Rheological Analysis of the Synthesis of High-Molecular-Weight Epoxy Resins from Modified Soybean Oil and Bisphenol A or BPA-Based Epoxy Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Epoxidation of the Soybean Oil
2.2. Hydroxylation of ESBO
2.3. Epoxy Fusion Process
2.4. Iodine, Epoxy, and Hydroxyl Values of Vegetable Oil and Its Derivatives Were Evaluated According to Standards
- Content of epoxy groups (epoxy value, EV) was evaluated according to PN-87/C-89085/13 standard: samples were dissolved in HCl/1,4-dioxane (POCh, Gliwice, Poland, reagent grade) solution and titrated by NaOH/methanol (POCh, Gliwice, Poland, reagent grade) in a presence of cresol red as an indicator to the visual change of tint to purple;
- The hydroxyl value (HV), samples of soybean oil, its epoxidized and fusion derivatives were dissolved in the solution of catalyst [4(dimethylamino) pyridine in DMF, POCh, Gliwice, Poland, reagent grade] and acetic anhydride in DMF (POCh, Gliwice, Poland, reagent grade). Followed by intensive stirring for another 15 min and titration with KOH aqueous solution in a presence of thymolphthalein until the tint change from colorless to blue;
- Iodine value (IV), describing the content of unsaturated bonds, was determined with Hanus method according to PN-EN ISO 3961:2011 standard (samples of products were dissolved in chloroform (POCh, Gliwice, Poland, reagent grade), where iodine bromide in glacial acetic acid (POCh, Gliwice, Poland, reagent grade) was added and prepared solution was titrated with sodium thiosulphate in a presence of starch aqueous solution to the moment of its discoloration).
2.5. Gel Permeation Chromatography
2.6. Rheological Properties
3. Results and Discussion
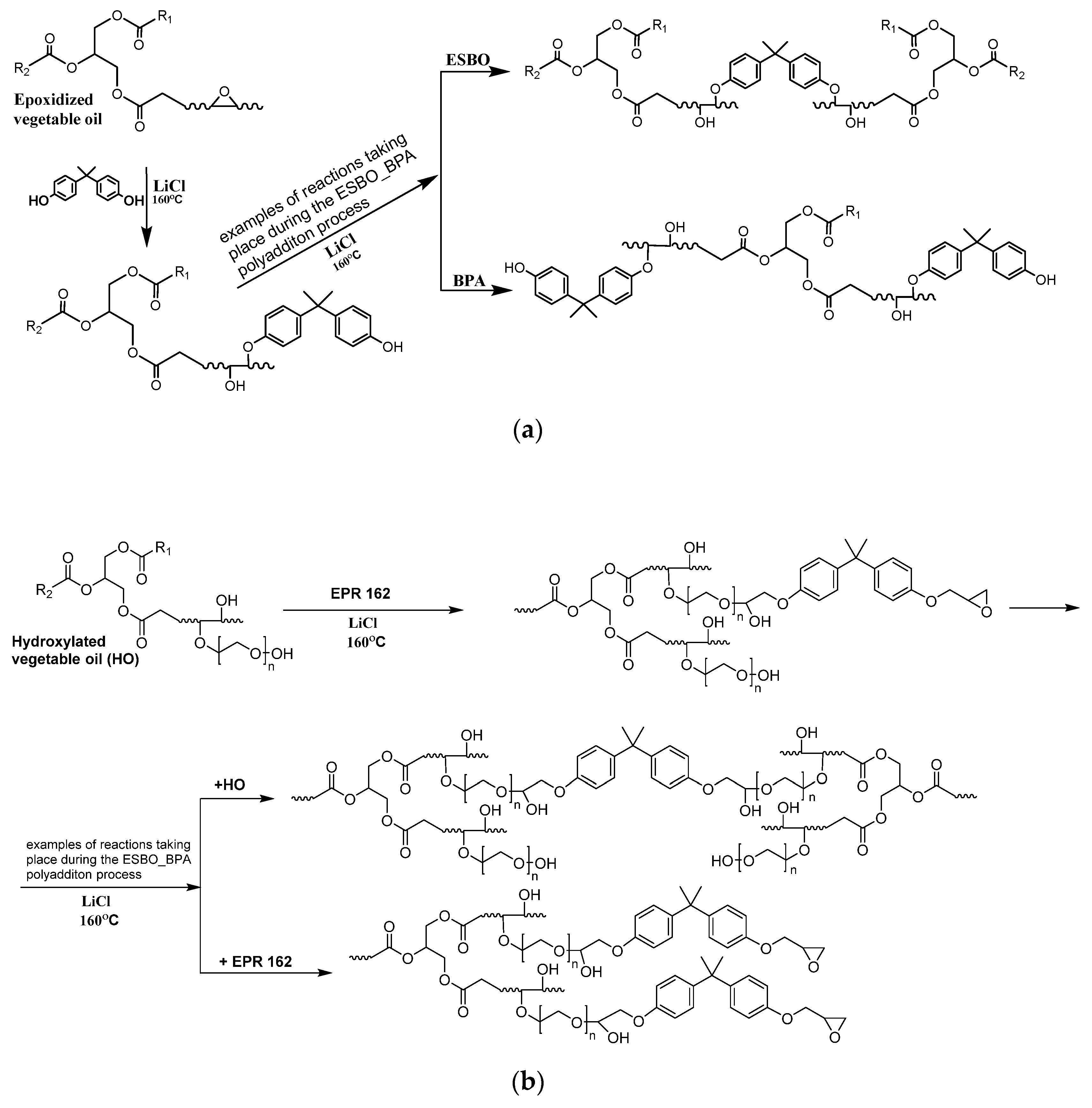
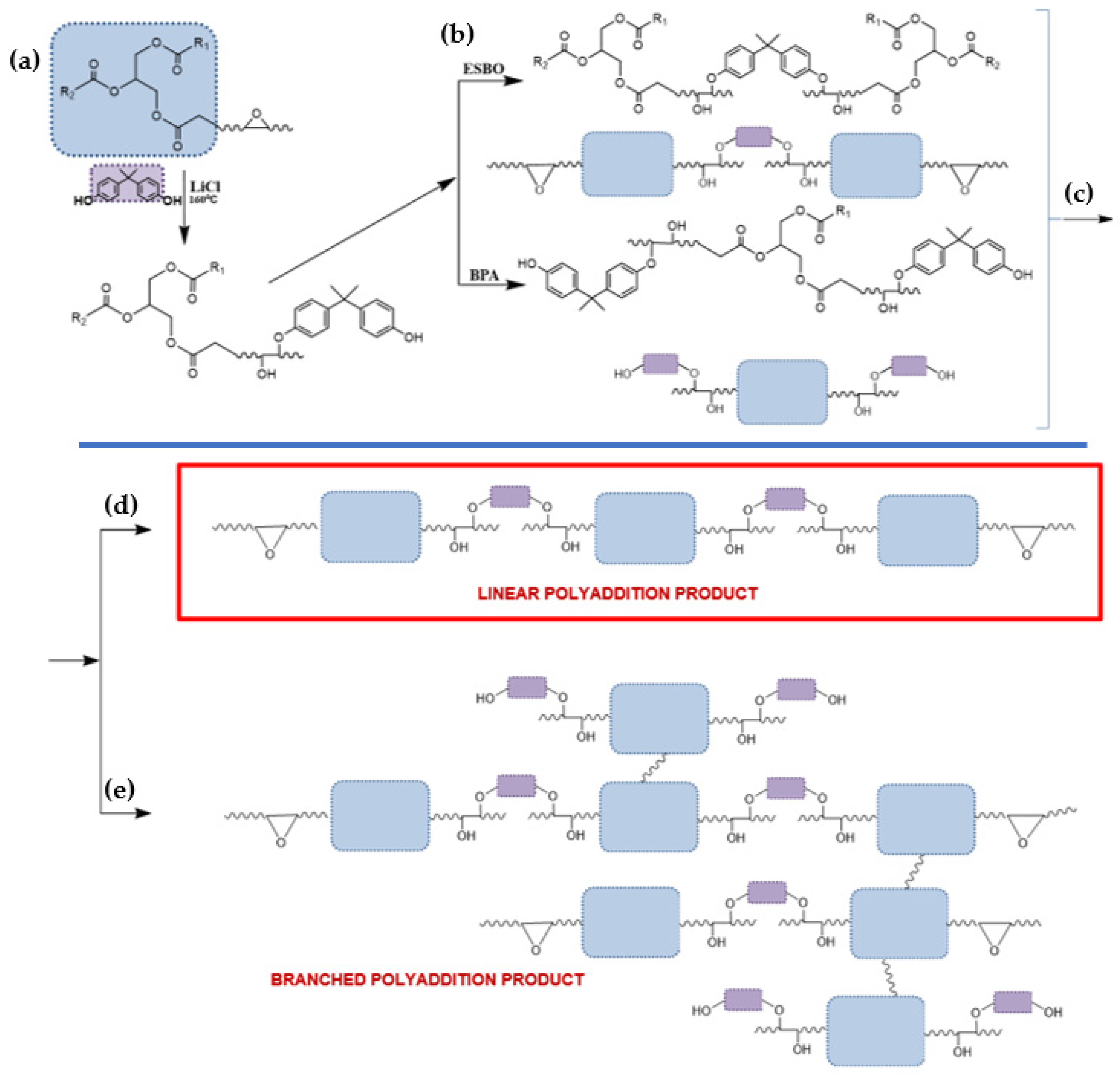
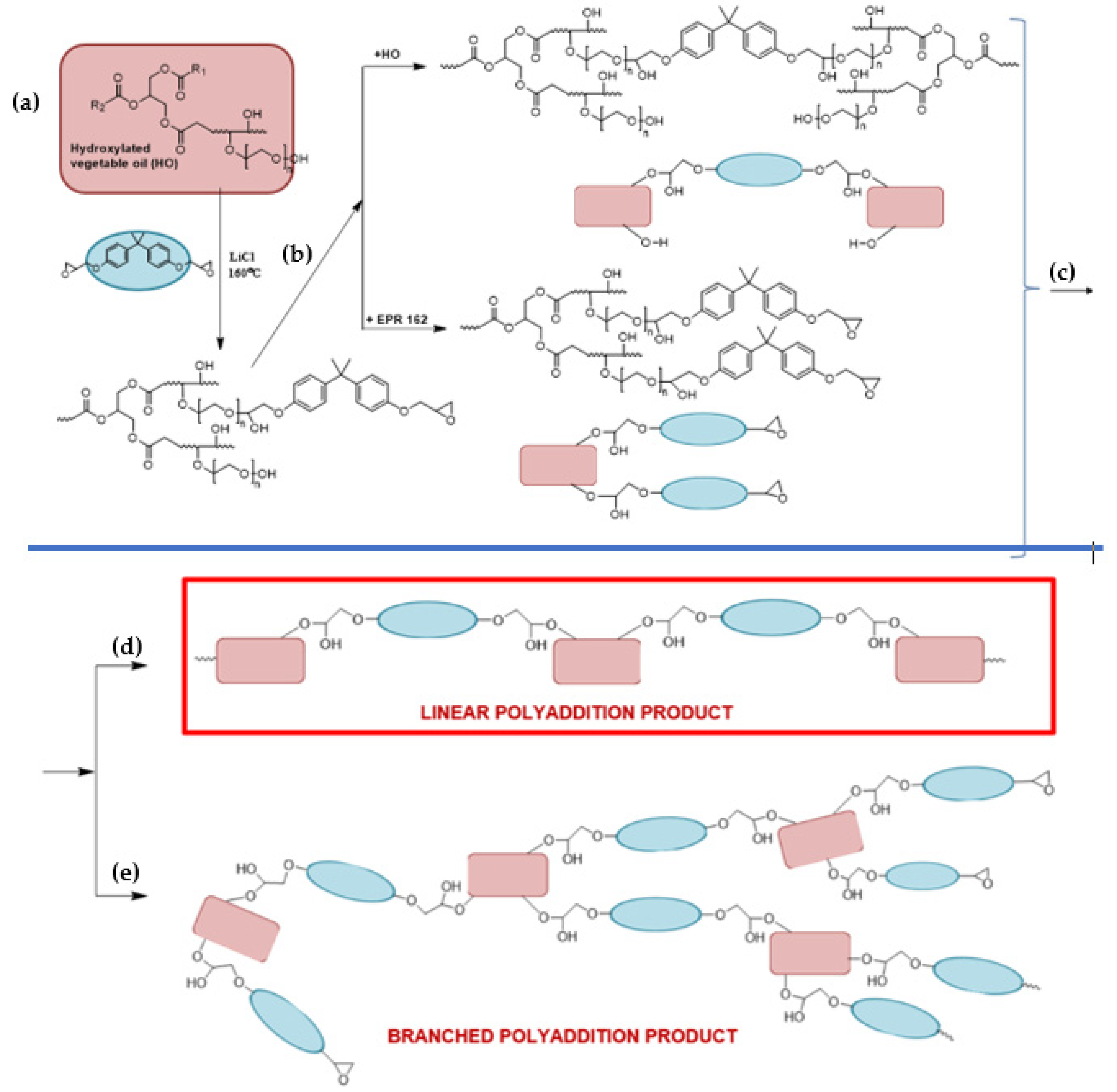
3.1. The Synthesis of Bio-Based Epoxy Resin Using Soybean Oil Derivatives
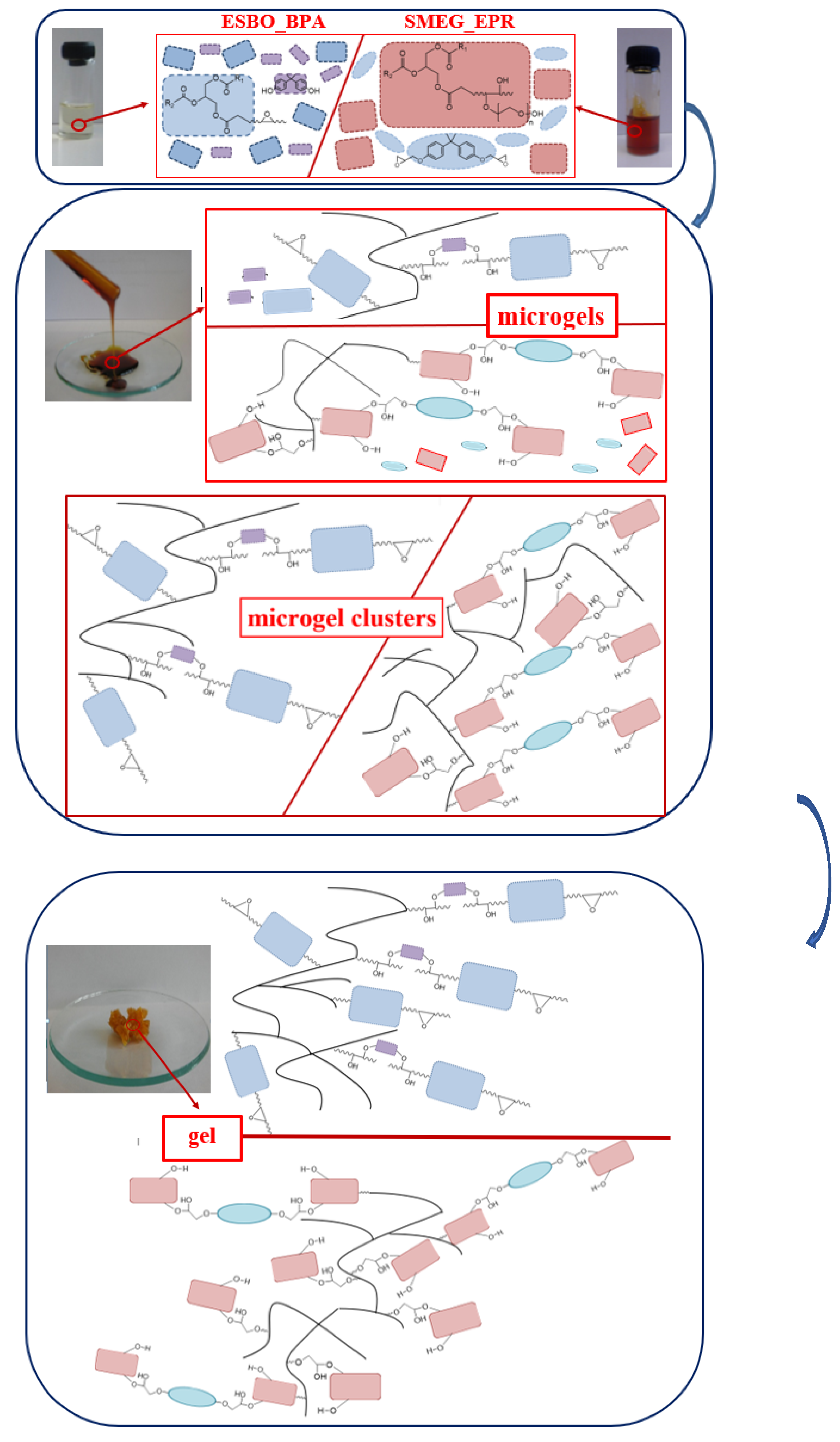
3.2. The Rheological Studies of Epoxy Fusion Process Using Soybean Oil Derivatives
3.2.1. Theoretical Determination of the Degree of Conversion at the Gel Point for the Polyaddition Process of Modified Soybean oil with BPA or EPR 0162, Carried out in Bulk, via the Fusion Process
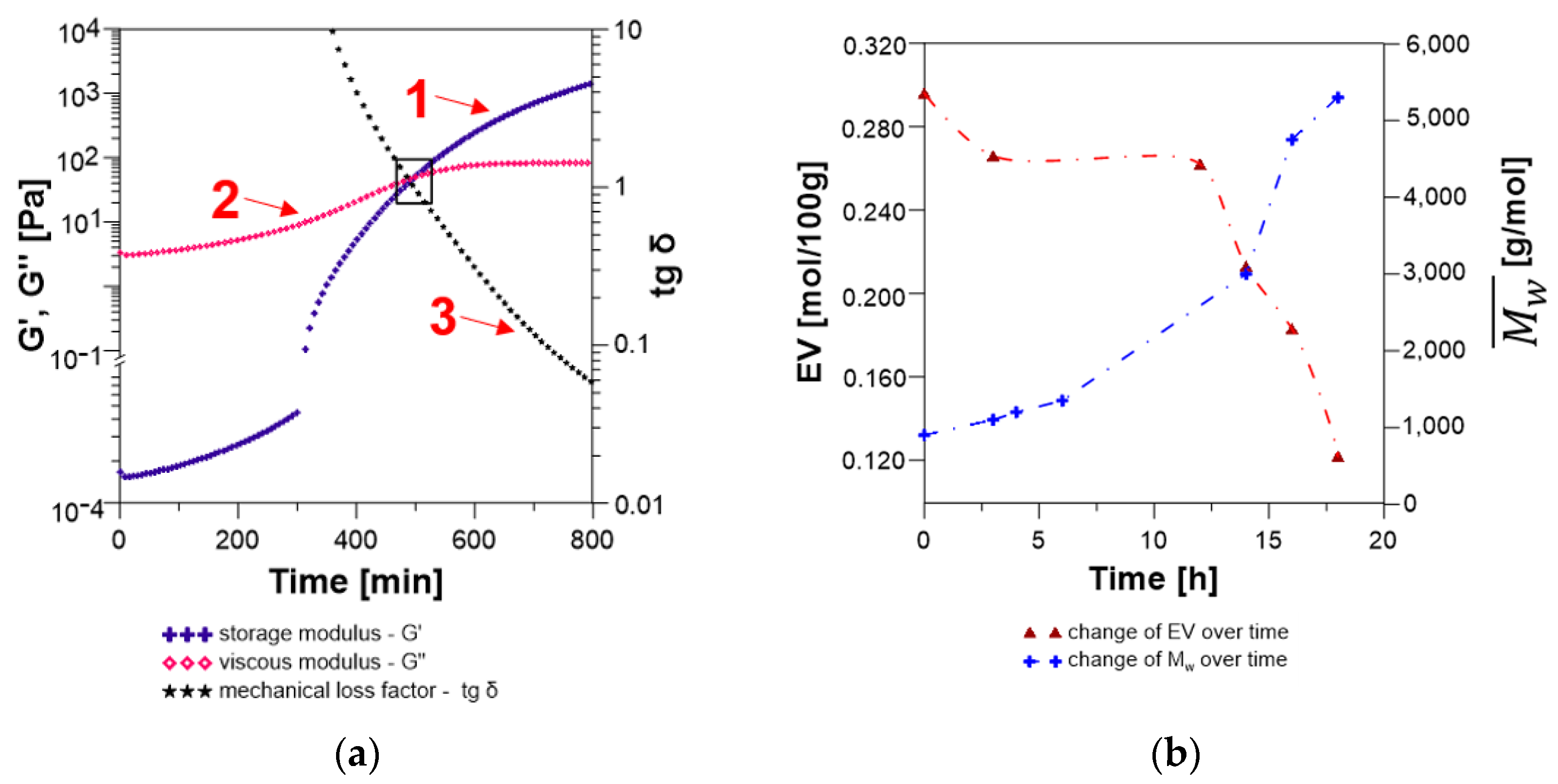
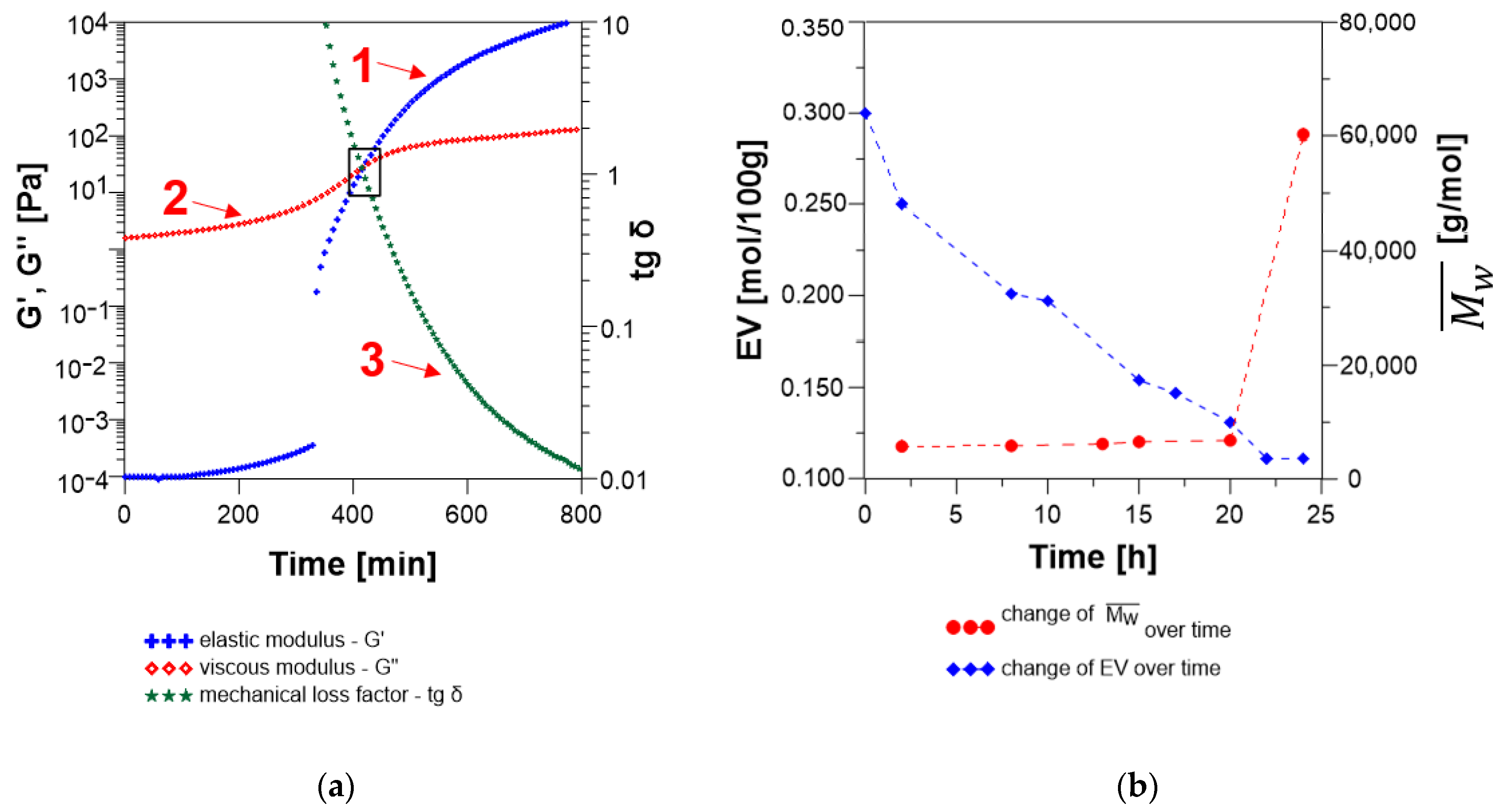
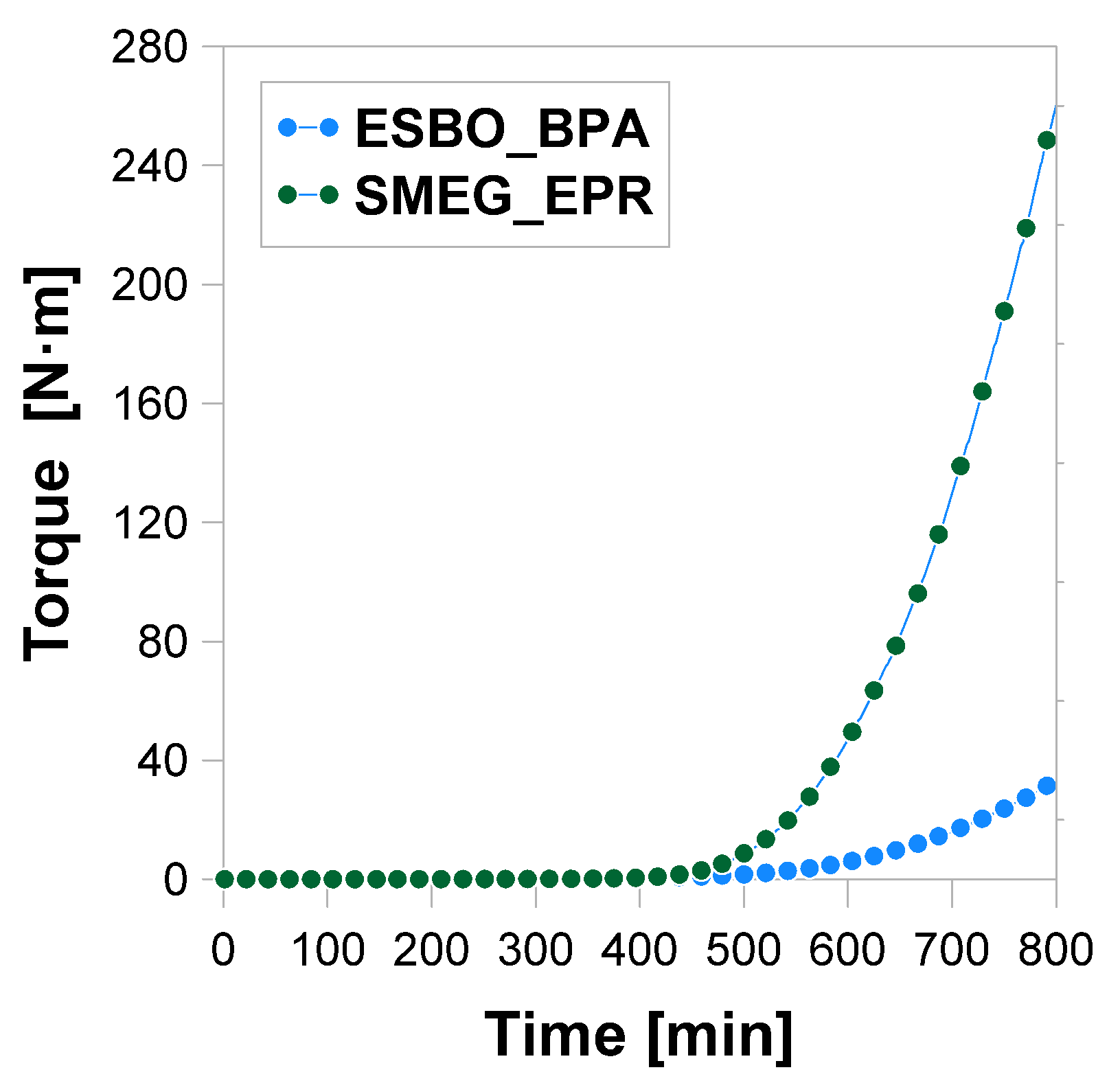
3.2.2. Analysis of the Changes of the Rheological Parameters That Occurred during the Polyaddition Process of Modified Soybean Oil with BPA or EPR0162
- ESBO_BPA Polyaddition Reaction, Carried out in Bulk, using the Epoxy Fusion Method
- 2.
- The SMEG_EPR Polyaddition Reaction Carried out in Bulk by the Fusion Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Prolongo, S.G.; Gude, M.R.; Sanchez, J.; Ureña, A. Nanoreinforced Epoxy Adhesives for Aerospace Industry. J. Adhes. 2009, 85, 180–199. [Google Scholar] [CrossRef]
- Ai, D.; Mo, R.; Wang, H.; Lai, Y.; Jiang, X.; Zhang, X. Preparation of waterborne epoxy dispersion and its application in 2K waterborne epoxy coatings. Prog. Org. Coat. 2019, 136, 105258. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, X.; Huang, Y.; Guo, Z.; Shao, L. Building Nanoporous Metal–Organic Frameworks “Armor” on Fibers for High-Performance Composite Materials. ACS Appl. Mater. Interfaces 2017, 9, 5590–5599. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Norizan, M.N.; Atikah, M.S.N.; Huzaifah, M.R.M.; Radzi, A.M.; Ishak, M.R.; Zainudin, E.S.; Izwan, S.; Azammi, A.N.; et al. Potential of natural fibre composites for transport industry: A review. In Proceedings of the Prosiding Seminar Enau Kebangsaan 2019, Bahau, Malaysia, 1 April 2019; pp. 2–11. [Google Scholar]
- Kumar, S.; Krishnan, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Toughening of Petroleum Based (DGEBA) Epoxy Resins with Various Renewable Resources Based Flexible Chains for High Performance Applications: A Review. Ind. Eng. Chem. Res. 2018, 57, 2711–2726. [Google Scholar] [CrossRef]
- Luo, X.; Yu, X.; Ma, Y.; Naito, K.; Zhang, Q. Preparation and cure kinetics of epoxy with nanodiamond modified with liquid crystalline epoxy. Thermochim. Acta 2018, 663, 1–8. [Google Scholar] [CrossRef]
- Czub, P. Characterization of an Epoxy Resin Modified with Natural Oil-Based Reactive Diluents. In Macromolecular Symposia; WILEY-VCH Verlag: Weinheim, Germany, 2006; Volume 245, pp. 533–538. [Google Scholar] [CrossRef]
- Czub, P. Synthesis of high-molecular-weight epoxy resins from modified natural oils and Bisphenol A or BisphenolA-based epoxy resins. Polym. Adv. Technol. 2008, 20, 194–208. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Czub, P. Synthesis of High-Molecular Weight Biobased Epoxy Resins: Determination of the Course of the Process by MALDI-TOF Mass Spectrometry. ACS Sustain. Chem. Eng. 2018, 6, 6084–6093. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Czub, P. Application of MALDI-TOF, 1H NMR and 13C NMR to follow the progress of the synthesis of high molecular weight epoxies from hydroxylated soybean oil and bisphenol A based epoxy resin. Polym. Test. 2018, 73, 200–207. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Czub, P. Novel bio-based epoxy-polyurethane materials from modified vegetable oils—Synthesis and characterization. Express Polym. Lett. 2017, 11, 308–319. [Google Scholar] [CrossRef]
- Qin, J.; Wolcott, M.; Zhang, J. Use of Polycarboxylic Acid Derived from Partially Depolymerized Lignin As a Curing Agent for Epoxy Application. ACS Sustain. Chem. Eng. 2013, 2, 188–193. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, D.Y.; Lim, C.S.; Seo, B.K. Studies of the physical properties of cycloaliphatic epoxy resin reacted with an-hydride curing agents. Key Eng. Mater. 2017, 737, 248–255. [Google Scholar] [CrossRef]
- Li, C.; Tan, J.; Gu, J.; Xue, Y.; Qiao, L.; Zhang, Q. Facile synthesis of imidazole microcapsules via thiol-click chemistry and their application as thermally latent curing agent for epoxy resins. Compos. Sci. Technol. 2017, 142, 198–206. [Google Scholar] [CrossRef]
- Lei, D.; Ma, W.; Wang, L.; Zhang, D. Preparation of 2-ethyl-4-methylimidazole derivatives as latent curing agents and their application in curing epoxy resin. J. Appl. Polym. Sci. 2015, 132, 42563. [Google Scholar] [CrossRef]
- Hart, K.R.; Sottos, N.R.; White, S.R. Repeatable self-healing of an epoxy matrix using imidazole initiated polymerization. Polymer 2015, 67, 174–184. [Google Scholar] [CrossRef]
- Guzmán, D.; Ramis, X.; Francos, X.F.; Serra, A. New catalysts for diglycidyl ether of bisphenol A curing based on thiol–epoxy click reaction. Eur. Polym. J. 2014, 59, 377–386. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Chen, S.; Zhang, J.; Cheng, J.; Miao, M.; Zhang, D. Preparation of Epoxy Resins with Excellent Comprehensive Performance by Thiol-Epoxy Click Reaction. Prog. Org. Coat. 2019, 139, 105436. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Vyazovkin, S.; Mititelu, A.; Sladic, C.; Vincent, L. A Study of Epoxy-Amine Cure Kinetics by Combining Isoconversional Analysis with Temperature Modulated DSC and Dynamic Rheometry. Macromol. Chem. Phys. 2003, 204, 1815–1821. [Google Scholar] [CrossRef]
- Vinnik, R.M.; Roznyatovsky, V.A. Kinetic Method by Using Calorimetry to Mechanism of Epoxy-amine Cure Reaction. J. Therm. Anal. Calorim. 2004, 75, 753–764. [Google Scholar] [CrossRef]
- Campaner, P.; D’Amico, D.; Longo, L.; Stifani, C.; Tarzia, A. Cardanol-based novolac resins as curing agents of epoxy resins. J. Appl. Polym. Sci. 2009, 114, 3585–3591. [Google Scholar] [CrossRef]
- Czub, P. Modified Natural Oils and the Products of Chemical Degradation of Waste Poly(Ethyleneterephthalate) as Environ-Mentally Friendly Raw Materials for Epoxy Resins; Publishing House of the Cracow University of Technology: Cracow, Polan, 2008; pp. 14–18. [Google Scholar]
- Sienkiewicz, A.; Czub, P. Blocked isocyanates as alternative curing agents for epoxy-polyurethane resins based on modified vegetable oils. Express Polym. Lett. 2019, 13, 642–655. [Google Scholar] [CrossRef]
- Wan, J.; Bu, Z.-Y.; Xu, C.-J.; Li, B.-G.; Fan, H. Preparation, curing kinetics, and properties of a novel low-volatile starlike aliphatic-polyamine curing agent for epoxy resins. Chem. Eng. J. 2011, 171, 357–367. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, X.; Yu, X. Reaction mechanism, cure behavior and properties of a multifunctional epoxy resin, TGDDM, with latent curing agent dicyandiamide. RSC Adv. 2018, 8, 8248–8258. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Duan, H.; Ji, S.; Ma, H. Novel phosphorus/nitrogen/boron-containing carboxylic acid as co-curing agent for fire safety of epoxy resin with enhanced mechanical properties. J. Hazard. Mater. 2020, 402, 123769. [Google Scholar] [CrossRef] [PubMed]
- Pascault, J.; Sautereau, H.; Verdu, J.; Williams, R. Thermosetting Polymers; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Sperling, L. Introduction to Physical Polymer Science; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Giacomazza, D.; Bulone, D.; Biagio, P.L.S.; Marino, R.; Lapasin, R. The role of sucrose concentration in self-assembly kinetics of high methoxyl pectin. Int. J. Biol. Macromol. 2018, 112, 1183–1190. [Google Scholar] [CrossRef]
- Münstedt, H. Rheological Measurements and Structural Analysis of Polymeric Materials. Polymers 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Hsissou, R.; Elharfi, A.; Hsissou, R.; Elharfi, A. Rheological behavior of three polymers and their hybrid composites (TGEEBA/MDA/PN), (HGEMDA/MDA/PN) and (NGHPBAE/MDA/PN). J. King Saud Univ. Sci. 2020, 32, 235–244. [Google Scholar] [CrossRef]
- Martin, J. Study of the curing process of a vinyl ester resin by means of TSR and DMTA. Polymer 2000, 41, 4203–4211. [Google Scholar] [CrossRef]
- Markovic, S.; Dunjic, B.; Zlatanic, A.; Djonlagic, J. Dynamic mechanical analysis study of the curing of phenol-formaldehyde novolac resins. J. Appl. Polym. Sci. 2001, 81, 1902–1913. [Google Scholar] [CrossRef]
- Zhang, Z.; Beatty, E.; Wong, C.P. Study on the Curing Process and the Gelation of Epoxy/Anhydride System for No-Flow Underfill for Flip-Chip Applications. Macromol. Mater. Eng. 2003, 288, 365–371. [Google Scholar] [CrossRef]
- Ditta, L.A.; Bulone, D.; Biagio, P.L.S.; Marino, R.; Giacomazza, D.; Lapasin, R. The degree of compactness of the incipient High Methoxyl Pectin networks. A rheological insight at the sol-gel transition. Int. J. Biol. Macromol. 2020, 158, 985–993. [Google Scholar] [CrossRef]
- Shaw, M.T. Introduction to Polymer Rheology; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Mortimer, S.; Ryan, A.; Stanford, J.L. Rheological Behavior and Gel-Point Determination for a Model Lewis Acid-Initiated Chain Growth Epoxy Resin. Macromolecules 2001, 34, 2973–2980. [Google Scholar] [CrossRef]
- Rao, B.; Reddy, K.R.; Pathak, S.K.; Pasala, A. Benzoxazine-epoxy copolymers: Effect of molecular weight and crosslinking on thermal and viscoelastic properties. Polym. Int. 2005, 54, 1371–1376. [Google Scholar] [CrossRef]
- Anantharaman, A.; Ajeesha, T.; Baby, J.; George, M. Effect of structural, electrical and magneto-optical properties of CeMnxFe1−xO3−δ perovskite materials. Solid State Sci. 2019, 99, 105846. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zadhoush, A. Investigation of the relation between viscoelastic properties of polysulfone solutions, phase inversion process and membrane morphology: The effect of solvent power. J. Membr. Sci. 2017, 532, 47–57. [Google Scholar] [CrossRef]
- Tung, C.-Y.M.; Dynes, P.J. Relationship between viscoelastic properties and gelation in thermosetting systems. J. Appl. Polym. Sci. 1982, 27, 569–574. [Google Scholar] [CrossRef]
- Domínguez, J.; Alonso, M.; Oliet, M.; Rodríguez, F. Chemorheological study of the curing kinetics of a phenolic resol resin gelled. Eur. Polym. J. 2010, 46, 50–57. [Google Scholar] [CrossRef]
- Chambon, F.; Winter, H.H. Linear Viscoelasticity at the Gel Point of a Crosslinking PDMS with Imbalanced Stoichiometry. J. Rheol. 1987, 31, 683–697. [Google Scholar] [CrossRef]
- Li, S.; Tang, M.; Huang, C.; Zhang, R.; Wu, J.; Ling, F.; Xu, Y.-X.; Huang, G. Branching function of terminal phosphate groups of polyisoprene chain. Polymer 2019, 174, 18–24. [Google Scholar] [CrossRef]
- Lewis, C.L.; Stewart, K.; Anthamatten, M. The Influence of Hydrogen Bonding Side-Groups on Viscoelastic Behavior of Linear and Network Polymers. Macromolecules 2014, 47, 729–740. [Google Scholar] [CrossRef]
- Baeurle, S.A.; Hotta, A.; Gusev, A.A. A new semi-phenomenological approach to predict the stress relaxation behavior of thermoplastic elastomers. Polymer 2005, 46, 4344–4354. [Google Scholar] [CrossRef]
- Ravve, A. Principles of Polymer Chemistry; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Lodge, T.P. Polymer Chemistry; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Stevens, M.P. Polymer Chemistry—An Introduction, 3rd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Vollmert, B. Polymer Chemistry; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Wu, H.; Morbidelli, M. A Model Relating Structure of Colloidal Gels to Their Elastic Properties. Langmuir 2001, 17, 1030–1036. [Google Scholar] [CrossRef]
- Yang, Z.; Xin, C.; Mughal, W.; Li, X.; He, Y. High-melt-elasticity poly(ethylene terephthalate) produced by reactive extrusion with a multi-functional epoxide for foaming. J. Appl. Polym. Sci. 2018, 135, 45805. [Google Scholar] [CrossRef]
- Tian, J.; Yu, W.; Zhou, C. The preparation and rheology characterization of long chain branching polypropylene. Polymer 2006, 47, 7962–7969. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sienkiewicz, A.; Czub, P. Rheological Analysis of the Synthesis of High-Molecular-Weight Epoxy Resins from Modified Soybean Oil and Bisphenol A or BPA-Based Epoxy Resins. Materials 2021, 14, 6770. https://doi.org/10.3390/ma14226770
Sienkiewicz A, Czub P. Rheological Analysis of the Synthesis of High-Molecular-Weight Epoxy Resins from Modified Soybean Oil and Bisphenol A or BPA-Based Epoxy Resins. Materials. 2021; 14(22):6770. https://doi.org/10.3390/ma14226770
Chicago/Turabian StyleSienkiewicz, Anna, and Piotr Czub. 2021. "Rheological Analysis of the Synthesis of High-Molecular-Weight Epoxy Resins from Modified Soybean Oil and Bisphenol A or BPA-Based Epoxy Resins" Materials 14, no. 22: 6770. https://doi.org/10.3390/ma14226770





