Gel-Based Luminescent Conductive Materials and Their Applications in Biosensors and Bioelectronics
Abstract
:1. Introduction
2. Gels, Luminescent Materials, and Conductive Materials
2.1. Gels
2.2. Luminescent Materials
2.3. Conductive Materials
3. Gel-Based Luminescent Conductive Materials
3.1. Luminescent Conductive Gels
3.2. Luminescent Conductive Gel Composites
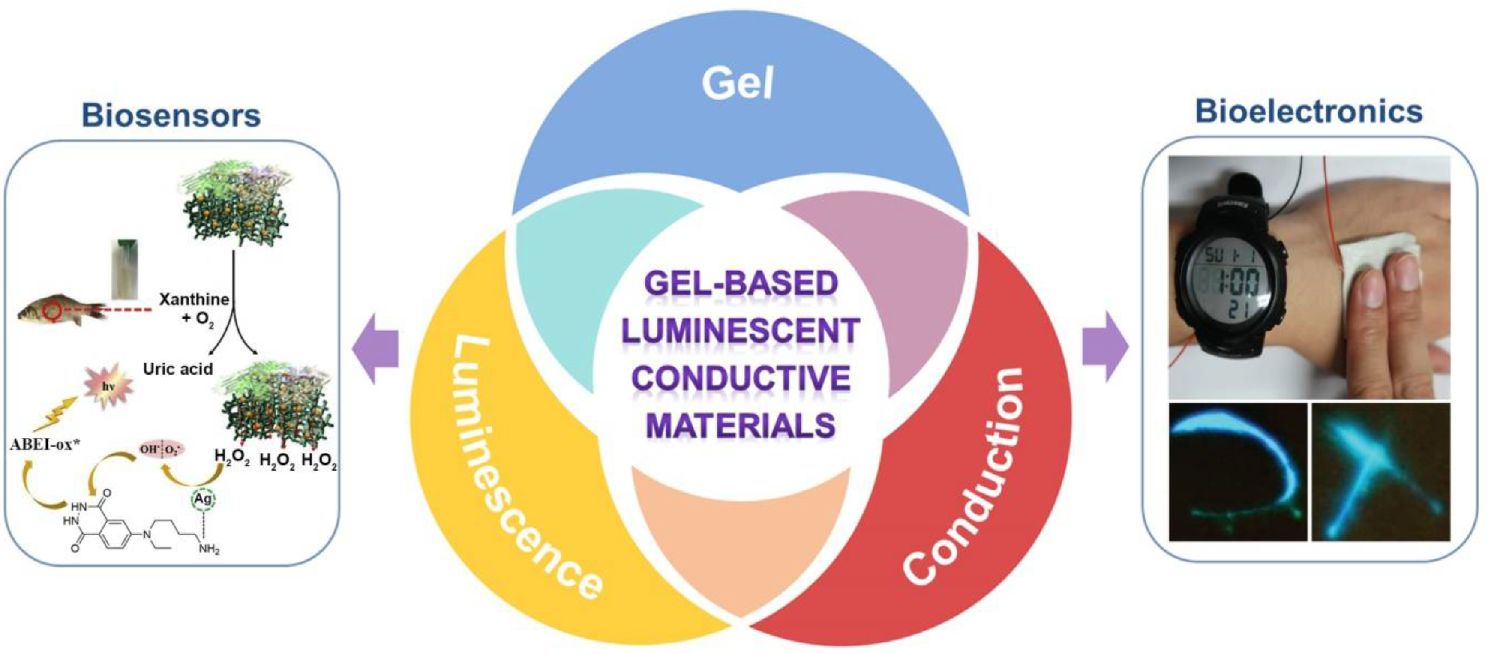
4. Applications in Biosensors and Bioelectronics
4.1. Biosensors
4.2. Bioelectronics
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, Z.; Lu, Y.-C.; Lai, J.J. Recent Advances in Biosensors for Nucleic Acid and Exosome Detection. Chonnam Med. J. 2019, 55, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Pohanka, M.; Skladal, P.; Kroea, M. Biosensors for biological warfare agent detection. Def. Sci. J. 2007, 57, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Yao, G.; Yin, C.; Wang, Q.; Zhang, T.; Chen, S.; Lu, C.; Zhao, K.; Xu, W.; Pan, T.; Gao, M.; et al. Flexible bioelectronics for physiological signals sensing and disease treatment. J. Mater. 2020, 6, 397–413. [Google Scholar] [CrossRef]
- Parlak, O.; Turner, A.P.F. Switchable bioelectronics. Biosens. Bioelectron. 2016, 76, 251–265. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; De Oliveira, N.; Wang, S. Implantable bioelectronics toward long-term stability and sustainability. Matter 2021, 4, 1125–1141. [Google Scholar] [CrossRef]
- Zavanelli, N.; Kim, J.; Yeo, W.-H. Recent Advances in High-Throughput Nanomaterial Manufacturing for Hybrid Flexible Bioelectronics. Materials 2021, 14, 2973. [Google Scholar] [CrossRef]
- Löffler, S.; Libberton, B.; Richter-Dahlfors, A. Organic Bioelectronic Tools for Biomedical Applications. Electronics 2015, 4, 879–908. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.Z.; Zhang, M.; Yao, M.Y.; Hua, T.; Li, L.; Yan, F. Flexible Organic Electronics in Biology: Materials and Devices. Adv. Mater. 2015, 27, 7493–7527. [Google Scholar] [CrossRef]
- Liu, Y.X.; Feig, V.R.; Bao, Z.A. Conjugated Polymer for Implantable Electronics toward Clinical Application. Adv. Healthc. Mater. 2021, 10, 2001916. [Google Scholar] [CrossRef]
- Fidanovski, K.; Mawad, D. Conjugated Polymers in Bioelectronics: Addressing the Interface Challenge. Adv. Healthc. Mater. 2019, 8, 1900053. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, K.; Xu, H.; Li, T.; Jin, Q.; Cui, D. Recent developments in sensors for wearable device applications. Anal. Bioanal. Chem. 2021, 413, 6037–6057. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Song, S.; Xu, H.; Fan, C.H. Potential diagnostic applications of biosensors: Current and future directions. Int. J. Nanomed. 2006, 1, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Sunwoo, S.H.; Ha, K.H.; Lee, S.; Lu, N.; Kim, D.H. Wearable and Implantable Soft Bioelectronics: Device Designs and Material Strategies. Annu. Rev. Chem. Biomol. 2021, 12, 359–391. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wu, S.J.; Qu, J.; Gong, L.; Tang, J.X. A Review of Conductive Hydrogel Used in Flexible Strain Sensor. Materials 2020, 13, 3947. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.R.; Xu, T.L.; Zhang, X.J. Multifunctional conductive hydrogel-based flexible wearable sensors. TrAC Trends Anal. Chem. 2021, 134, 116130. [Google Scholar] [CrossRef]
- Fu, F.F.; Wang, J.L.; Zeng, H.B.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Lei, K.; Li, Z.; Zhu, D.D.; Sun, C.Y.; Sun, Y.L.; Yang, C.C.; Zheng, Z.; Wang, X.L. Polysaccharide-based recoverable double-network hydrogel with high strength and self-healing properties. J. Mater. Chem. B 2020, 8, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.F.; Li, Z.; Liu, X.L.; Peng, H.Q.; Song, F.Y.; Qi, J.; Lam, J.W.Y.; Long, L.L.; Sessler, J.L.; Tang, B.Z. A Functioning Macroscopic “Rubik’s Cube” Assembled via Controllable Dynamic Covalent Interactions. Adv. Mater. 2019, 31, 1902365. [Google Scholar] [CrossRef]
- Xiong, L.; Feng, J.; Hu, R.; Wang, S.Q.; Li, S.Y.; Li, Y.; Yang, G.Q. Sensing in 15 s for Aqueous Fluoride Anion by Water-Insoluble Fluorescent Probe Incorporating Hydrogel. Anal. Chem. 2013, 85, 4113–4119. [Google Scholar] [CrossRef]
- An, R.; Zhang, X.Y.; Han, L.L.; Wang, X.D.; Zhang, Y.L.; Shi, L.Y.; Ran, R. Healing, flexible, high thermal sensitive dual-network ionic conductive hydrogels for 3D linear temperature sensor. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110310. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, J.; Pan, L.J.; Shi, Y.; Yu, G.H. Energy gels: A bio-inspired material platform for advanced energy applications. Nano Today 2016, 11, 738–762. [Google Scholar] [CrossRef] [Green Version]
- Kageyama, R.; Kagami, S.; Inaba, M.; Inoue, H. A soft tactile sensor made of conductive-gel and its applications. Adv. Robot. 1999, 13, 301–302. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.C.; Ji, X.F.; Gong, J.Y.; Hu, Y.B.; Wu, W.J.; Wang, X.N.; Peng, H.Q.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Bioinspired Simultaneous Changes in Fluorescence Color, Brightness, and Shape of Hydrogels Enabled by AIEgens. Adv. Mater. 2020, 32, 1906493. [Google Scholar] [CrossRef]
- Xu, L.H.; Li, J.J.; Zeng, H.B.; Zhang, X.J.; Cosnier, S.; Marks, R.S.; Shan, D. ATMP-induced three-dimensional conductive polymer hydrogel scaffold for a novel enhanced solid-state electrochemiluminescence biosensor. Biosens. Bioelectron. 2019, 143, 111601. [Google Scholar] [CrossRef]
- Liang, G.J.; Ruan, Z.H.; Liu, Z.X.; Li, H.F.; Wang, Z.F.; Tang, Z.J.; Mo, F.N.; Yang, Q.; Ma, L.T.; Wang, D.H.; et al. Toward Multifunctional and Wearable Smart Skins with Energy-Harvesting, Touch-Sensing, and Exteroception-Visualizing Capabilities by an All-Polymer Design. Adv. Electron. Mater. 2019, 5, 1900553. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Witzmann, F.A.; Bai, F.J.; Hong, S.M.; Liu, S.; Pedrick, N.; Ringham, H.; Tan, J. Gels and more gels: Probing toxicity. Curr. Opin. Mol. Ther. 2004, 6, 608–615. [Google Scholar] [PubMed]
- Clark, A.H. Biopolymer gels. Curr. Opin. Colloid Interface Sci. 1996, 1, 712–717. [Google Scholar] [CrossRef]
- Scherer, G.W. Drying gels. 5. Rigid gels. J. Non-Cryst. Solids 1987, 92, 122–144. [Google Scholar]
- Flory, P.J. Gels and gelling processes—Introduction. Faraday Discuss. 1975, 57, 7–18. [Google Scholar] [CrossRef]
- Li, Z.; Su, S.; Yu, L.; Zheng, Z.; Wang, X.L. Preparation of a photo- and thermo-responsive topological gel from anthracene-modified polyrotaxanes. Soft Matter 2018, 14, 2767–2771. [Google Scholar] [CrossRef] [PubMed]
- Teale, F.W.J. Exciting stuff. Nature 1984, 307, 486. [Google Scholar] [CrossRef]
- Thomas, D.G. Electroluminescence. Phys. Today 1968, 21, 43. [Google Scholar] [CrossRef]
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef]
- Hastings, J.W.; Johnson, C.H. [3] Bioluminescence and chemiluminescence. In Methods Enzymology; Marriott, G., Parker, I., Eds.; Elsvier Inc.: Amsterdam, The Netherlands, 2003; Volume 360, pp. 75–104. [Google Scholar]
- Rossello, J.M.; Dellavale, D.; Bonetto, F.J. Stable tridimensional bubble clusters in multi-bubble sonoluminescence (MBSL). Ultrason. Sonochemistry 2015, 22, 59–69. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, Q. Transforming the behavior of conventional chromophores from aggregation-caused quenching to aggregation-induced emission. J. Funct. Mater. 2015, 46, 14001–14011. [Google Scholar]
- Cai, X.L.; Liu, B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef]
- Omote, Y.; Miyake, T.; Ohmori, S.; Sugiyama, N. Chemiluminescence of luminol and acetyl-luminol. Bull. Chem. Soc. Jpn. 1967, 40, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.W.; Guo, Z.H.; Zhang, Z.J. Flow-injection electrogenerated chemiluminescence determination of epinephrine using luminol. Anal. Chim. Acta 2001, 441, 81–86. [Google Scholar] [CrossRef]
- Hall, C.; Perutz, R.N. Transition metal alkane complexes. Chem. Rev. 1996, 96, 3125–3146. [Google Scholar] [CrossRef]
- Zhao, Q.; Huang, C.H.; Li, F.Y. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.L.; He, H.Z.; Leung, K.H.; Chan, D.S.H.; Leung, C.H. Bioactive Luminescent Transition-Metal Complexes for Biomedical Applications. Angew. Chem. Int. Ed. 2013, 52, 7666–7682. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Chiu, Y.C.; Chi, Y.; Cheng, Y.M.; Lai, C.H.; Chou, P.T.; Wong, K.T.; Tsai, M.H.; Wu, C.C. Phosphorescent iridium(III) complexes with nonconjugated cyclometalated ligands. Chem. A Eur. J. 2008, 14, 5423–5434. [Google Scholar] [CrossRef]
- Borgstrom, M.; Ott, S.; Lomoth, R.; Bergquist, J.; Hammarstrom, L.; Johansson, O. Photoinduced energy transfer coupled to charge separation in a Ru(II)-Ru(II)-acceptor triad. Inorg. Chem. 2006, 45, 4820–4829. [Google Scholar] [CrossRef]
- Kumar, P.; Soumya, S.; Prasad, E. Enhanced Resonance Energy Transfer and White-Light Emission from Organic Fluorophores and Lanthanides in Dendron-based Hybrid Hydrogel. ACS Appl. Mater. Interfaces 2016, 8, 8068–8075. [Google Scholar] [CrossRef] [PubMed]
- Bekiari, V.; Thiakou, K.A.; Raptopoulou, C.P.; Perlepes, S.P.; Lianos, P. Structure and photophysical behavior of 2,2′-bipyrimidine/lanthanide ion complexes in various environments. J. Lumin. 2008, 128, 481–488. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum Dots in Bioanalysis: A Review of Applications Across Various Platforms for Fluorescence Spectroscopy and Imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [Green Version]
- Coe-Sullivan, S.; Liu, W.H.; Allen, P.; Steckel, J.S. Quantum Dots for LED Downconversion in Display Applications. ECS J. Solid State Sci. Technol. 2013, 2, R3026–R3030. [Google Scholar] [CrossRef]
- Fang, Y.X.; Guo, S.J.; Li, D.; Zhu, C.Z.; Ren, W.; Dong, S.J.; Wang, E.K. Easy Synthesis and Imaging Applications of Cross-Linked Green Fluorescent Hollow Carbon Nanoparticles. ACS Nano 2012, 6, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable Preparation of Supported Metal Nanoparticles and Their Applications in Catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, S.; Hadjipanayis, G.C.; Sorensen, C.M.; Klabunde, K.J. Effect of particle-size and surface-chemistry on the interactions among fine metallic particles. IEEE Trans. Magn. 1993, 29, 2619–2621. [Google Scholar] [CrossRef]
- Orbach, R.; Guo, W.W.; Wang, F.; Lioubashevski, O.; Willner, I. Self-Assembly of Luminescent Ag Nanocluster-Functionalized Nanowires. Langmuir 2013, 29, 13066–13071. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.W.; Yuan, J.P.; Dong, Q.Z.; Wang, E.K. Highly Sequence-Dependent Formation of Fluorescent Silver Nanoclusters in Hybridized DNA Duplexes for Single Nucleotide Mutation Identification. J. Am. Chem. Soc. 2010, 132, 932–934. [Google Scholar] [CrossRef]
- Jin, R.C. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Liu, P.R.; Cheng, Y.B.; Zhao, H.J.; Yang, H.G. Functionalization of perovskite thin films with moisture-tolerant molecules. Nat. Energy 2016, 1, 15016. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Q.; Zhong, F. Progress in Research of Inorganic-Organic Hybrid Materials. Petrochem. Technol. 2008, 37, 649–655. [Google Scholar]
- Zhu, Y.C.; Bando, Y.; Uemura, Y. ZnS-Zn nanocables and ZnS nanotubes. Chem. Commun. 2003, 836–837. [Google Scholar] [CrossRef]
- Borodina, T.A.; Minakova, T.S. Quantum yield of oxygen photoadsorption on ZNS, ZNS-CU and ZNS-AG. Zhurnal Fiz. Khimii 1978, 52, 1203–1205. [Google Scholar]
- Han, H. Preparation of fast ionic conductors and its application. New Chem. Mater. 2003, 31, 22–25. [Google Scholar]
- Keplinger, C.; Sun, J.Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z.G. Stretchable, Transparent, Ionic Conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Wang, R.; Li, Z.; Deng, Y. Advances of Ionic Liquid Application in Green Catalysis and Clean Synthesis. Petrochem. Technol. 2007, 36, 975–984. [Google Scholar]
- Qian, J.; Li, X.; Luan, H.; Li, H.; Li, P. Electrochemical Properties of Ionic Liquids and Solubility of IrCl_3. Chin. J. Rare Met. 2015, 39, 136–143. [Google Scholar]
- Kausar, A. Research Progress in Frontiers of Poly(Ionic Liquid)s: A Review. Polym.-Plast. Technol. Eng. 2017, 56, 1823–1838. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Wang, X.L.; Tu, J.P. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: Challenges, opportunities, and future vision. J. Mater. Chem. A 2017, 5, 8209–8229. [Google Scholar] [CrossRef]
- Hu, P.; Jiang, W.; Zhong, L. Study on properties of deep eutectic solvents and their applications. Mod. Chem. Ind. 2018, 38, 53–57. [Google Scholar]
- Feng, X.D.; Kawabata, K.; Cowan, M.G.; Dwulet, G.E.; Toth, K.; Sixdenier, L.; Haji-Akbari, A.; Noble, R.D.; Elimelech, M.; Gin, D.L.; et al. Single crystal texture by directed molecular self-assembly along dual axes. Nat. Mater. 2019, 18, 1235–1243. [Google Scholar] [CrossRef]
- Kumar, S. Self-organization of disc-like molecules: Chemical aspects. Chem. Soc. Rev. 2006, 35, 83–109. [Google Scholar] [CrossRef]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-Tech Applications of Self-Assembling Supramolecular Nanostructured Gel-Phase Materials: From Regenerative Medicine to Electronic Devices. Angew. Chem. Int. Ed. 2008, 47, 8002–8018. [Google Scholar] [CrossRef] [PubMed]
- Szollosi, A.; Hoschke, A.; Rezessy-Szabo, J.M.; Bujna, E.; Kun, S.; Nguyen, Q.D. Formation of novel hydrogel bio-anode by immobilization of biocatalyst in alginate/polyaniline/titanium-dioxide/graphite composites and its electrical performance. Chemosphere 2017, 174, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Patel, M.; Koh, W.G. Incorporation of Conductive Materials into Hydrogels for Tissue Engineering Applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.H.; Cooper, R.C.; An, S.J.; Lee, S.; van der Zande, A.; Petrone, N.; Hammerherg, A.G.; Lee, C.; Crawford, B.; Oliver, W.; et al. High-Strength Chemical-Vapor Deposited Graphene and Grain Boundaries. Science 2013, 340, 1073–1076. [Google Scholar] [CrossRef]
- Xu, Y.X.; Sheng, K.X.; Li, C.; Shi, G.Q. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, H.; Ding, L.; Wu, C. Research progress of a new organic conductive and luminescent material poly(3,4-ethylenedioxythiophene) (PEDOT). New Chem. Mater. 2005, 33, 17–20. [Google Scholar]
- Masi, J.V. New developments in polymers: Conductive and active (magnetic, luminescent and electronic) applications. In Proceedings of the Electrical Insulation Conference and Electrical Manufacturing Expo, Indianapolis, IN, USA, 23–26 October 2005; pp. 299–306. [Google Scholar]
- Zhou, Y.; Wan, C.J.; Yang, Y.S.; Yang, H.; Wang, S.C.; Dai, Z.D.; Ji, K.J.; Jiang, H.; Chen, X.D.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Wei, S.X.; Li, Z.; Lu, W.; Liu, H.; Zhang, J.W.; Chen, T.; Tang, B.Z. Multicolor Fluorescent Polymeric Hydrogels. Angew. Chem. Int. Ed. 2021, 60, 8608–8624. [Google Scholar] [CrossRef]
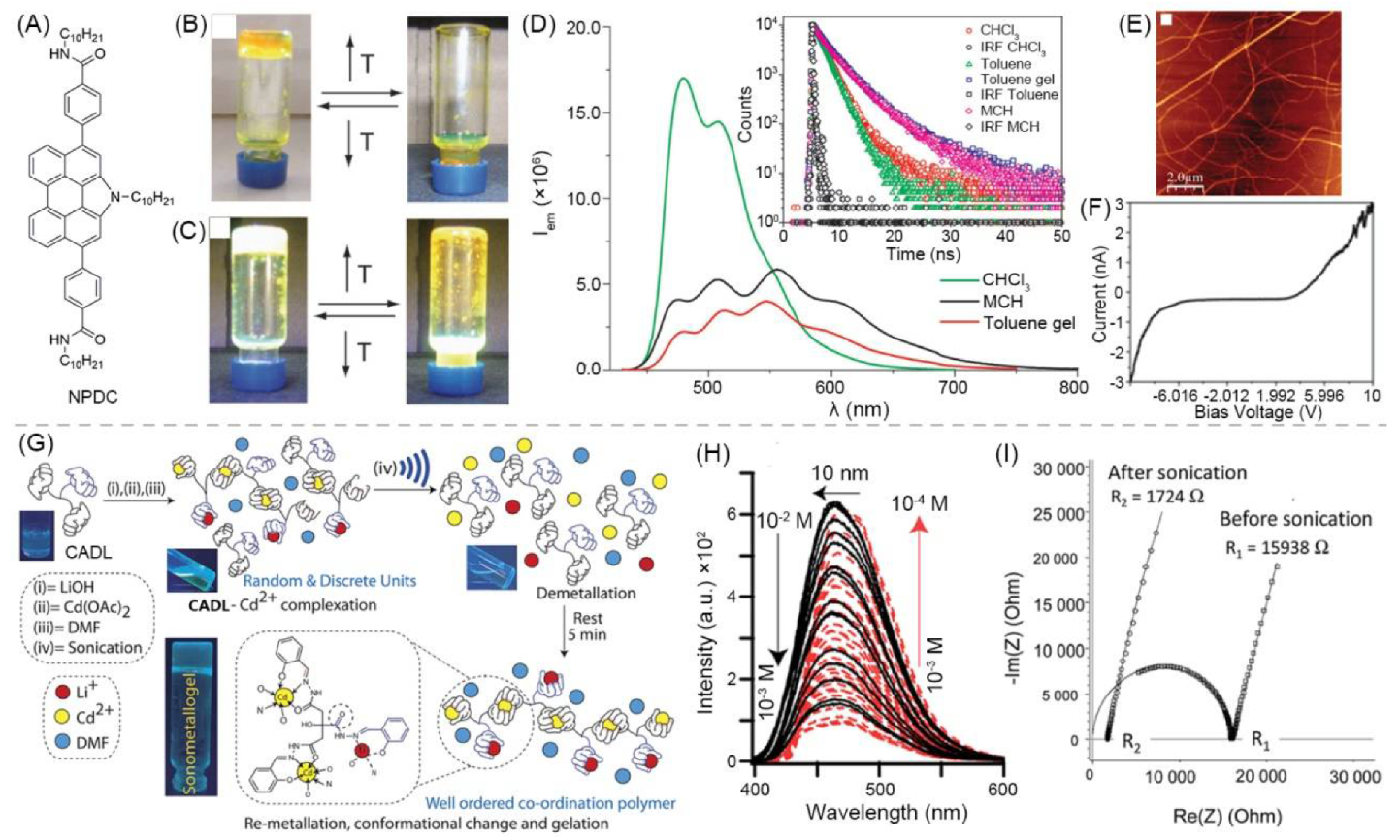
- Garcia, F.; Buendia, J.; Ghosh, S.; Ajayaghosh, A.; Sanchez, L. Luminescent and conductive supramolecular polymers obtained from an N-annulated perylenedicarboxamide. Chem. Commun. 2013, 49, 9278–9280. [Google Scholar] [CrossRef]
- Pandey, V.K.; Dixit, M.K.; Manneville, S.; Bucher, C.; Dubey, M. A multi-stimuli responsive conductive sonometallogel: A mechanistic insight into the role of ultrasound in gelation. J. Mater. Chem. A 2017, 5, 6211–6218. [Google Scholar] [CrossRef]
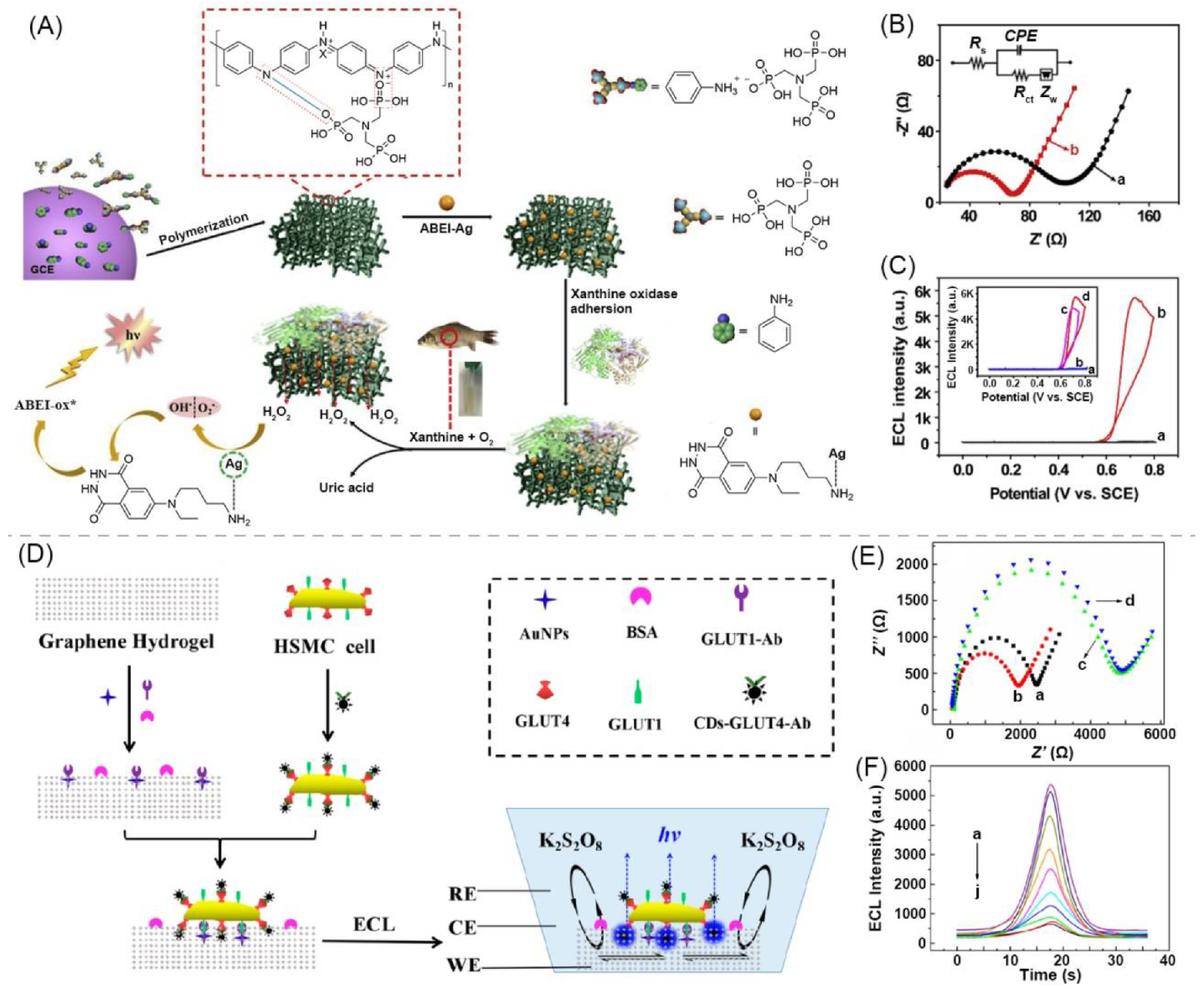
- Liu, G.; Ma, C.; Jin, B.K.; Chen, Z.X.; Cheng, F.L.; Zhu, J.J. Electrochemiluminescence Investigation of Glucose Transporter 4 Expression at Skeletal Muscle Cells Surface Based on a Graphene Hydrogel Electrode. Anal. Chem. 2019, 91, 3021–3026. [Google Scholar] [CrossRef]
- Mohanan, V.V.; Pradhan, B.; Sridurai, V.; Yelamaggad, C.V.; Achalkumar, A.S.; Nair, G.G. Giant enhancement and facile tuning of photoluminescence in a soft anisotropic magneto-gel. Nanoscale 2018, 10, 15686–15695. [Google Scholar] [CrossRef]
- Tong, X.; Zhao, Y.; An, B.K.; Park, S.Y. Fluorescent liquid-crystal gels with electrically switchable photoluminescence. Adv. Funct. Mater. 2006, 16, 1799–1804. [Google Scholar] [CrossRef]
- Tong, X.; Chung, J.W.; Park, S.Y.; Zhao, Y. Self-Assembled Liquid-Crystal Gels in an Emulsion. Langmuir 2009, 25, 8532–8537. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Bhattacharya, S. Aggregation induced emission switching and electrical properties of chain length dependent pi-gels derived from phenylenedivinylene bis-pyridinium salts in alcohol-water mixtures. J. Mater. Chem. 2012, 22, 25277–25287. [Google Scholar] [CrossRef]
- De, J.; Devi, M.; Shah, A.; Gupta, S.P.; Bala, I.; Singh, D.P.; Douali, R.; Pal, S.K. Luminescent Conductive Columnar pi-Gelators for Fe(II) Sensing and Bio-Imaging Applications. J. Phys. Chem. B 2020, 124, 10257–10265. [Google Scholar] [CrossRef]
- Xue, P.C.; Lu, R.; Zhao, L.; Xu, D.F.; Zhang, X.F.; Li, K.C.; Song, Z.G.; Yang, X.C.; Takafuji, M.; Ihara, H. Hybrid Self-Assembly of a pi Gelator and Fullerene Derivative with Photoinduced Electron Transfer for Photocurrent Generation. Langmuir 2010, 26, 6669–6675. [Google Scholar] [CrossRef]
- Dixit, M.K.; Chery, D.; Mahendar, C.; Bucher, C.; Dubey, M. Nanofabrication of Au nanoparticles over conductive metallohydrogel nanofibers for nanocatalysis application. Inorg. Chem. Front. 2020, 7, 991–1002. [Google Scholar] [CrossRef]
- Shukla, J.; Kumar, Y.; Dixit, M.K.; Mahendar, C.; Sharma, V.K.; Kalam, A.; Dubey, M. Investigation of the Mechanism Behind Conductive Fluorescent and Multistimuli-responsive Li+-enriched Metallogel Formation. Chem. Asian J. 2020, 15, 3020–3028. [Google Scholar] [CrossRef]
- Mahendar, C.; Kumar, Y.; Dixit, M.K.; Mukherjee, M.; Kalam, A.; Dubey, M. Conductive Zn(ii)-metallohydrogels: The role of alkali metal cation size in gelation, rheology and conductance. Mol. Syst. Des. Eng. 2021, 6, 654–661. [Google Scholar] [CrossRef]
- Le, N.H.; Seema, H.; Kemp, K.C.; Ahmed, N.; Tiwari, J.N.; Park, S.; Kim, K.S. Solution-processable conductive micro-hydrogels of nanoparticle/graphene platelets produced by reversible self-assembly and aqueous exfoliation. J. Mater. Chem. A 2013, 1, 12900–12908. [Google Scholar] [CrossRef]
- Hao, N.; Zhang, X.; Zhou, Z.; Hua, R.; Zhang, Y.; Liu, Q.; Qian, J.; Li, H.; Wang, K. AgBr nanoparticles/3D nitrogen-doped graphene hydrogel for fabricating all-solid-state luminol-electrochemiluminescence Escherichia coli aptasensors. Biosens. Bioelectron. 2017, 97, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Wang, H.J.; Yuan, R.; Chai, Y.Q. Functional Three-Dimensional Porous Conductive Polymer Hydrogels for Sensitive Electrochemiluminescence In Situ Detection of H2O2 Released from Live Cells. Anal. Chem. 2018, 90, 8462–8469. [Google Scholar] [CrossRef]
- Ding, C.F.; Li, Y.X.; Wang, L.; Luo, X.L. Ratiometric Electrogenerated Chemiluminescence Cytosensor Based on Conducting Polymer Hydrogel Loaded with Internal Standard Molecules. Anal. Chem. 2019, 91, 983–989. [Google Scholar] [CrossRef]
- Han, C.Y.; Guo, W.W. Fluorescent Noble Metal Nanoclusters Loaded Protein Hydrogel Exhibiting Anti-Biofouling and Self-Healing Properties for Electrochemiluminescence Biosensing Applications. Small 2020, 16, 2002621. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qin, Z.J.; Zheng, Y.K.; Liu, L.; Wang, X.M. Aggregation-Induced Electrochemiluminescence by Metal-Binding Protein Responsive Hydrogel Scaffolds. Small 2019, 15, 1901170. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Huang, C.Z.; Li, Y.F. Silver-based metal-organic gels as novel coreactant for enhancing electrochemiluminescence and its biosensing potential. Biosens. Bioelectron. 2019, 134, 29–35. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Liu, W.S.; Chen, J.S.; Niu, H.L.; Mao, C.J.; Jin, B.K. Metal-organic gel and metal-organic framework based switchable electrochemiluminescence RNA sensing platform for Zika virus. Sens. Actuators B Chem. 2020, 321, 128456. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, M.H.; Li, C.C.; Wang, Q.B.; Luo, X.L.; Zhang, C.Y. A Host-Guest Interaction-Based and Metal-Organic Gel-Based Biosensor with Aggregation-Induced Electrochemiluminescence Enhancement for Methyltransferase Assay. Anal. Chem. 2021, 93, 2974–2981. [Google Scholar] [CrossRef]
- Wang, C.; Han, Q.; Liu, P.K.; Zhang, G.; Song, L.; Zou, X.C.; Fu, Y.Z. A Superstable Luminescent Lanthanide Metal Organic Gel Utilized in an Electrochemiluminescence Sensor for Epinephrine Detection with a Narrow Potential Sweep Range. ACS Sens. 2021, 6, 252–258. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, Y.X.; Zhang, Q.; Liang, Z.H.; Wang, P.L.; Ma, Q. Polydopamine nanoparticles@MoS2 nanosheet aerogel-based ECL sensing system for MiRNA-126 detection. Chem. Eng. J. 2021, 411, 128428. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, Y.M.; Moon, H.C. Porous Ion Gel: A Versatile Ionotronic Sensory Platform for High-Performance, Wearable Ionoskins with Electrical and Optical Dual Output. ACS Nano 2021, 15, 15132–15141. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.C.; Lodge, T.P.; Frisbie, C.D. Electrochemiluminescent displays based on ion gels: Correlation between device performance and choice of electrolyte. J. Mater. Chem. C 2016, 4, 8448–8453. [Google Scholar] [CrossRef]
- Berestetsky, N.; Vaganova, E.; Wachtel, E.; Leitus, G.; Goldberg, A.; Yitzchaik, S. Photoactive proton conductor: Poly(4-vinyl pyridine) gel. J. Phys. Chem. B 2008, 112, 3662–3667. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.C.; Zhao, J.; Li, X.W.; Li, C.Z.; Yuan, W.K. Synthesis and characterization of electrically conductive and fluorescent poly(N-5-(8-hydroxyquinoline)methyl aniline)/V2O5 xerogel hybrids. Synth. Met. 2009, 159, 366–371. [Google Scholar] [CrossRef]
- Xie, Z.L.; Xu, H.B.; Gessner, A.; Kumke, M.U.; Priebe, M.; Fromm, K.M.; Taubert, A. A transparent, flexible, ion conductive, and luminescent PMMA ionogel based on a Pt/Eu bimetallic complex and the ionic liquid Bmim N(Tf)(2). J. Mater. Chem. 2012, 22, 8110–8116. [Google Scholar] [CrossRef] [Green Version]
- Gokceoren, A.T.; Alveroglu, E. Synthesis and investigation of poly(N-isopropylacrylamide-co-N-vinylcarbazole) hydrogels morphological, fluorescence and electrical properties. J. Mol. Struct. 2016, 1108, 25–32. [Google Scholar] [CrossRef]
- Chen, F.; Tang, Z.Q.; Lu, S.P.; Zhu, L.; Wang, Q.L.; Gang, Q.; Yang, J.; Chen, Q. Fabrication and mechanical behaviors of novel supramolecular/polymer hybrid double network hydrogels. Polymer 2019, 168, 159–167. [Google Scholar] [CrossRef]
- Liu, H.T.; Huang, C.L.; Liou, G.S. Design, Synthesis, and Electrofluorochromism of New Triphenylamine Derivatives with AIE-Active Pendent Groups. ACS Appl. Mater. Interfaces 2019, 11, 11684–11690. [Google Scholar] [CrossRef]
- Cho, K.G.; Lee, J.I.; Lee, S.J.; Hong, K.Y.; Kang, M.S.; Lee, K.H. Light-Emitting Devices Based on Electrochemiluminescence Gels. Adv. Funct. Mater. 2020, 30, 1907936. [Google Scholar] [CrossRef]
- Nobeshima, T.; Nakamura, K.; Kobayashi, N. Electrochemical Materials for Novel Light Emitting Device and Dual Mode Display. J. Photopolym. Sci. Technol. 2013, 26, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Qi, W.J.; Xu, G.B. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015, 44, 3117–3142. [Google Scholar] [CrossRef] [Green Version]
- Daimon, T.; Nihei, E. Fabrication of organic electrochemiluminescence devices with pi-conjugated polymer materials. J. Mater. Chem. C 2013, 1, 2826–2833. [Google Scholar] [CrossRef]
- Park, J.M.; Park, J.; Kim, Y.H.; Zhou, H.; Lee, Y.; Jo, S.H.; Ma, J.; Lee, T.W.; Sun, J.Y. Aromatic nonpolar organogels for efficient and stable perovskite green emitters. Nat. Commun. 2020, 11, 4638. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.J.; Liu, Z.X.; Mo, F.N.; Tang, Z.J.; Li, H.F.; Wang, Z.F.; Sarangi, V.; Pramanick, A.; Fan, J.; Zhi, C.Y. Self-healable electroluminescent devices. Light Sci. Appl. 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.W.; Yuan, W. Highly Stretchable and Transparent Double-Network Hydrogel Ionic Conductors as Flexible Thermal-Mechanical Dual Sensors and Electroluminescent Devices. ACS Appl. Mater. Interfaces 2019, 11, 16765–16775. [Google Scholar] [CrossRef]
- Yin, T.H.; Wu, L.; Wu, T.H.; Mao, G.Y.; Nian, G.D.; Chen, Z.; Hu, X.C.; Wang, P.; Xiang, Y.H.; Yu, H.H.; et al. Ultrastretchable and conductive core/sheath hydrogel fibers with multifunctionality. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 272–280. [Google Scholar] [CrossRef]
- Kwon, D.K.; Myoung, J.M. Wearable and Semitransparent Pressure-Sensitive Light-Emitting Sensor Based on Electrochemiluminescence. ACS Nano 2020, 14, 8716–8723. [Google Scholar] [CrossRef]
- Kimura, M.; Miki, N.; Suzuki, D.; Adachi, N.; Tatewaki, Y.; Shirai, H. Wrapping of Self-Organized Fluorescent Nanofibers with a Silica Wall. Langmuir 2009, 25, 776–780. [Google Scholar] [CrossRef]


| No. | Gel Type | Network a | Dispersing Medium b | Conductive Species | Electrical Parameter | Luminescent Species | λex/λem (nm) c | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | supramolecular bulk organogel | 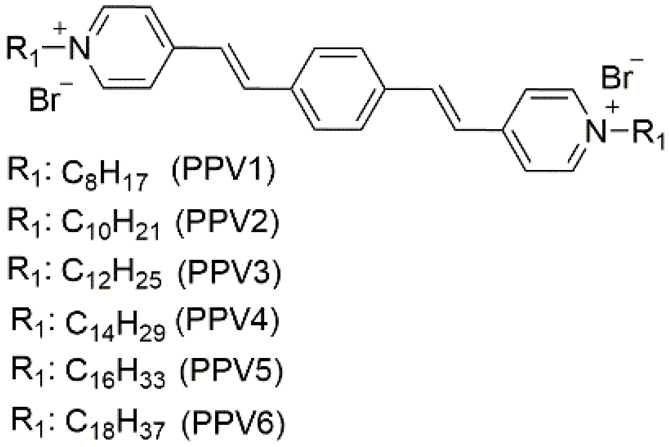 | AN (PPV4), EG (PPV3–5), EtOH/H2O (PPV1–7), MeOH/H2O (PPV3–5), PrOH/H2O (PPV3–5), t-BuOH/H2O (PPV3–5) | PPV1–7 | Resistance (Ω) (10V) (xerogel): 1.1 × 107 (PPV1) 3.5 × 107 (PPV2) 4.7 × 107 (PPV3) 9.1 × 107 (PPV4) 2.7 × 107 (PPV5) 4.0 × 107 (PPV6) | PPV1–7 | 470/~600 (PPV4) (EtOH/H2O) | - | [87] |
| 2 | supramolecular bulk organogel | 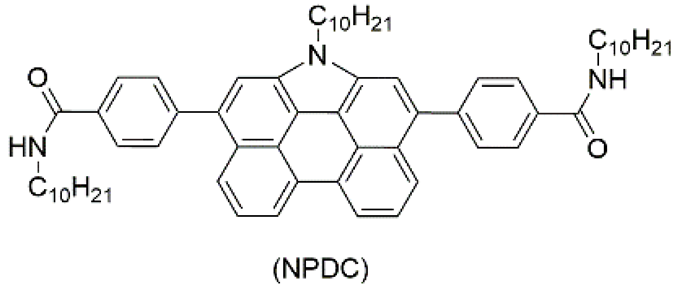 | toluene | DNPC | Conductivity (S·cm−1): 2.16 × 10−6 (C-AFM), 1.92 × 10−6 (FPC) | DNPC | 420/~546 | - | [81] |
| 3 | supramolecular bulk organogel |  | hexane, decane, dodecane, hexadecane | PPV1–5 | Hole mobility (cm2·V−1·s−1) (80 V): 3.07 × 10−3 (TPV2); 3.19 × 10−3 (TPV3); 3.30 × 10−3 (TPV4); 3.47 × 10−3 (TPV5) | PPV1–5 | -/~433 | Fe2+ sensing, pollen grains imaging | [88] |
| 4 | supramolecular bulk xerogel | 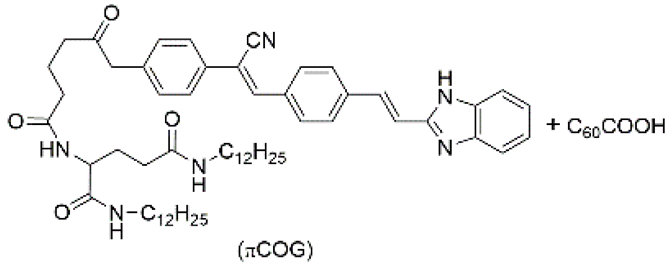 | air | πCOG, C60COOH | Photocurrent (μA): ~4.5 | πCOG | -/~530 | - | [89] |
| 5 | supramolecular bulk organogel | 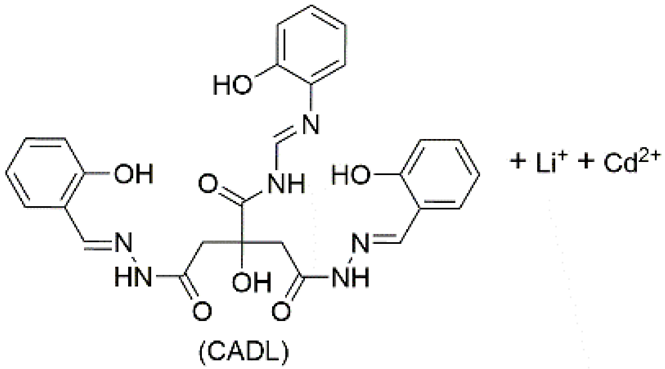 | DMF | Li+, Cd2+, OH−, CH3COO−, CADL | Conductivity (S cm−1): 4.06 × 10−4 Resistance (Ω): 1724 | CADL | 378/~475 | - | [82] |
| 6 | supramolecular bulk hydrogel | 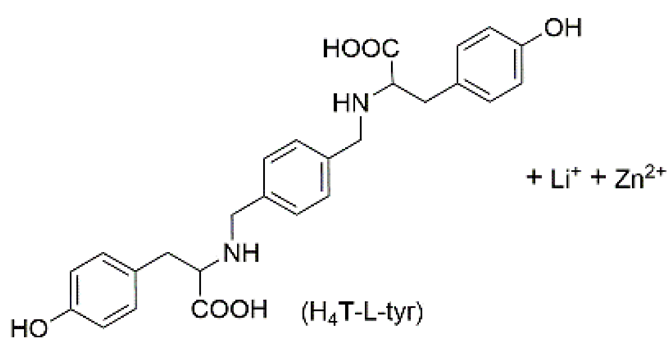 | H2O | Li+, Zn2+, OH−, NO3−, H4T–L–tyr, Au nanoparticle (NP) | Conductivity (S·cm−1): 5.05 × 10−3 | H4T–L–tyr | -/~420 | catalysis | [90] |
| 7 | supramolecular bulk organogel | 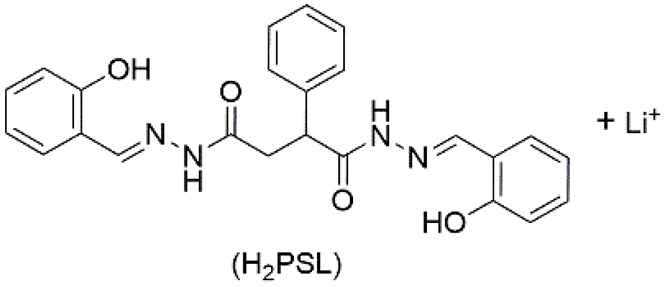 | DMF | Li+, OH−, H2PSL | Resistance (Ω): 7.4 × 103 | H2PSL | 320/~472 | - | [91] |
| 8 | supramolecular bulk hydrogel |  | H2O | M+, Zn2+, OH−, NO3−, o–H4TEP | Conductivity (S cm−1): 1.36 × 10−2 (MH–Li); 1.44 × 10−2 (MH–Na); 1.53 × 10−2 (MH–K); 1.60 × 10−2 (MH–Cs) Resistance (Ω): 237 (MH–Li); 137(MH–Na); 92 (MH–K); 75 (MH–Cs) | o–H4TEP | 363/~500 | - | [92] |
| 9 | supramolecular micro-hydrogel | SnO2 + chemically converted graphene (CCG) | H2O | CCG | Conductivity (S·cm−1): 350 | rhodamine B | 555/~573 | DNA detection | [93] |
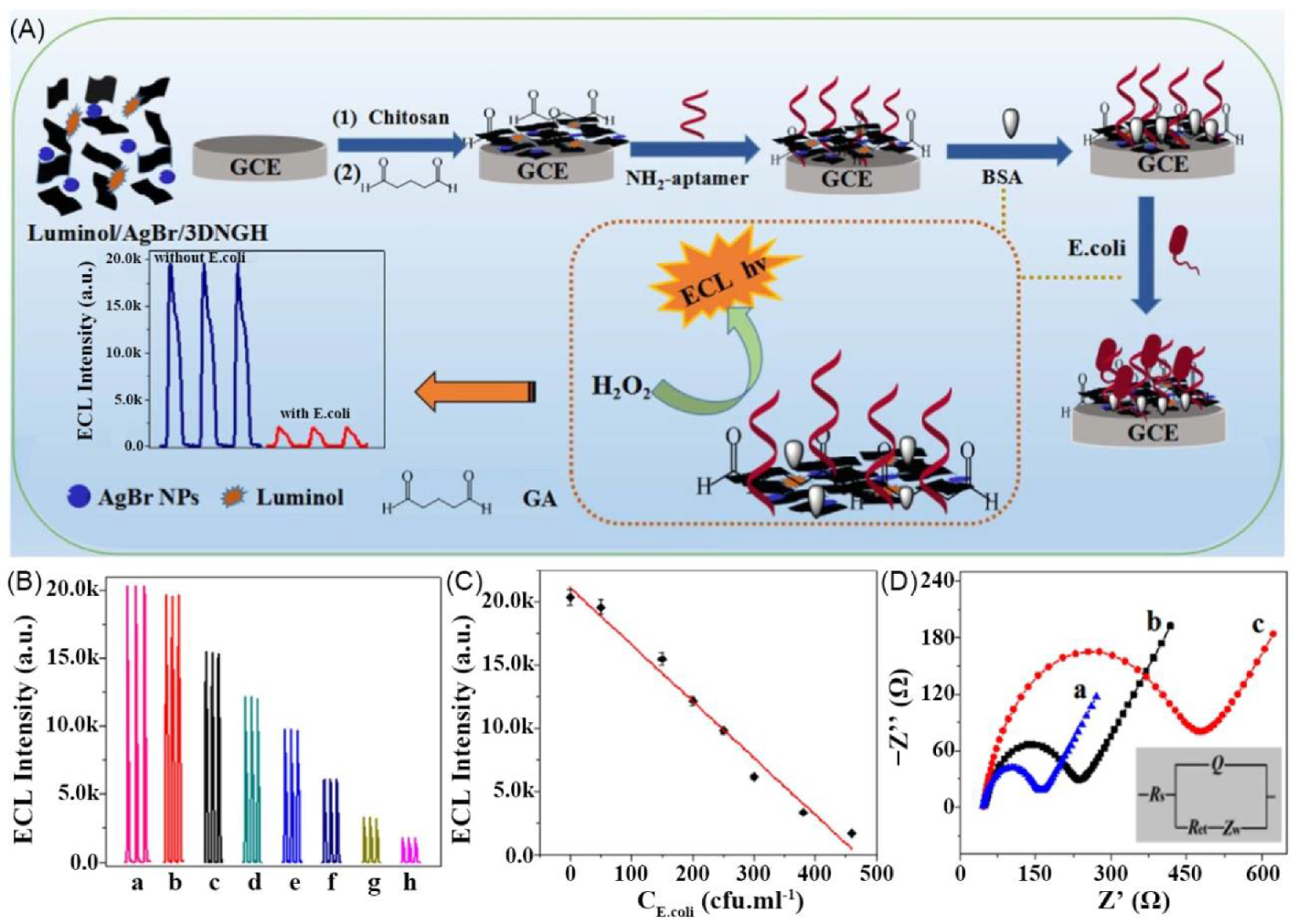
| 10 | supramolecular bulk hydrogel | nitrogen-doped graphene (NG) | H2O | NG | - | luminol | - | Escherichia coli sensing | [94] |
| 11 | polymeric bulk hydrogel | polyaniline (PAni) + phytic acid (PA) | H2O | PAni, PA | - | N-(aminobutyl)-N-(ethylisoluminol) (ABEI) | - | Live cell H2O2 detection | [95] |
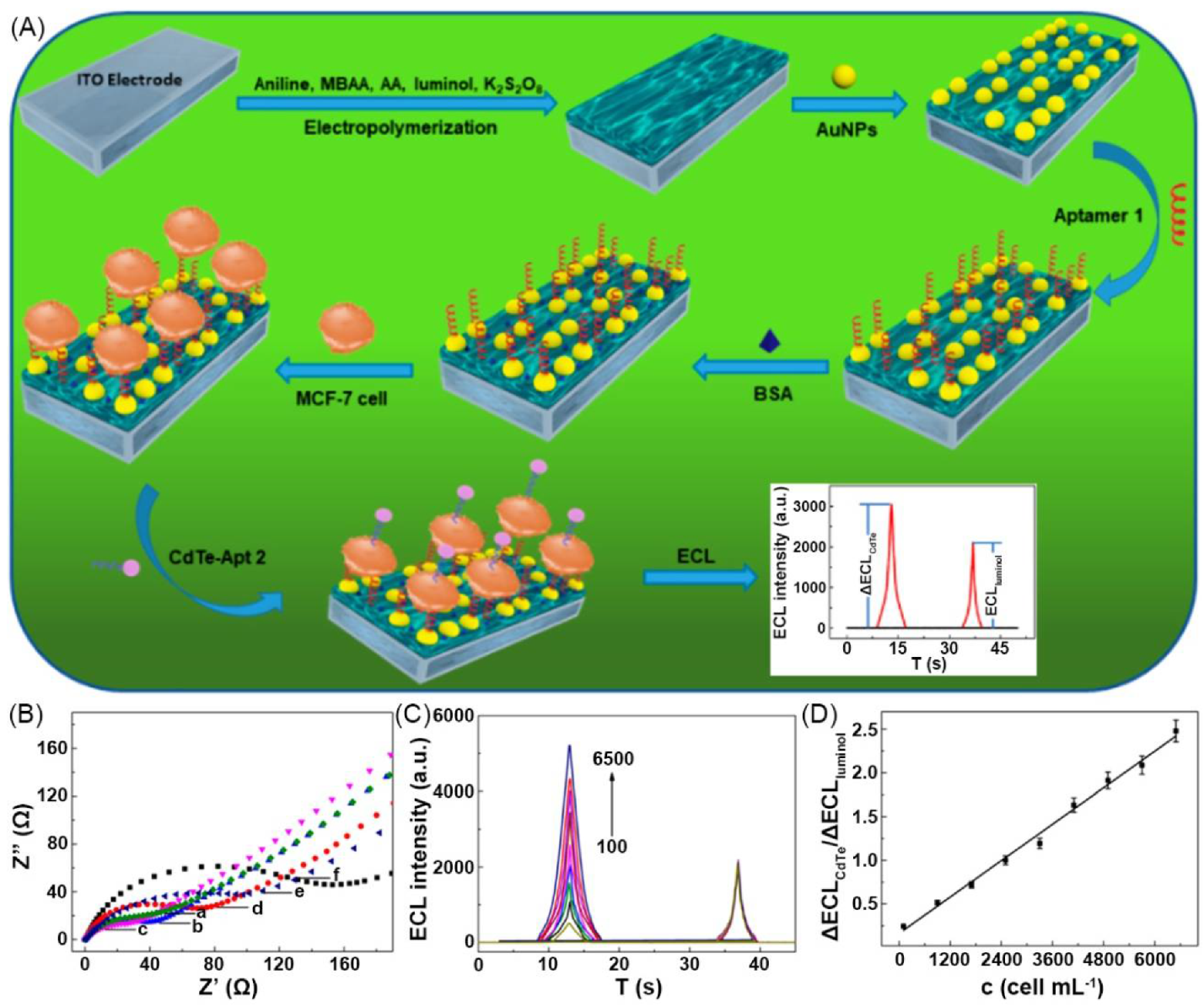
| 12 | polymeric bulk hydrogel | PAni + poly(acrylic acid) (PAA) | H2O | PAni, AuNP | Resistance (Ω): 24 | luminol, CdTe, quantum dot (QD) | - | cytosensor | [96] |
| 13 | supramolecular bulk hydrogel | graphene | H2O | graphene, AuNP | - | carbon dot (CD) | 410/~517 | cytosensor, glucose transporter 4 expression evaluation | [83] |
| 14 | polymeric bulk hydrogel | PAni + ATMP | H2O | PAni | - | ABEI | - | xanthine detection | [26] |
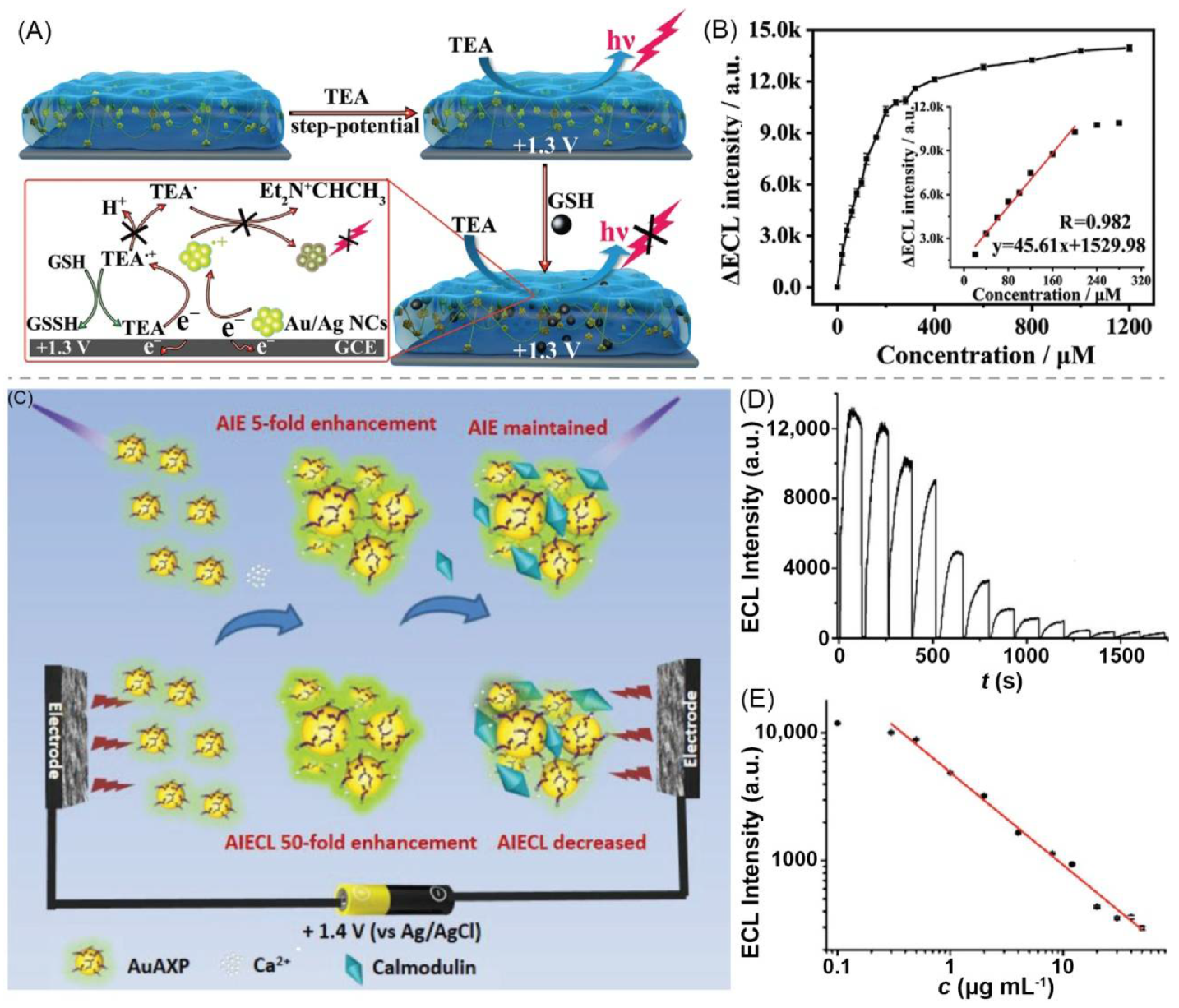
| 15 | polymeric bulk hydrogel | BSA + Au/Ag nanocluster (NC) | H2O | Na+, K+, PO43−, Cl−, NO3− | - | Au/Ag NC | 500/~620 | detection of glutathione (GSH) | [97] |
| 16 | supramolecular bulk hydrogel | AuAXP nanocluster (NC) + Ca2+ | H2O | Ca2+, Cl− | - | AuAXP NC | ~500 nm ECL | detection of calmodulin | [98] |
| 17 | supramolecular bulk hydrogel | Ag-melamine metal-organic gel (Ag-MOG) | H2O | Ag-MOG | - | Tri(2,2’-bipyridyl)dichlororuthenium(II) (Ru(bpy)32+);dichlorotris (1,10-phenanthroline) ruthenium (II) (Ru(phen)32+) | - | DNA detection | [99] |
| 18 | supramolecular bulk hydrogel | Au NP | H2O | graphite-like carbon nitride (g-C3N4), Au NP | - | Au NP | ~465 ECL | Zika Virus DNA detection | [100] |
| 19 | supramolecular bulk hydrogel | Ag9 NC | H2O | Ag9 NC | Resistance (Ω): 586 | Ag9 NC | 234/~575 | methyltransferase Assay | [101] |
| 20 | supramolecular bulk hydrogel | tris(4,4′-dicarboxylicacid-2,2′-bipyridyl) ruthenium (II) dichloride (Ru(dcbpy)32+) + 4′-(4-carboxyphenyl)-2,2′:6′,2′’-terpyridine (Hcptpy) + Tb3+ | H2O | Tb3+, Ru2+, Cl−, NO3− | - | Ru(dcbpy)32+, Tb complex | -/~608.4; ~679 ECL | epinephrine detection | [102] |
| 21 | Supramolecular bulk aerogel | MoS2 nanosheet (NS) | air | MoS2 NS | Resistance (Ω): 115 | polydopamine NP with phenylboronic acid (PBA) (PDA-PBA NP) | -/~457 (PDA-PBA NP); ~600, 562, 552 ECL (PDA-PBA NP) | MiRNA-126 detection | [103] |
| 22 | polymeric bulk organogel | poly(ethyl acrylate-r-styrene-r-divinylbenzene) (PEA-r-PS-r-PDVB) | 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide ([EMI][TFSI]) | [EMI][TFSI] | Resistance (Ω): 77,520 | Ru(bpy)3(PF6)2 | ~612 ECL | wearable ionoskin | [104] |
| 23 | polymeric bulk organogel | poly(vinylidene fluoride-co-hexafluoropropylene) (P(VDF-co-HFP)) | 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([AMI][TFSI] including [EMI][TFSI], [BMI][TFSI], [HMI][TFSI], and [DMI][TFSI]) | [AMI][TFSI] | Conductivity (S·cm−1): 2.5 × 10−3 ([EMI][TFSI]); 1.5 × 10−3 ([BMI][TFSI]); 0.60 × 10−3 ([HMI][TFSI]); 0.28 × 10−3 ([DMI][TFSI]) Resistance (Ω): 226 ([EMI][TFSI]) 381 ([BMI][TFSI]) 939 ([HMI][TFSI]) 2018 ([DMI][TFSI]) | 2,2′-bipyridyl-bis[2-(2′,4′-difluorophenyl)pyridine]-iridium(III) hexafluorophosphate Ir(diFppy)2(bpy)PF6; Ru(bpy)3(PF6)2 | green ECL; red ECL | display material | [105] |
| 24 | polymeric bulk organogel | poly(4-vinyl pyridine) (P4VP) | pyridine | P4VP | Resistance (MΩ) 9–10 (after 385 nm radiation) | P4VP | 280/~364, 440 | - | [106] |
| 25 | polymeric bulk xerogel | poly(N-[5-(8-hydroxyquinoline)methyl]aniline) (PNQA) + V2O5 | air | PNQA | Conductivity (S·cm−1) (after aging): 3.1×10−3 | PNQA | 373/~471 | - | [107] |
| 26 | polymeric bulk organogel | poly(methyl methacrylate) (PMMA) | 1-butyl-3-methylimidazolium bis(trifluoromethane sulfonyl)imide ([Bmim][N(Tf)2]) | [Bmim][N(Tf)2] | Conductivity (S·cm−1) 10−3 | [(Bu’2bpy)Pt(C≡CC6H4tpy)][Eu(hfac)3]2 | 310/~616 | - | [108] |
| 27 | polymeric bulk organogel | poly(N-isopropylacrylamide-co-N-vinylcarbazole) (P(NVC-co-NIPA)) | dioxane | P(NVC-co-NIPA) | Conductivity (S·cm−1) (treated by CAN): 0.017 (S1); 0.19 (S2); 0.20 (S3); 0.22 (S4); 0.25 (S5); 0.43 (S6) | P(NVC-co-NIPA) | 300/- (S1); 300/~373 (S2); 300/~373 (S3); 300/~380, 410 (S4); 300/~380, 410 (S5); 300/~420 (S6) | - | [109] |
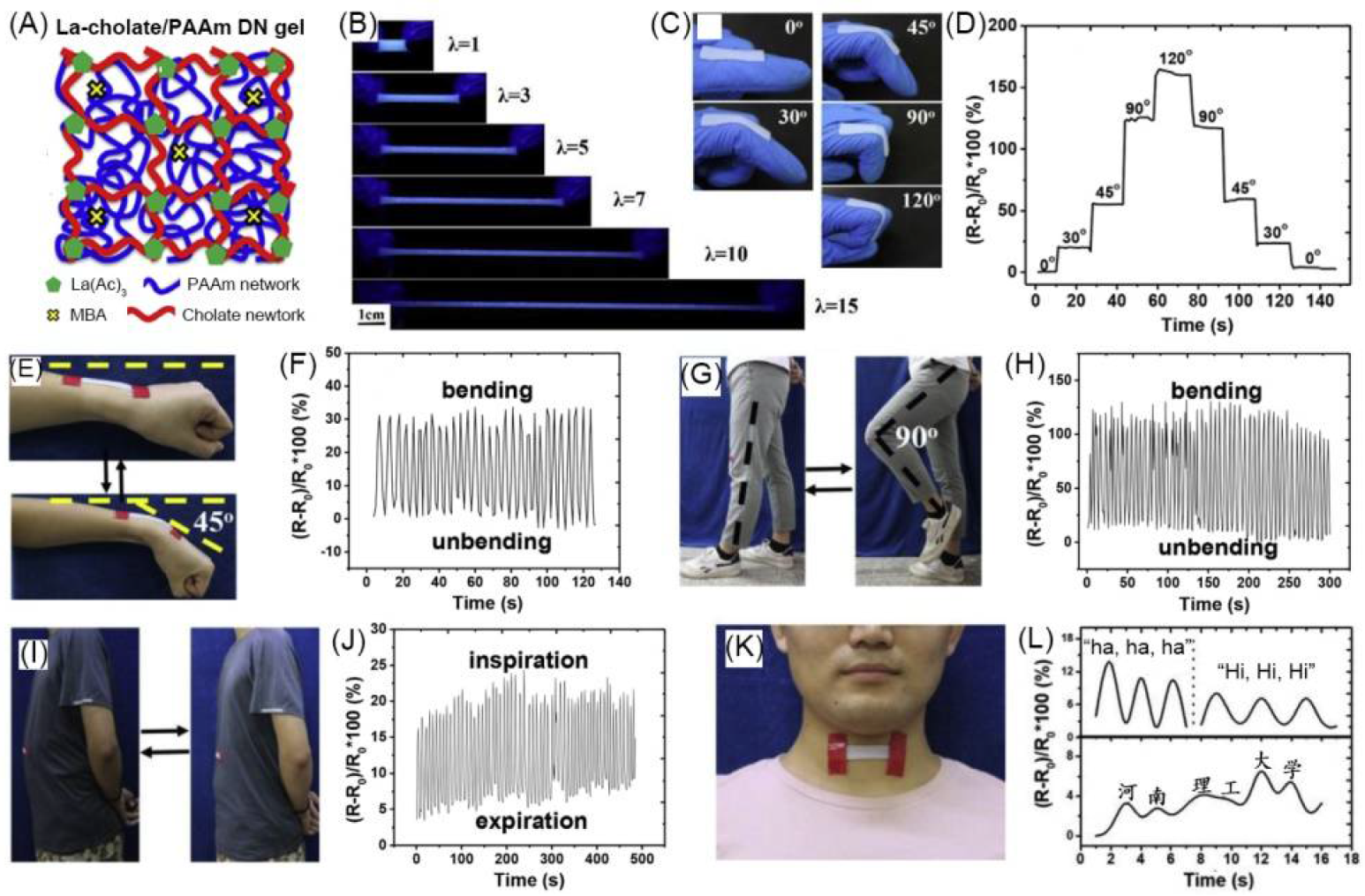
| 28 | polymeric bulk hydrogel | La-cholate/poly(acrylamimde) double network | H2O | La3+, Na+, CH3COO−, Cl− | Conductivity (S·cm−1): 3 × 10−3 | La complex | -/~430 | strain sensor | [110] |
| 29 | polymeric bulk organogel | poly(MMA–HEMA) | PC-γ-GBL mixture | TBABF4 | - | TPETPAOMe BTOTPAOMe | -/~505; -/~551 | electrofluorechromic devices | [111] |
| No. | Layer I | Layer II | Layer III | Conductive Species | Electrical Parameter | Luminescent Species | Luminescent Parameter | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | polystyrene organic gel + MAPbBr3 PNC (luminescent layer) | Ecoflex + ZnS:Cu + BaTiO3 (luminescent layer) | PAAm hydrogel + LiCl (ionic conductive layer) | Li+, Cl− | - | MAPbBr3 PNC; ZnS:Cu | λex(PL)/λem(PL): 365/~525 (V-PNC@AINO); EL color: blue (ZnS:Cu-based layer) | soft EL devices | [116] |
| 2 | PAA hydrogel + NaCl (ionic conductive layer) | polyurethane + ZnS particle + boron nitride nanosheet (luminescent layer) | PAA hydrogel + NaCl (ionic conductive layer) | Na+, Cl− | Conductivity (S·cm−1): 2 × 10−3 | ZnS particle | λem(EL): ~450; ~500; ~588 | light-emitting device | [117] |
| 3 | Ecoflex + ZnS:Cu (luminescent layer) | PAA hydrogel + NaCl (ionic conductive layer) | Ecoflex | Na+, Cl− | - | ZnS:Cu | λem(EL): ~520 | wearable smart skin | [27] |
| 4 | agarose-polyacrylamide (PAAm) hydrogel + LiCl (ionic conductive layer) | polydimethylsiloxane (PDMS) elastomer + ZnS:Cu (luminescent layer) | agarose-polyacrylamide hydrogel + LiCl (ionic conductive layer) | Li+, Cl− | Resistance (Ω): ~20,000 | ZnS:Cu | EL color: blue | wearable sensor; flexible EL device | [118] |
| 5 | PAAm hydrogel + LiCl (ionic conductive fiber core) | PSPI elastomer + ZnS; PSPI elastomer + CdTe/ZnS QD (luminescent sheath) | - | Li+, Cl− | Conductivity (S·cm−1): 0.16 | ZnS; CdTe/ZnS QD | EL color: blue (ZnS-based sheath); PL color: pink (QD-based sheath) | wearable motion sensor | [119] |
| 6 | PDMS + carbon nanotube (CNT) (conductive layer) | red: Ru(bpy)3(PF6)2 + [EMI][TFSI] + polymethyl methacrylate (PMMA) + polyethylene glycol (PEG); green: bis-[2-(2,4-difluorophenyl) pyridin ate](2,2′-dimethyl-4,4′-bipyridine)iridium(III) hexafluorophosphate ([Ir-(Fppy)2(dmb)]PF6) + [EMI][TFSI] + PMMA + PEG; blue: 9,10-diphenylanthracene (DPA) + PMMA + LiCF3SO3 (luminescent conductive organogel layer) | - | CNT; [EMI][TFSI; LiCF3SO3 | Resistance (Ω·sq−1): 805.2–73.5 (CNT solution volume 250–2000 μL) (conductive layer) | Ru(bpy)3(PF6)2, [Ir-(Fppy)2(dmb)]PF6, DPA | λem(EL): ~616 (red layer); ~532 (green layer); ~430 (blue layer) | wearable sensor | [120] |
| 7 | single-walled carbon nanotube (SWNT) (conductive fiber core) | supramolecular organic gel of pyrene-based LMWG (luminescent middle layer) 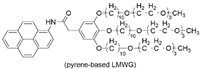 | silica wall | SWNT | - | pyrene-based LMWG | λex(PL)/λem(PL): -/~370, 390 | - | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Su, G.; Li, Z. Gel-Based Luminescent Conductive Materials and Their Applications in Biosensors and Bioelectronics. Materials 2021, 14, 6759. https://doi.org/10.3390/ma14226759
Qi J, Su G, Li Z. Gel-Based Luminescent Conductive Materials and Their Applications in Biosensors and Bioelectronics. Materials. 2021; 14(22):6759. https://doi.org/10.3390/ma14226759
Chicago/Turabian StyleQi, Jiajin, Gongmeiyue Su, and Zhao Li. 2021. "Gel-Based Luminescent Conductive Materials and Their Applications in Biosensors and Bioelectronics" Materials 14, no. 22: 6759. https://doi.org/10.3390/ma14226759
APA StyleQi, J., Su, G., & Li, Z. (2021). Gel-Based Luminescent Conductive Materials and Their Applications in Biosensors and Bioelectronics. Materials, 14(22), 6759. https://doi.org/10.3390/ma14226759