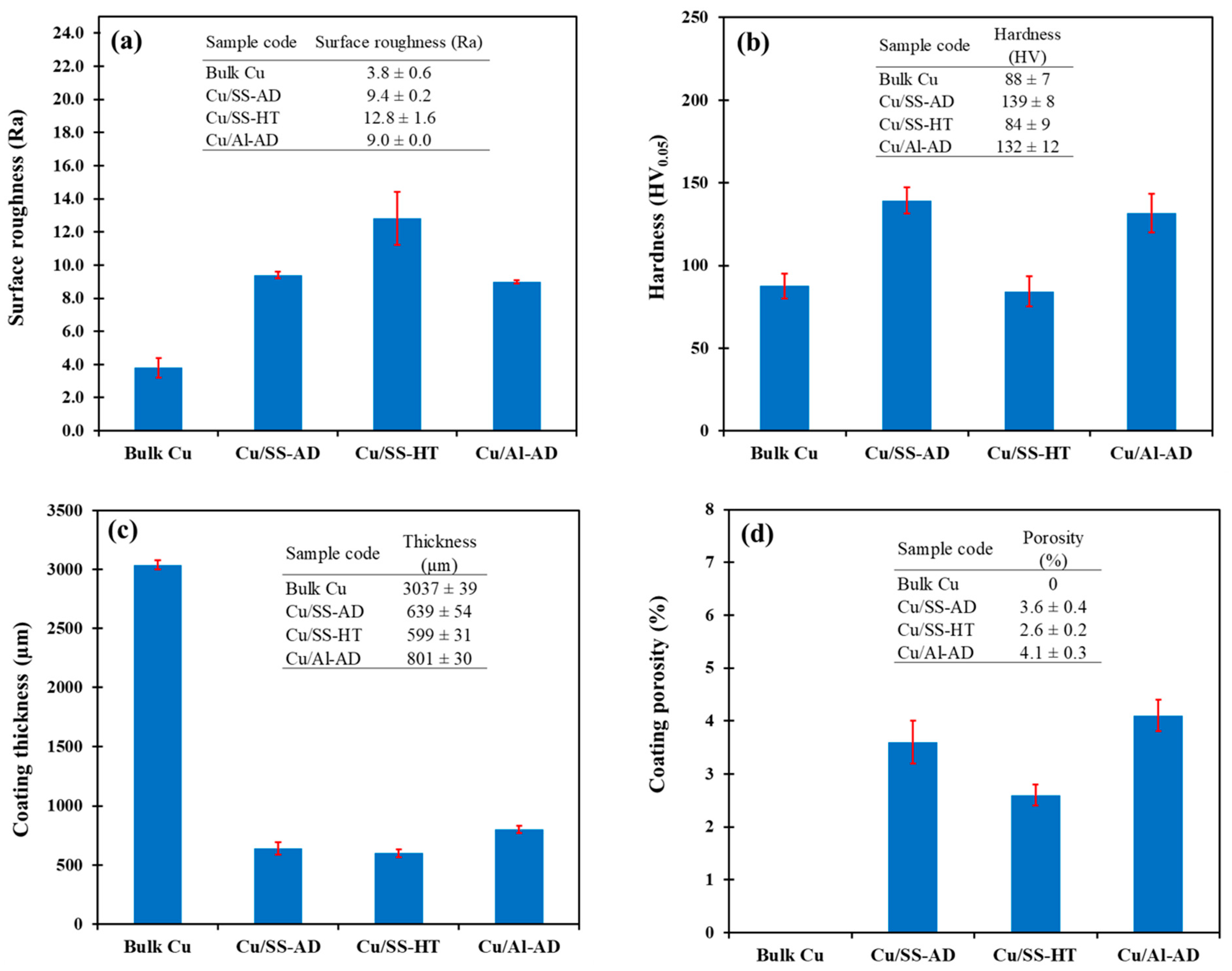
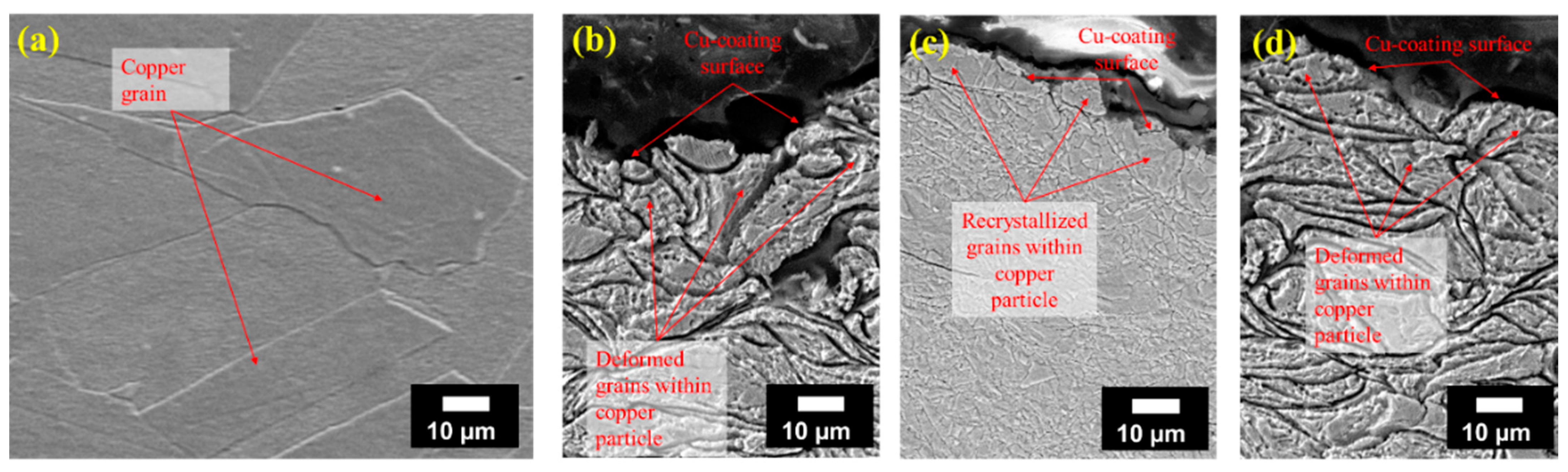
3.3. Copper Coating and Bulk Copper Characterization
4. Discussion
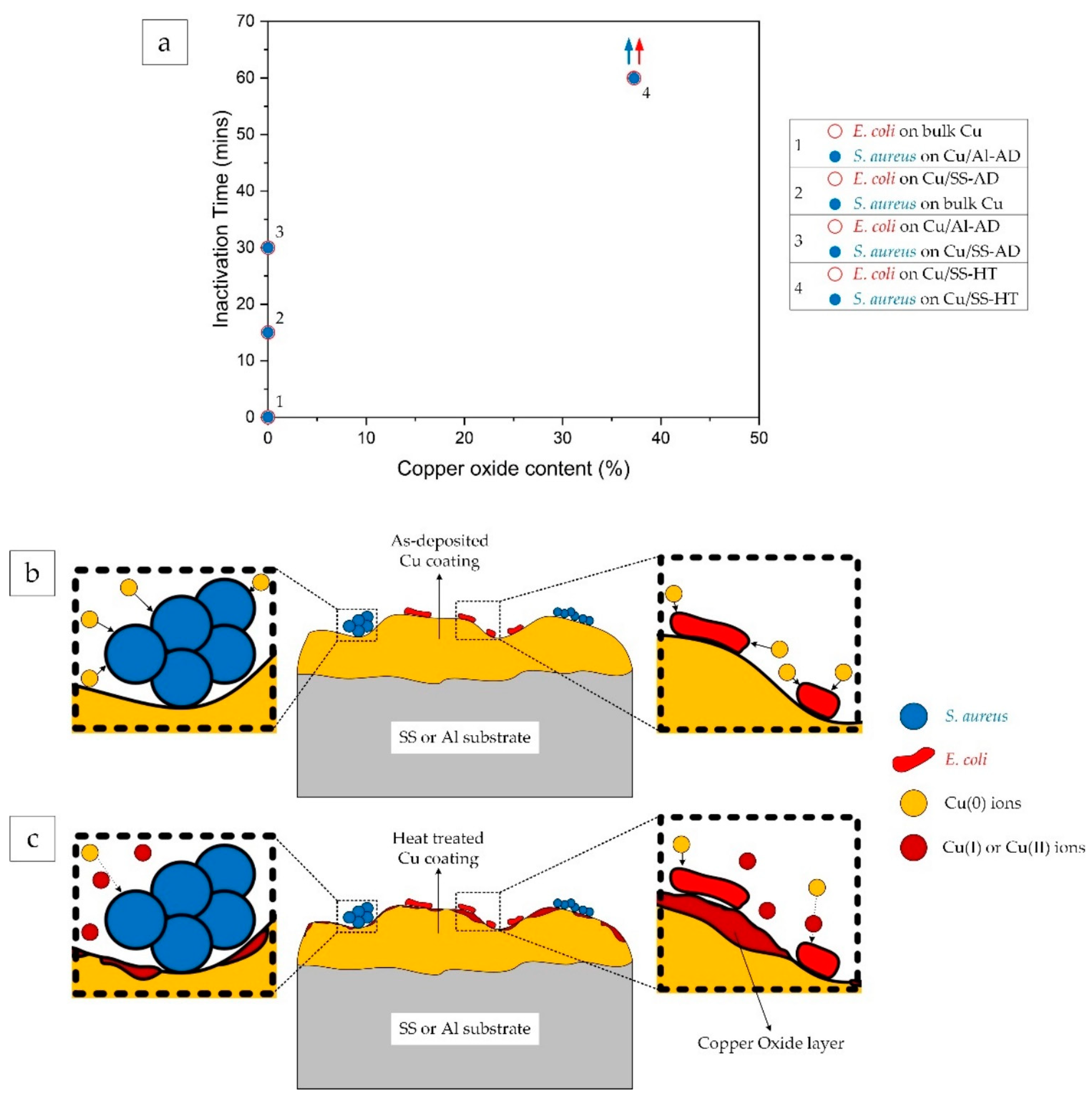
4.1. Effect of Cu Ionic Species on Antibacterial Efficacy
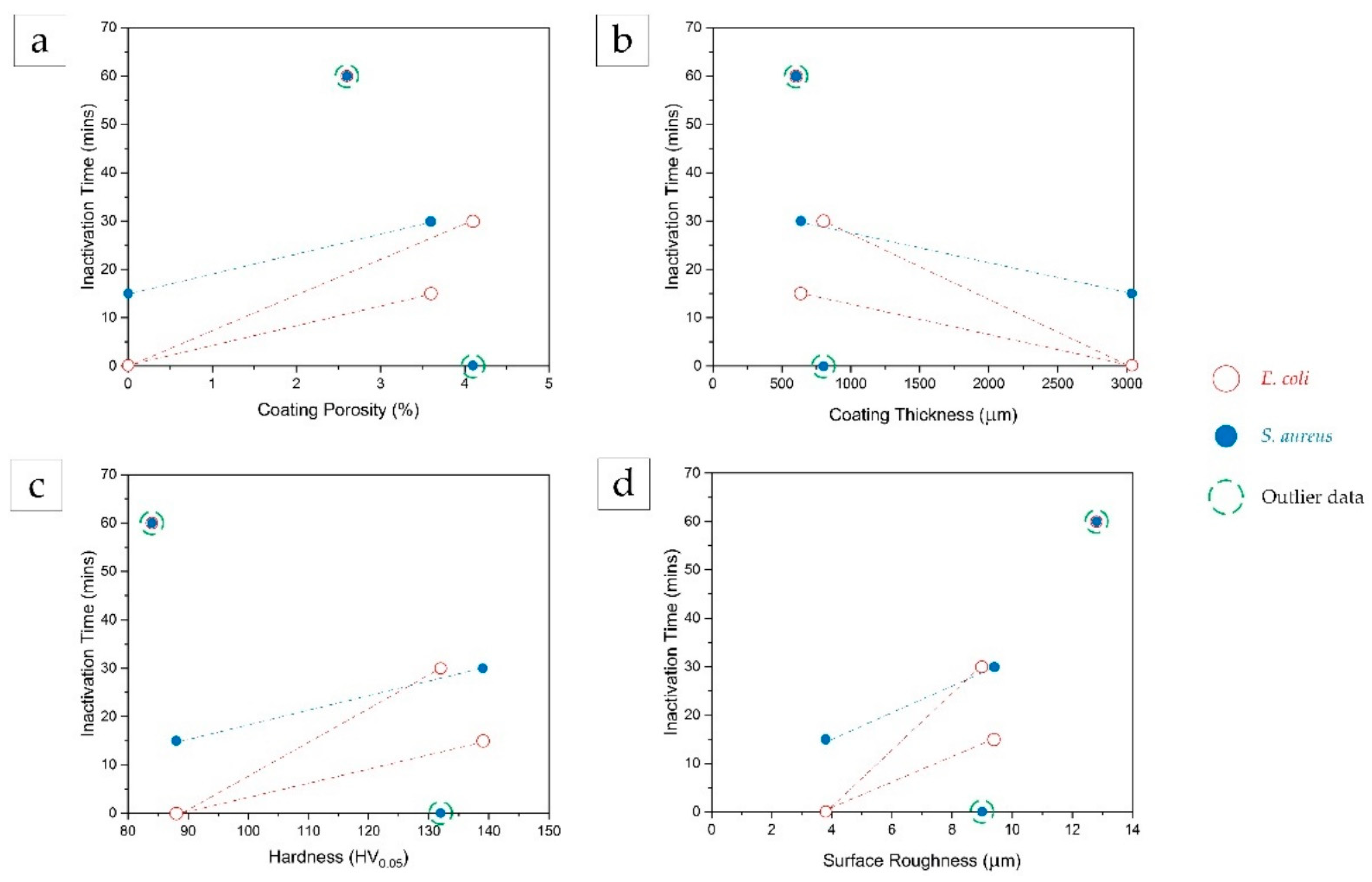
4.2. Effect of Cu Coating Properties on Antibacterial Efficacy
- The inactivation time for both bacterial cells increased with an increase in coating porosity, coating hardness, and surface roughness of the coatings.
- The inactivation time for both bacterial cells decreased with an increase in coating thickness.
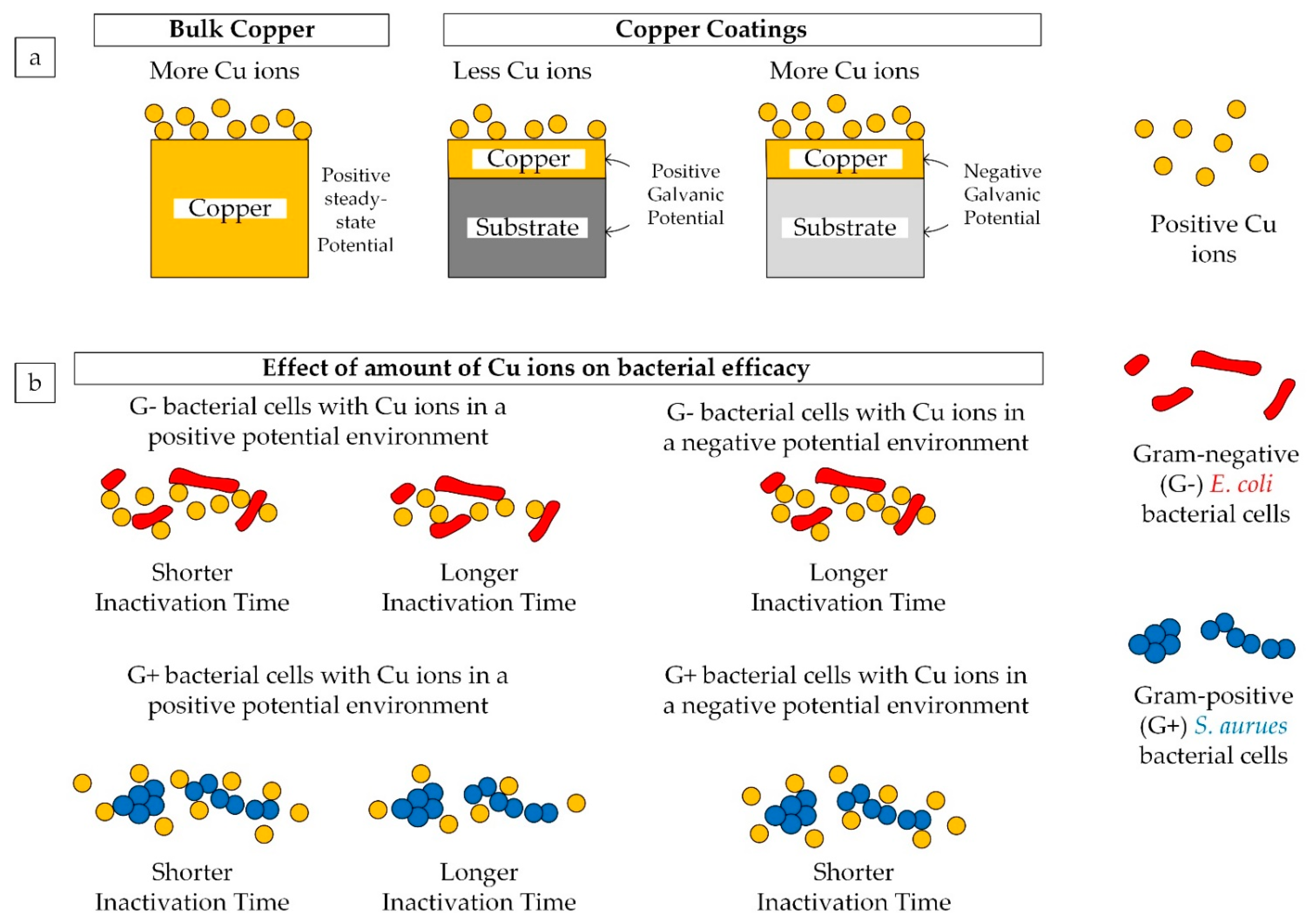
4.3. Effect of Galvanic Coupling Due to Different Coating/Substrate Configurations
4.4. Bacteria Killing Mechanisms
5. Future Works
6. Conclusions
- It was found that there was a presence of copper oxides (Cu2O and CuO) on the surface of heat-treated samples. This resulted in reduced antibacterial efficacy due to the presence of Cu(I) and Cu(II) ions and lower concentrations of Cu(0)—metallic copper ions.
- There was an insignificant difference in the bacterial cell morphology (shape and dimensions) of both E. coli and S. aureus exposed to different types of copper samples. Moreover, both bacterial cells showed signs of damage resulting in abnormal shapes, stained cells, disrupted cell membranes, and release of cell debris when seen under an SEM.
- The inactivation time for both E. coli and S. aureus appeared to increase with an increase in coating porosity, hardness, and surface roughness, but decrease with an increase in thickness of copper material.
- Cu ions from bulk copper surface react more aggressively towards the Gram-negative E. coli bacterial cells than Gram-positive S. aureus cells, inactivating E. coli faster than S. aureus. However, in the case of bimetallic galvanic coupling with a negative potential, such as Cu/Al coating/substrate configuration, it is possible that the Cu ions react more aggressively towards Gram-positive S. aureus resulting in shorter inactivation time compared to E. coli.
- Copper coating/substrate configuration with a positive galvanic coupling potential (i.e., Cu/SS in this study) could yield less Cu ions compared to bulk copper samples which resulted in higher inactivation time for both types of bacterial cells.
- Different coating/substrate galvanic coupling configurations respond differently to different types of bacteria. Therefore, it is possible to tailor coating properties based on various factors such as galvanic coupling, post heat treatment, coating porosity, thickness, etc. to render them effective towards targeted bacteria.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borkow, G. Using copper to fight microorganisms. Curr. Chem. Biol. 2012, 6, 93–103. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Inactivation of influenza a virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E remains infectious on common touch surface materials. mBio 2015, 6, e01697-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Hutasoit, N.; Kennedy, B.; Hamilton, S.; Luttick, A.; Rashid, R.A.R.; Palanisamy, S. SARS-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020, 25, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Duval, R.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Molteni, C.; Abicht, H.; Solioz, M. Killing of bacteria by copper surfaces involves dissolved copper. Appl. Environ. Microbiol. 2010, 76, 4099–4101. [Google Scholar] [CrossRef] [Green Version]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Luo, J.; Hein, C.; Ghanbaja, J.; Pierson, J.-F.; Mücklich, F. Bacteria accumulate copper ions and inhibit oxide formation on copper surface during antibacterial efficiency test. Micron 2019, 127, 102759. [Google Scholar] [CrossRef]
- Hassan, I.A.; Parkin, I.P.; Nair, S.P.; Carmalt, C.J. Antimicrobial activity of copper and copper(i) oxide thin films deposited via aerosol-assisted CVD. J. Mater. Chem. B 2014, 2, 2855–2860. [Google Scholar] [CrossRef]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235, 265–270. [Google Scholar] [CrossRef]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Kocaman, A.; Keles, O. Antibacterial efficacy of wire arc sprayed copper coatings against various pathogens. J. Therm. Spray Technol. 2019, 28, 504–513. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Salimijazi, H.; Fathi, M.; Mostaghimi, J.; Pershin, L. Relationship between surface properties and antibacterial behavior of wire arc spray copper coatings. Surf. Coat. Technol. 2013, 233, 74–79. [Google Scholar] [CrossRef]
- Champagne, V.; Sundberg, K.; Helfritch, D. Kinetically deposited copper antimicrobial surfaces. Coatings 2019, 9, 257. [Google Scholar] [CrossRef] [Green Version]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial killing by dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef] [Green Version]
- Champagne, V.K.; Helfritch, D.J. A demonstration of the antimicrobial effectiveness of various copper surfaces. J. Biol. Eng. 2013, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.W.; Lößlein, S.; Terriac, E.; Brix, K.; Siems, K.; Moeller, R.; Kautenburger, R.; Mücklich, F. Increasing antibacterial efficiency of Cu surfaces by targeted surface functionalization via ultrashort pulsed direct laser interference patterning. Adv. Mater. Interfaces 2021, 8, 2001656. [Google Scholar] [CrossRef]
- Davis, J.R. Metals Handbook, Desk Edition, 2nd ed.; ASM International: Washington, DC, USA, 1998. [Google Scholar]
- Ueda, M.; Yokota, T.; Honda, M.; Lim, P.N.; Osaka, N.; Makita, M.; Nishikawa, Y.; Kasuga, T.; Aizawa, M. Regulating size of silver nanoparticles on calcium carbonate via ultrasonic spray for effective antibacterial efficacy and sustained release. Mater. Sci. Eng. C 2021, 125, 112083. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-Y.; Hsieh, Y.-J.; Lu, P.-L.; Lin, L.; Wang, L.-C.; Wang, H.-Y.; Tsai, T.-H.; Shih, C.-J.; Tseng, S.-P. In vitro and in vivo assessments of inspired Ag/80S bioactive nanocomposites against carbapenem-resistant Klebsiella pneumoniae. Mater. Sci. Eng. C 2021, 125, 112093. [Google Scholar] [CrossRef]
- An, S.; Joshi, B.; Yarin, A.L.; Swihart, M.T.; Yoon, S.S. Supersonic cold spraying for energy and environmental applications: One-step scalable coating technology for advanced micro- and nanotextured materials. Adv. Mater. 2020, 32, e1905028. [Google Scholar] [CrossRef]
- Hou, W.; Oheil, M.; Shen, Z.; Shen, Y.; Jahed, H.; Gerlich, A. Enhanced strength and ductility in dissimilar friction stir butt welded Al/Cu joints by addition of a cold-spray Ni interlayer. J. Manuf. Process. 2020, 60, 573–577. [Google Scholar] [CrossRef]
- Assadi, H.; Gärtner, F.; Stoltenhoff, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Assadi, H.; Kreye, H.; Gärtner, F.; Klassen, T. Cold spraying—A materials perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.; Gaertner, F.; Assadi, H.; Kreye, H. Development of a generalized parameter window for cold spray deposition. Acta Mater. 2006, 54, 729–742. [Google Scholar] [CrossRef]
- Joshi, A.; James, S. Molecular dynamics simulation study of cold spray process. J. Manuf. Process. 2018, 33, 136–143. [Google Scholar] [CrossRef]
- Yin, S.; Cavaliere, P.; Aldwell, B.; Jenkins, R.; Liao, H.; Li, W.; Lupoi, R. Cold spray additive manufacturing and repair: Fundamentals and applications. Addit. Manuf. 2018, 21, 628–650. [Google Scholar] [CrossRef]
- Hutasoit, N.; Rashid, R.A.R.; Palanisamy, S.; Duguid, A. Effect of build orientation and post-build heat treatment on the mechanical properties of cold spray additively manufactured copper parts. Int. J. Adv. Manuf. Technol. 2020, 110, 1–17. [Google Scholar] [CrossRef]
- Hutasoit, N.; Javed, M.A.; Rashid, R.A.R.; Wade, S.; Palanisamy, S. Effects of build orientation and heat treatment on microstructure, mechanical and corrosion properties of Al6061 aluminium parts built by cold spray additive manufacturing process. Int. J. Mech. Sci. 2021, 204, 106526. [Google Scholar] [CrossRef]
- Mimura, K.; Lim, J.-W.; Isshiki, M.; Zhu, Y.; Jiang, Q. Brief review of oxidation kinetics of copper at 350 °C to 1050 °C. Met. Mater. Trans. A 2006, 37, 1231–1237. [Google Scholar] [CrossRef]
- Zhu, Y.; Mimura, K.; Isshiki, M. Oxidation mechanism of copper at 623–1073 K. Mater. Trans. 2002, 43, 2173–2176. [Google Scholar] [CrossRef] [Green Version]
- ASTM E2197-17e1. Standard Quantitative Disk Carrier Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal, and Sporicidal Activities of Chemicals; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- United States Environmental Protection Agency. Interim Method for Evaluating the Efficacy of Antimicrobial Surface Coatings; Office of Pesticide Programs: Washington, DC, USA, 2020.
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnes, S.L.; Caves, V.; Keevil, C. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ. Microbiol. 2011, 14, 1730–1743. [Google Scholar] [CrossRef]
- Zahiri, S.H.; Fraser, D.; Gulizia, S.; Jahedi, M. Effect of processing conditions on porosity formation in cold gas dynamic spraying of copper. J. Therm. Spray Technol. 2006, 15, 422–430. [Google Scholar] [CrossRef]
- Phani, P.S.; Rao, D.S.; Joshi, S.; Sundararajan, G. Effect of process parameters and heat treatments on properties of cold sprayed copper coatings. J. Therm. Spray Technol. 2007, 16, 425–434. [Google Scholar] [CrossRef]
- Hong, R.; Kang, T.Y.; Michels, C.A.; Gadura, N. Membrane Lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 1776–1784. [Google Scholar] [CrossRef] [Green Version]
- Dupont, C.L.; Grass, G.; Rensing, C. Copper toxicity and the origin of bacterial resistance—new insights and applications. Metallomics 2011, 3, 1109–1118. [Google Scholar] [CrossRef]
- Noyce, J.; Michels, H.; Keevil, C. Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef]
- Santo, C.E.; Taudte, N.; Nies, D.H.; Grass, G. Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces. Appl. Environ. Microbiol. 2008, 74, 977–986. [Google Scholar] [CrossRef] [Green Version]
- Elguindi, J.; Moffitt, S.; Hasman, H.; Andrade, C.; Raghavan, S.; Rensing, C. Metallic copper corrosion rates, moisture content, and growth medium influence survival of copper ion-resistant bacteria. Appl. Microbiol. Biotechnol. 2010, 89, 1963–1970. [Google Scholar] [CrossRef] [Green Version]
- Mazurkow, J.M.; Yüzbasi, N.S.; Domagala, K.W.; Pfeiffer, S.; Kata, D.; Graule, T. Nano-sized copper (oxide) on alumina granules for water filtration: Effect of copper oxidation state on virus removal performance. Environ. Sci. Technol. 2020, 54, 1214–1222. [Google Scholar] [CrossRef]
- Sundberg, K.; Walde, C.; Sousa, B.; Mohanty, S.; Lee, J.-H.; Champagne, V.; Sisson, R.; Danielle, C. Microstructural characterization of conventional and nanomaterial copper cold spray coatings. J. Biotechnol. Biomater. 2020, 10. [Google Scholar]
- Sundberg, K.; Wang, Y.; Mishra, B.; Carl, A.; Grimm, R.; Te, A.; Lozeau, L.; Sisson, R.; Cote, D. The effect of corrosion on conventional and nanomaterial copper cold spray surfaces for antimicrobial applications. Biomed. J. Sci. Tech. Res. 2019, 22, 16753–16763. [Google Scholar] [CrossRef]
- Jing, H.; Yu, Z.; Li, L. Antibacterial properties and corrosion resistance of Cu and Ag/Cu porous materials. J. Biomed. Mater. Res. Part. A 2008, 87A, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Das, P.; Natan, M.; Jacobi, G.; Banin, E.; Luong, J.H.T.; Gedanken, A. Antimicrobial activities of Zn-doped CuO microparticles decorated on polydopamine against sensitive and antibiotic-resistant bacteria. ACS Appl. Polym. Mater. 2020, 2, 5878–5888. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of corrosion engineering and corrosion control. In Principles of Corrosion Engineering and Corrosion Control; Elsevier: Amsterdam, The Netherlands, 2006; pp. 438–478. [Google Scholar]
- Ghosh, M.; Roy, A.; Ghosh, A.; Kumar, H.; Saha, G. Antibacterial and antimicrobial coatings on metal substrates by cold spray technique: Present and future perspectives. In Green Approaches in Medicinal Chemistry for Sustainable Drug Design; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–45. [Google Scholar]
- Gottenbos, B.; Grijpma, D.W.; Van Der Mei, H.C.; Feijen, J.; Busscher, H.J. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 2001, 48, 7–13. [Google Scholar] [CrossRef]

| Bacteria | Source of Efficacy | Form of Copper | Reference | |
|---|---|---|---|---|
| Species | Cell Wall | |||
| Escherichia coli O157 | Gram-negative | High copper element content (>70%) | Possibly plate form | [7] |
| Acinetobacter baumannii | Gram-negative | |||
| Enterobacter spp. | Gram-negative | |||
| Klebsiella pneumoniae | Gram-negative | |||
| Pseudomonas aeruginosa | Gram-negative | |||
| Clostridium difficile | Gram-positive | |||
| Enterococcus hirae | Gram-positive | Cu(0) and growth media | Solid copper, possibly in a plate form | [8] |
| Enterococcus hirae | Gram-positive | Cu(0); Cu(I) found as effective as Cu(0) | Sheet | [9] |
| Escherichia coli (NBRC3972) | Gram-negative | Cu(I) | Powder | [12] |
| Staphylococcus aureus | Gram-positive | |||
| Escherichia coli K12 | Gram-negative | Cu(0) | Solid copper, possibly in a plate form | [10] |
| Escherichia coli (ATCC 25922) | Gram-negative | Cu(0), Cu(I) found slightly less effective as Cu(0) | Thin film, manufactured via chemical vapor deposition | [11] |
| Staphylococcus aureus (8325-4) | Gram-positive | |||
| Escherichia coli | Gram-negative | Cu(I) and Cu(II) | Copper oxides nano-particle | [13] |
| Escherichia coli (W3110) | Gram-negative | Copper ion | Solid copper, possibly in a plate form | [17] |
| Bacillus cereus L8 | Gram-positive | |||
| Deinococcus radiodurans DSM 20539 | Gram-positive | |||
| Staphylococcus aureus | Gram-positive | Oxides and surface roughness | Coating, manufactured via wire arc spray | [15] |
| Escherichia coli | Gram-negative | |||
| Staphylococcus aureus | Gram-positive | Cu(0) and Cu(I) | Coating, manufactured via wire arc spray | [14] |
| Escherichia coli | Gram-negative | |||
| P. aeruginosa | Gram-negative | |||
| Vancomycin-resistant Enterococcus | Gram-positive | |||
| Methicillin-resistant Staphylococcus aureus | Gram-positive | |||
| Staphylococcus aureus | Gram-positive | Strain-hardened particle | Deposit, manufactured via cold spray process | [16,18] |
| Escherichia coli | Gram-negative | Surface roughness | Laser patterning | [19] |
| Material | Composition (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Cr | Cu | Fe | Mn | Ni | P | S | Si | |
| Cu powder | - | - | >99.90 | - | - | - | - | - | - |
| SS304 | <0.08 | 18.16 | - | 71.54 | 1.07 | 8.21 | - | - | 0.48 |
| Al5005 | 98.89 | 0.79 | 0.223 | 0.041 | 0.033 | ||||
| Sample Code | Condition |
|---|---|
| Bulk Cu | As-received copper plate |
| Cu/SS-AD | As-deposited copper coating on SS304 substrate plate |
| Cu/SS-HT | Copper coating on SS304 substrate annealed at 400 °C for 10 min |
| Cu/Al-AD | As-deposited copper coating on Al5005 substrate plate |
| Bacteria | Time (min) | Log Reduction | |||
|---|---|---|---|---|---|
| Bulk Cu | Cu/SS-AD | Cu/SS-HT | Cu/Al-AD | ||
| S. aureus | 0 | 4.11 ± 0.17 | 2.70 ± 0.07 | 0.11 ± 0.07 | (5) |
| 15 | (5) 1 | 3.76 ± 0.10 | 1.37 ± 0.04 | (5) | |
| 30 | (5) | (5) | 1.63 ± 0.08 | (5) | |
| 45 | (5) | (5) | 1.75 ± 0.02 | (5) | |
| 60 | (5) | (5) | 2.44 ± 0.25 | (5) | |
| E. coli | 0 | (5) | 2.67 ± 0.07 | 0.13 ± 0.07 | 0.09 ± 0.05 |
| 15 | (5) | (5) | 1.39 ± 0.04 | 1.42 ± 0.17 | |
| 30 | (5) | (5) | 1.49 ± 0.08 | (5) | |
| 45 | (5) | (5) | 1.63 ± 0.02 | (5) | |
| 60 | (5) | (5) | 2.11 ± 0.25 | (5) | |
| Sample Code | S. aureus | E. coli | |||
|---|---|---|---|---|---|
| Shape | Dimensions (μm ± SD) | Shape | Dimensions (μm ± SD) | ||
| Diameter | Length | Diameter | |||
| Glass | Spherical (cocci) | 0.9 ± 0.1 | Rod | 2.2 ± 0.7 | 0.7 ± 0.1 |
| Bulk Cu | 1.0 ± 0.1 | 2.1 ± 0.4 | 0.6 ± 0.1 | ||
| Cu/SS-AD | 1.0 ± 0.1 | 2.4 ± 0.5 | 0.7 ± 0.1 | ||
| Cu/SS-HT | 0.9 ± 0.2 | 2.4 ± 0.7 | 0.6 ± 0.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutasoit, N.; Topa, S.H.; Javed, M.A.; Rahman Rashid, R.A.; Palombo, E.; Palanisamy, S. Antibacterial Efficacy of Cold-Sprayed Copper Coatings against Gram-Positive Staphylococcus aureus and Gram-Negative Escherichia coli. Materials 2021, 14, 6744. https://doi.org/10.3390/ma14226744
Hutasoit N, Topa SH, Javed MA, Rahman Rashid RA, Palombo E, Palanisamy S. Antibacterial Efficacy of Cold-Sprayed Copper Coatings against Gram-Positive Staphylococcus aureus and Gram-Negative Escherichia coli. Materials. 2021; 14(22):6744. https://doi.org/10.3390/ma14226744
Chicago/Turabian StyleHutasoit, Novana, Sanjida Halim Topa, Muhammad Awais Javed, Rizwan Abdul Rahman Rashid, Enzo Palombo, and Suresh Palanisamy. 2021. "Antibacterial Efficacy of Cold-Sprayed Copper Coatings against Gram-Positive Staphylococcus aureus and Gram-Negative Escherichia coli" Materials 14, no. 22: 6744. https://doi.org/10.3390/ma14226744
APA StyleHutasoit, N., Topa, S. H., Javed, M. A., Rahman Rashid, R. A., Palombo, E., & Palanisamy, S. (2021). Antibacterial Efficacy of Cold-Sprayed Copper Coatings against Gram-Positive Staphylococcus aureus and Gram-Negative Escherichia coli. Materials, 14(22), 6744. https://doi.org/10.3390/ma14226744







