Thermal Decomposition of Brominated Butyl Rubber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Specimen Preparation
2.3. TG Analysis
3. Result and Discussion
3.1. Thermal Decomposition
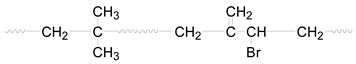

| Bond | –C=C– | –C–H | –C–C– | –C–Br |
|---|---|---|---|---|
| Bond energy (KJ/mol) | 605 | 420 | 346 | 292 |
3.2. Activation Energy and Reaction Order of Thermal Decomposition
3.2.1. TG Method
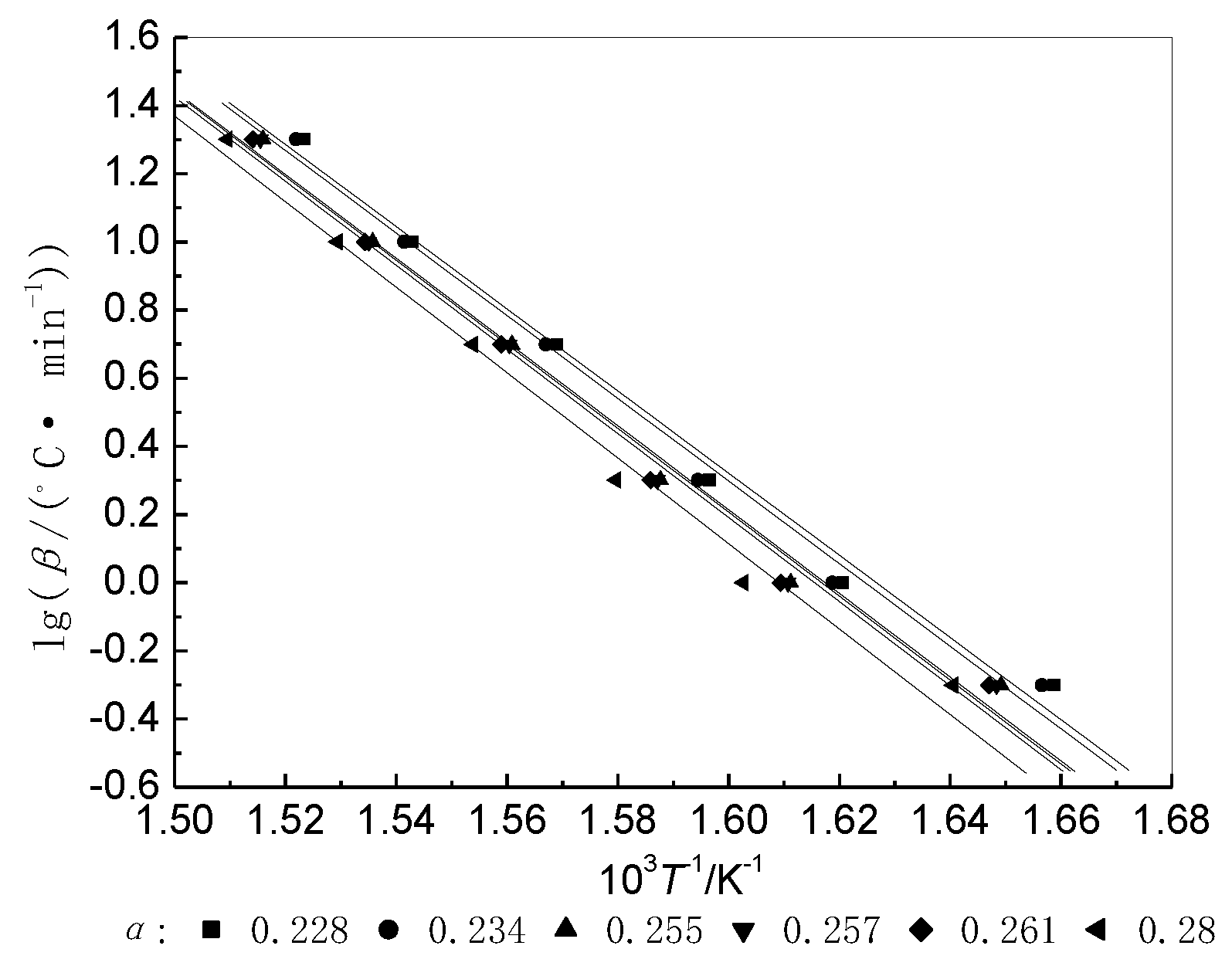
3.2.2. Isoconversional Procedure (Integral Method)
3.3. Lifetime of Thermal Decomposition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dziemidkiewicz, A.; Maciejewska, M.; Pingot, M. Thermal analysis of halogenated rubber cured with a new cross-linking system. J. Therm. Anal. Calorim. 2019, 138, 4395–4405. [Google Scholar] [CrossRef] [Green Version]
- Charles, J.; Muthusamy, S. Comparative Study of Butyl Rubber (IIR) and Bromobutyl Rubber (BIIR) Based On FTIR, Dielectric and Thermal Studies. J. Appl. Sci. Eng. Methodol. 2016, 2, 206–211. [Google Scholar]
- Arabiourrutia, M.; Lopez, G.; Artetxe, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Waste Tyre Valorization by Catalytic Pyrolysis—A Review. Renew. Sustain. Energy Rev. 2020, 129, 109932. [Google Scholar] [CrossRef]
- Liu, S.; Jie, Y.; Bikane, K.; Chen, T.; Ma, C.; Wang, B.; Sun, L. Rubber Pyrolysis: Kinetic Modeling and Vulcanization Effects. Energy 2018, 155, 215–225. [Google Scholar] [CrossRef]
- Hu, Q.; Tang, Z.; Yao, D.; Yang, H.; Shao, J.; Chen, H. Thermal Behavior, Kinetics and Gas Evolution Characteristics for the Co-Pyrolysis of Real-World Plastic and Tyre Wastes. J. Clean. Prod. 2020, 260, 121102. [Google Scholar] [CrossRef]
- Basha, S.K.T.; Divya, R.; Menon, A.U.; Ashok, N.; Balachandran, M. Cure and Degradation Kinetics of Sulfur Cured Nanocomposites of EPDM-NBR Rubber Blends. Mater. Today Proc. 2018, 5, 23586–23595. [Google Scholar] [CrossRef]
- Rasam, S.; Moshfegh Haghighi, A.; Azizi, K.; Soria-Verdugo, A.; Keshavarz Moraveji, M. Thermal Behavior, Thermodynamics and Kinetics of Co-Pyrolysis of Binary and Ternary Mixtures of Biomass Through Thermogravimetric Analysis. Fuel 2020, 280, 118665. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal Degradation Kinetics of Plastics and Model Selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal Degradation of Typical Plastics Under High Heating Rate Conditions by TG-FTIR: Pyrolysis Behaviors and Kinetic Analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Wang, J.; Jia, H.; Tang, Y.; Xiong, X.; Ding, L. Thermal Stability and Non-Isothermal Crystallization Kinetics of Metallocene Poly (Ethylene-Butene-Hexene)/High Fluid Polypropylene Copolymer Blends. Thermochim. Acta 2017, 647, 55–61. [Google Scholar] [CrossRef]
- IRS Group. World Rubber Statistics Handbook. Synth. Rubber Ind. 2020, 43, 59. [Google Scholar]
- Steehler, K.J. Ewing’s Analytical Instrumentation Handbook. J. Chem. Educ. 2005, 82, 1315. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, B. Rubber Compounding: Chemistry and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Weast, R.C. Handbook of Chemistry and Physics. Anal. Chem. 2016, 44, 1321. [Google Scholar]
- Wang, Y.; Huang, Z.; Zhang, L. Study on Vulcanizing and Thermal Behavior of Polymethy/Vinylsiloxane/Butyl Rubber Blend. Adhesion 2007, 28, 18–20. [Google Scholar]
- Zhang, L.; Xue, Q.; Mo, Z.; Jin, X. Modern Research Methods of Polymer Physics; Wuhan University Press: Wuhan, China, 2003; pp. 233–234. [Google Scholar]
- Rosu, D.; Cascavaf, C.N.; Ciobanu, C.; Rosu, L. An Investigation of the Thermal Degradation of Epoxy Maleate of Bisphenol A. J. Anal. Appl. Pyrol. 2004, 72, 191–196. [Google Scholar] [CrossRef]
- Sreehitha, P.R.; Durga, B.; Balachandran, M. Dielectric Properties, Thermal Characteristics and Degradation Kinetics of PMMA Nanodielectrics. Mater. Today Proc. 2020, 24, 772–781. [Google Scholar] [CrossRef]
- Run, M.; Dayu, Z.; Sizhu, W.; Gang, W. Studies on Thermal Decomposition of Poly(Ethylene Terephthalate)/Mesoporous Molecular Sieve Composite. Polym. Mater. Sci. Eng. 2006, 22, 64–67. [Google Scholar]
- Zhiguo, Z.; Hong, Y. Non-Isothermal Decomposition Kinetics and Lifetime of Polyoxypropylene Ether. J. Zhejiang Univ. Eng. Sci. 2006, 40, 689–692. [Google Scholar]
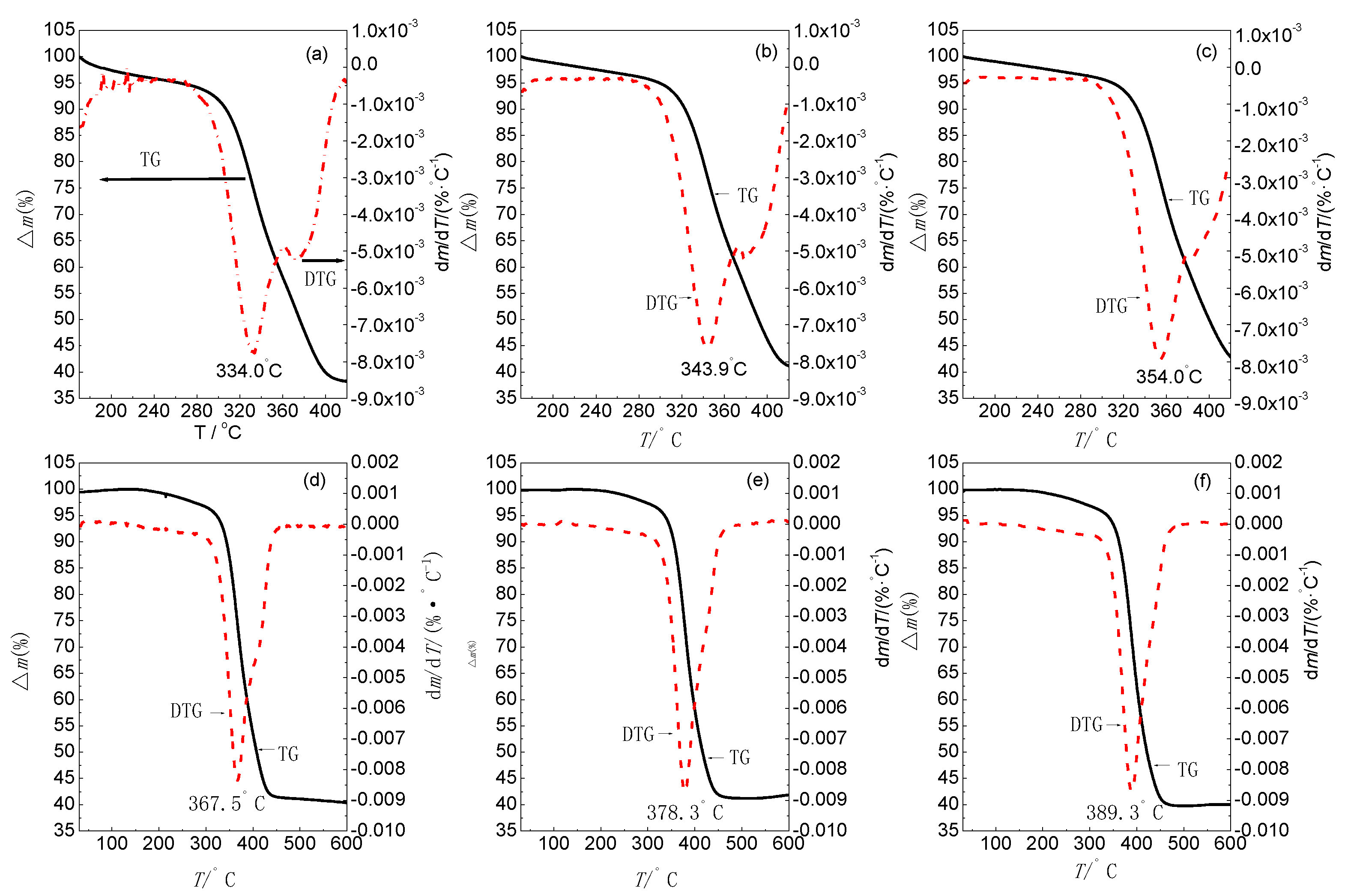


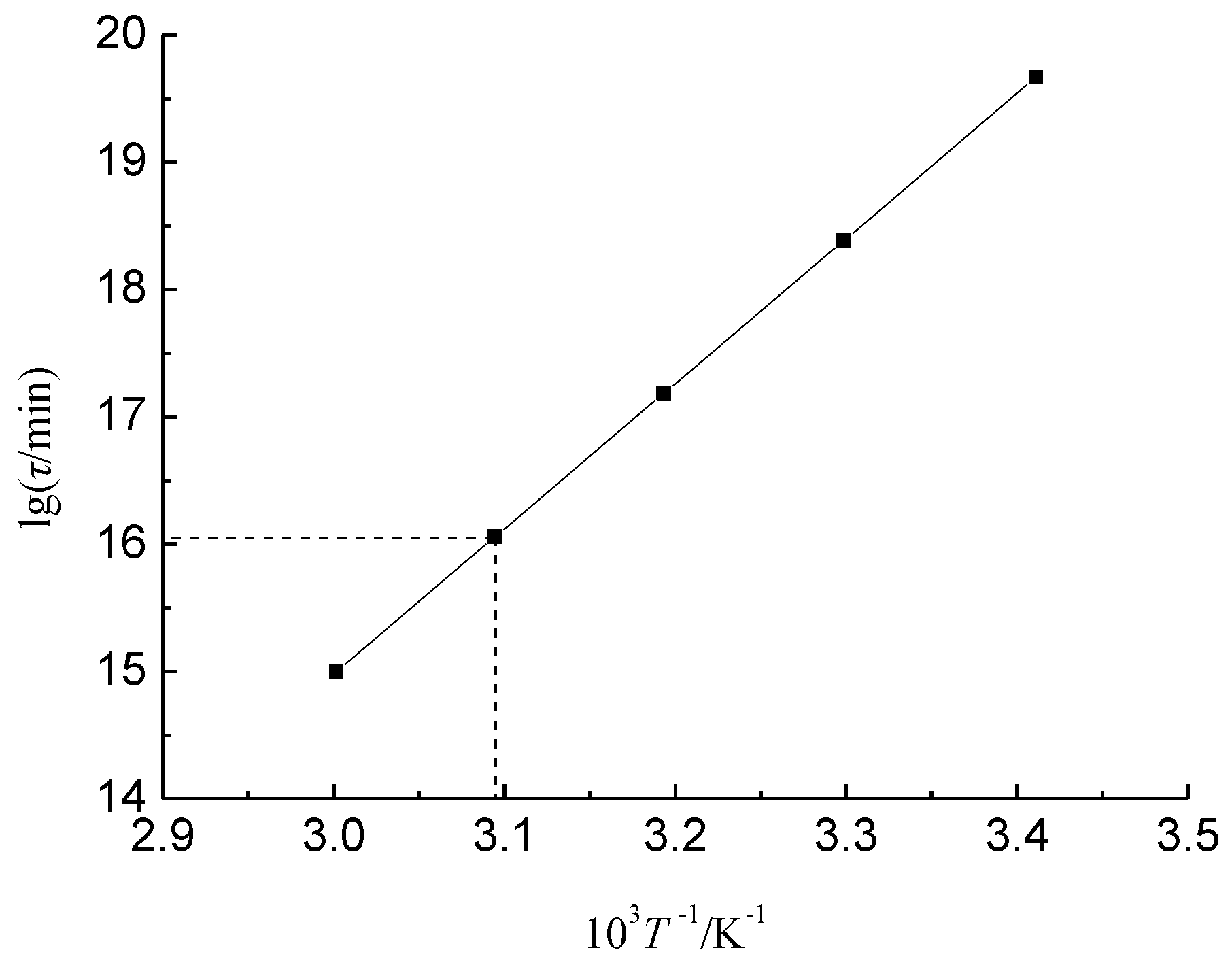
| β (°C·min−1) | Δm (%) | Tpi (°C) |
|---|---|---|
| 0.5 | 73.9 | 334.0 |
| 1 | 77.2 | 343.9 |
| 2 | 76.6 | 354.0 |
| 5 | 74.5 | 367.5 |
| 10 | 74.3 | 378.3 |
| 20 | 72.0 | 389.3 |
| β (°C min−1) | TG Method | |||
|---|---|---|---|---|
| E (kJ·mol−1) | R | lnA (min−1) | n | |
| 0.5 | 224.41 | −0.9946 | 40.67 | 1.05 |
| 1 | 219.31 | −0.9949 | 39.87 | 1.03 |
| 2 | 220.55 | −0.9946 | 40.08 | 1.04 |
| 5 | 223.64 | −0.9943 | 40.56 | 1.05 |
| 10 | 224.01 | −0.9945 | 40.62 | 1.05 |
| 20 | 228.13 | −0.9940 | 41.29 | 1.07 |
| 1 − α | 0.9 | 0.8 | 0.7 | 0.6 | 0.5 |
|---|---|---|---|---|---|
| E (kJ·mol−1) | 145.32 | 215.33 | 232.66 | 266.50 | 286.75 |
| lnA (min−1) | 34.43 | 38.27 | 41.24 | 46.90 | 49.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zang, Y.; Lu, Y.; Lin, W.; Zhao, S.; Xiong, J. Thermal Decomposition of Brominated Butyl Rubber. Materials 2021, 14, 6767. https://doi.org/10.3390/ma14226767
Zhang W, Zang Y, Lu Y, Lin W, Zhao S, Xiong J. Thermal Decomposition of Brominated Butyl Rubber. Materials. 2021; 14(22):6767. https://doi.org/10.3390/ma14226767
Chicago/Turabian StyleZhang, Wei, Yang Zang, Yanli Lu, Weisheng Lin, Shengyun Zhao, and Jinping Xiong. 2021. "Thermal Decomposition of Brominated Butyl Rubber" Materials 14, no. 22: 6767. https://doi.org/10.3390/ma14226767






