Evaluation of the Effects of Surface Treatment Methods on the Properties of Coral Aggregate and Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Crush Index
2.2.2. Water Absorption
2.2.3. Slump
2.2.4. Microhardness
2.2.5. Compressive Strength
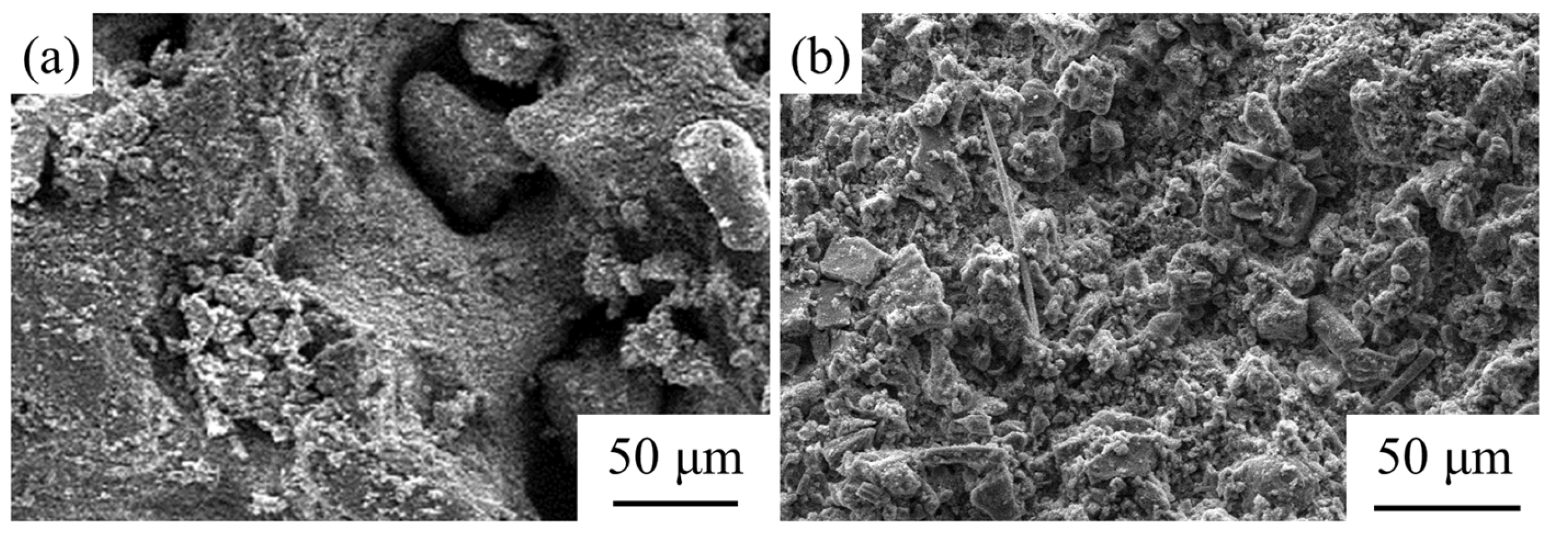
2.2.6. SEM
2.2.7. Aggregate Modification Method
- (1)
- Modification of the granulated blast furnace slag solution
- (2)
- Modification of the sodium silicate solution
- (3)
- Compound modification
3. Results and Discussion
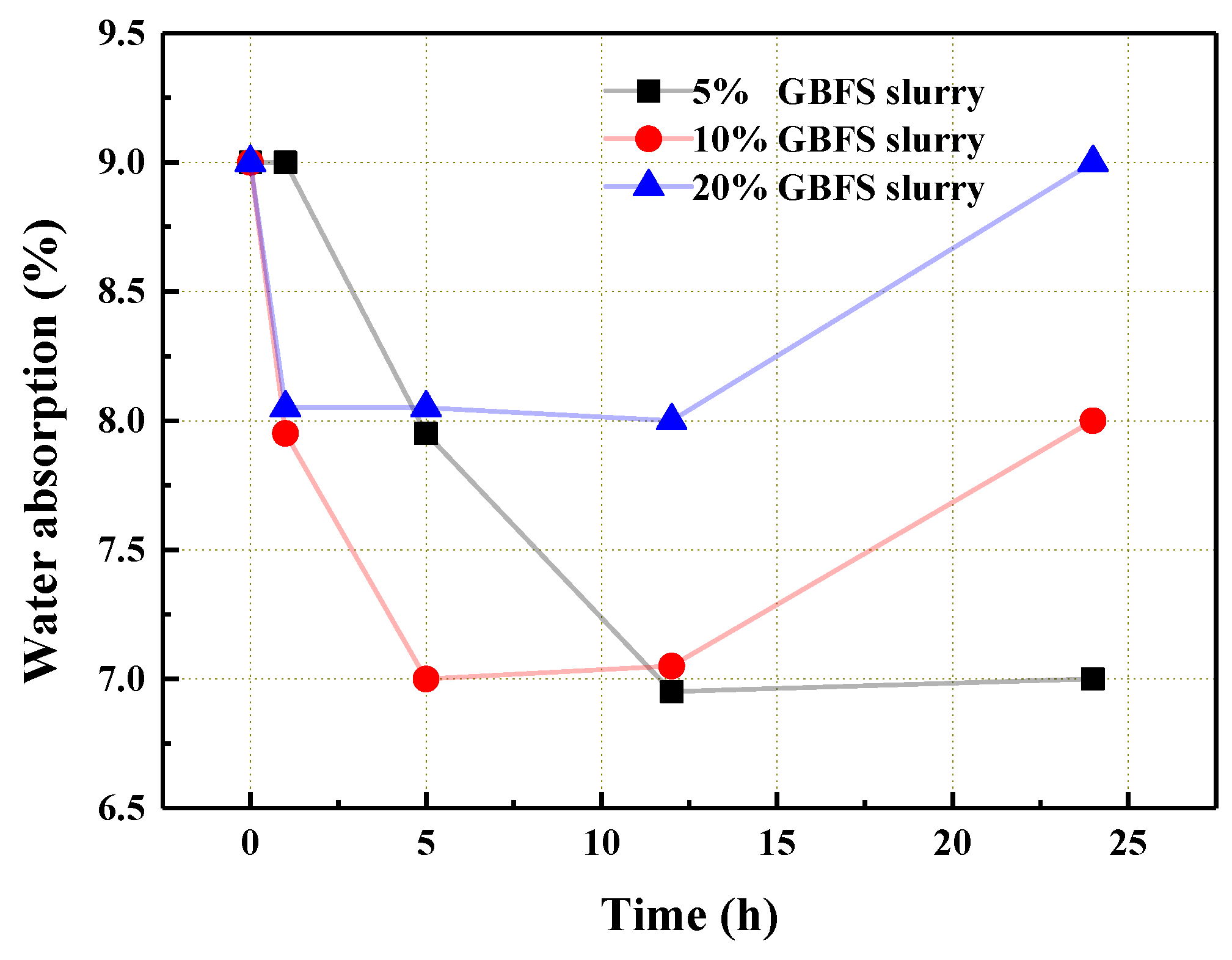
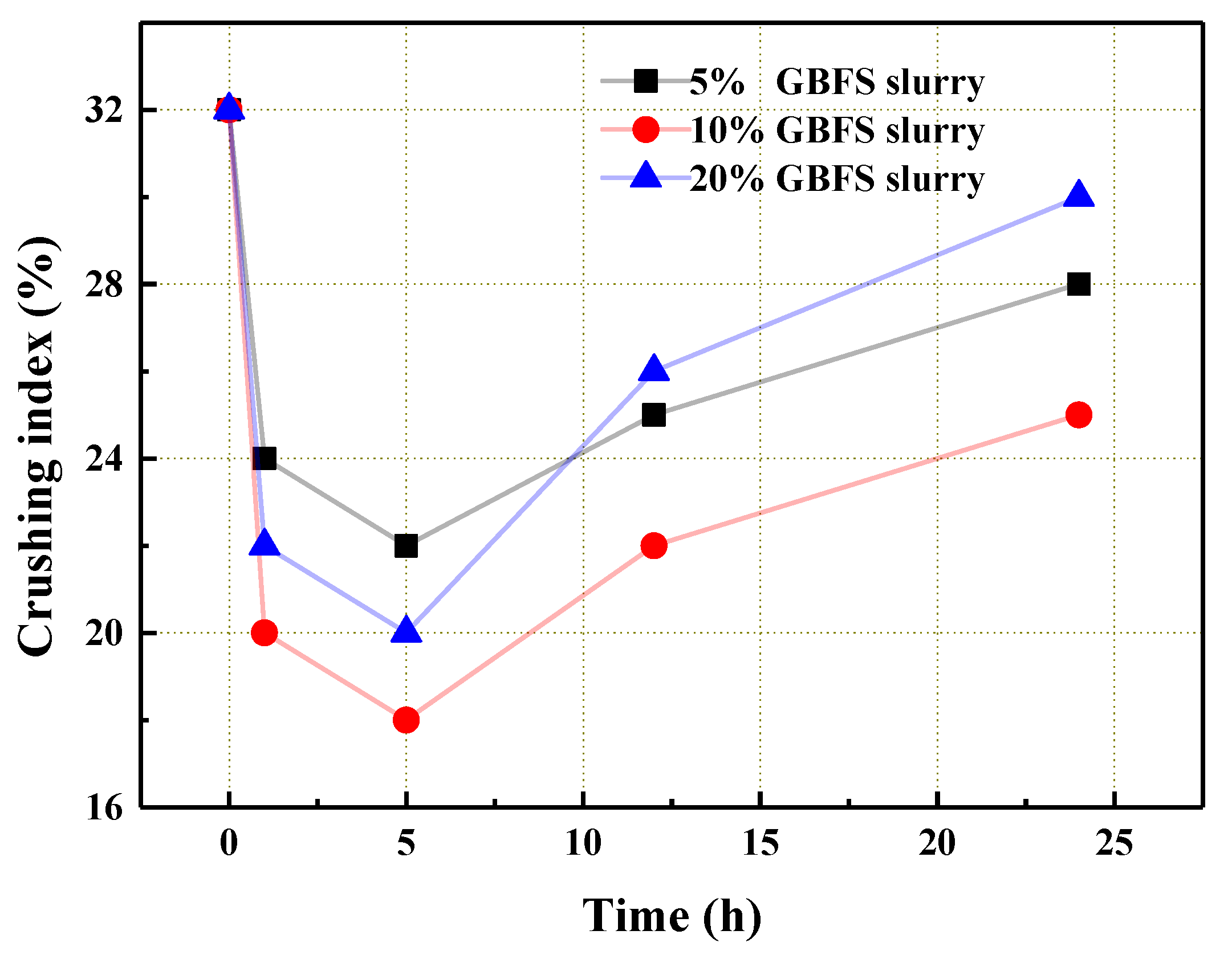
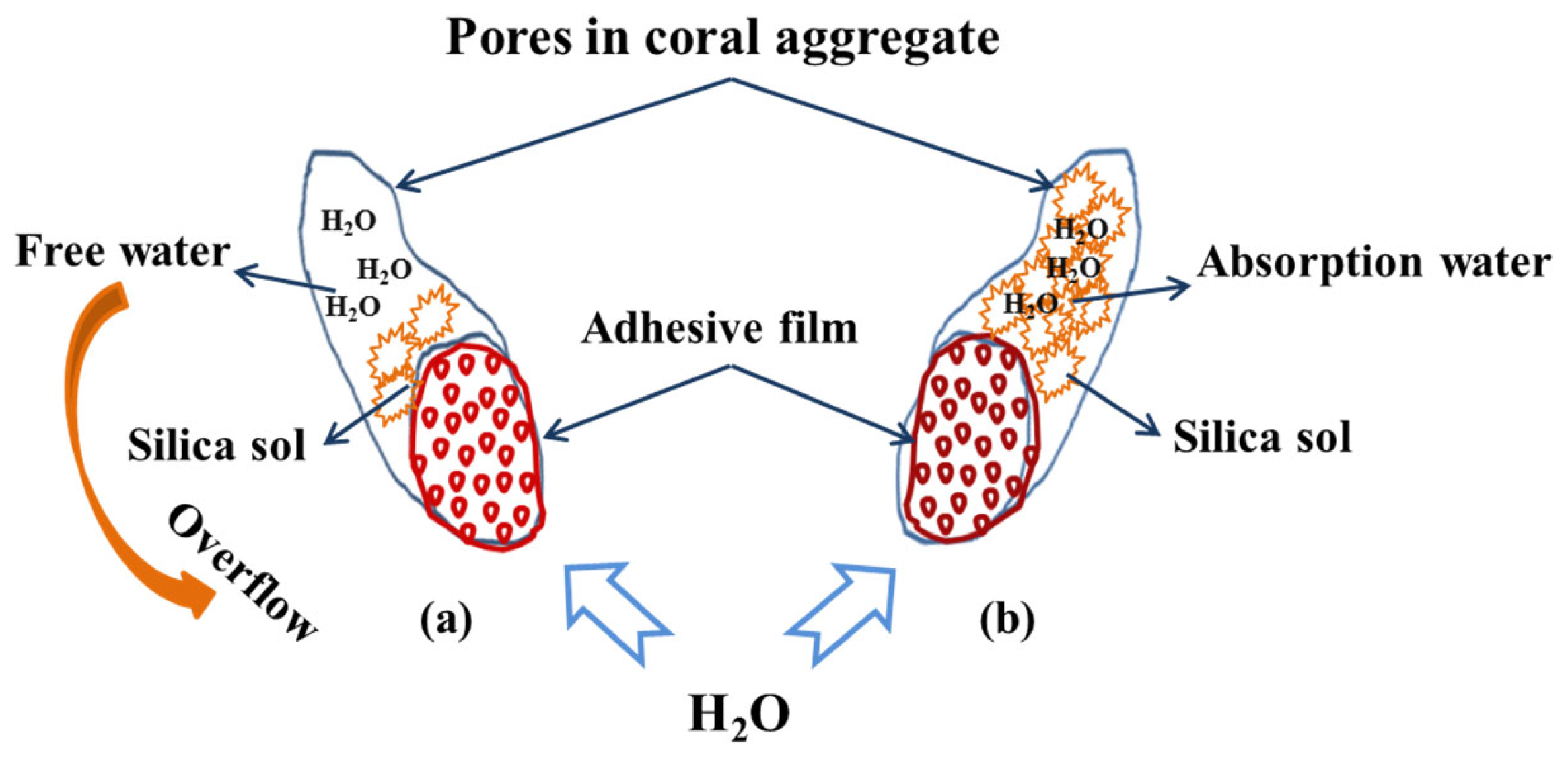
3.1. The Influence of GBFS Slurry Modification on the Performance of the Aggregate
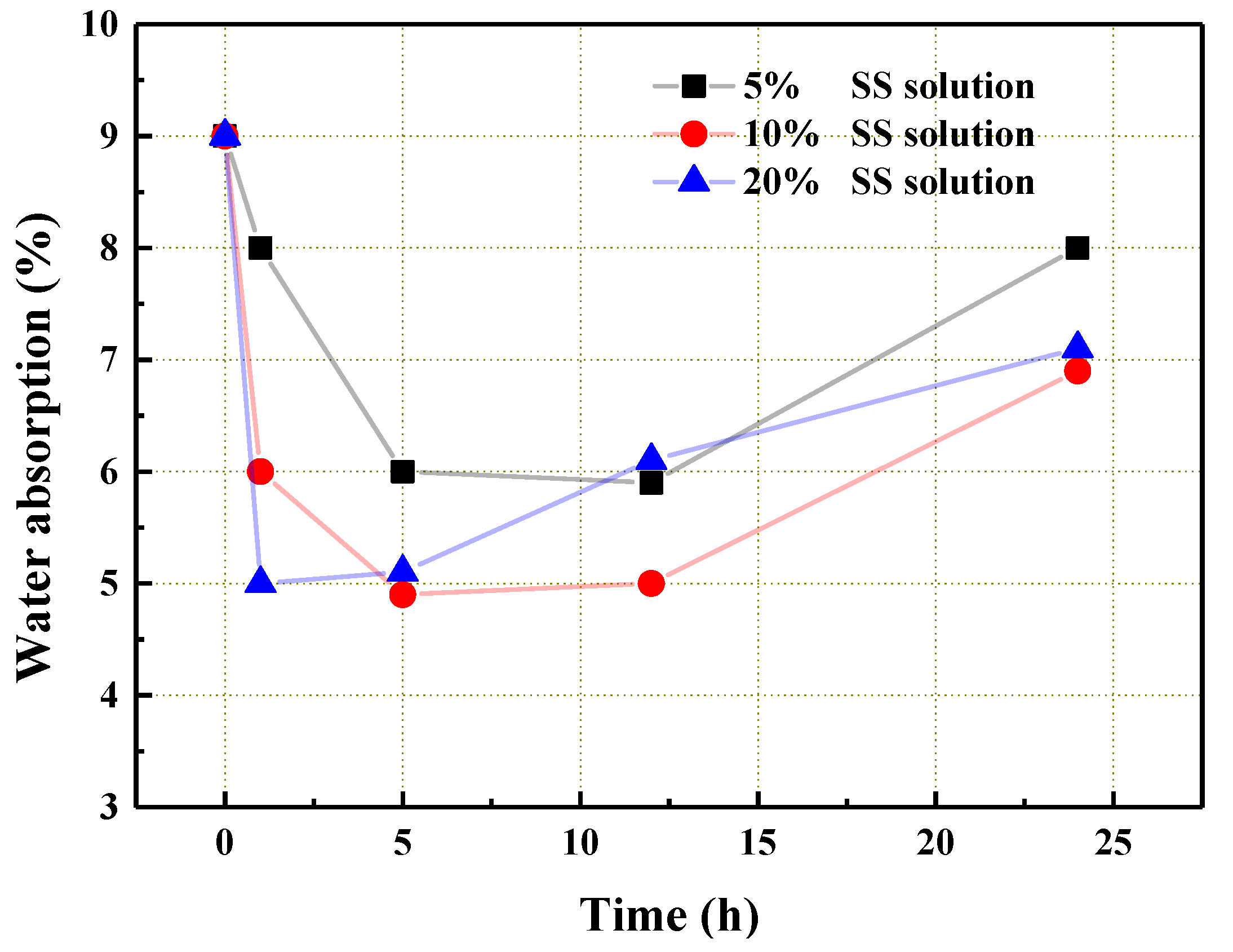
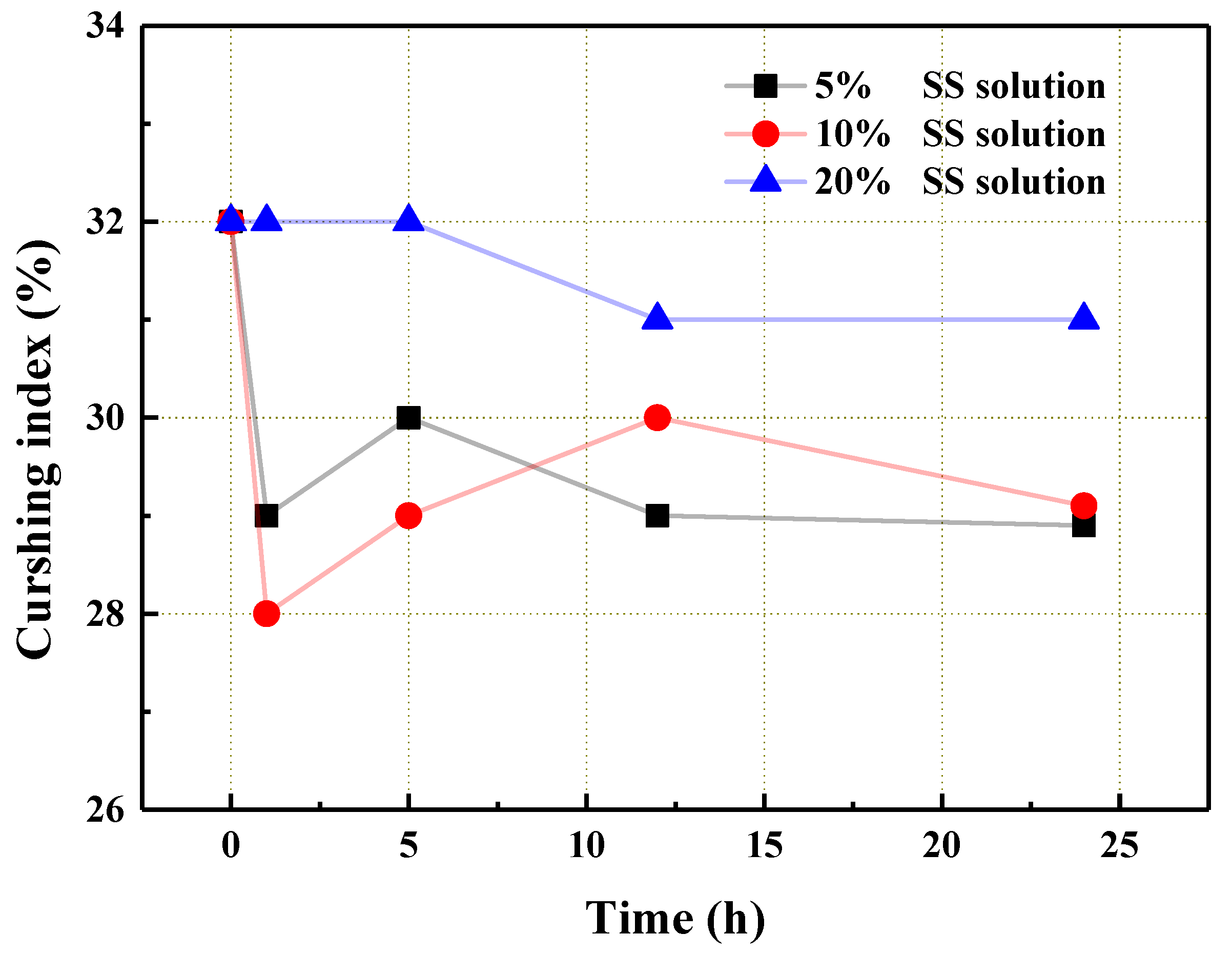
3.2. The Influence of SS Solution Modification on the Performance of the Aggregate
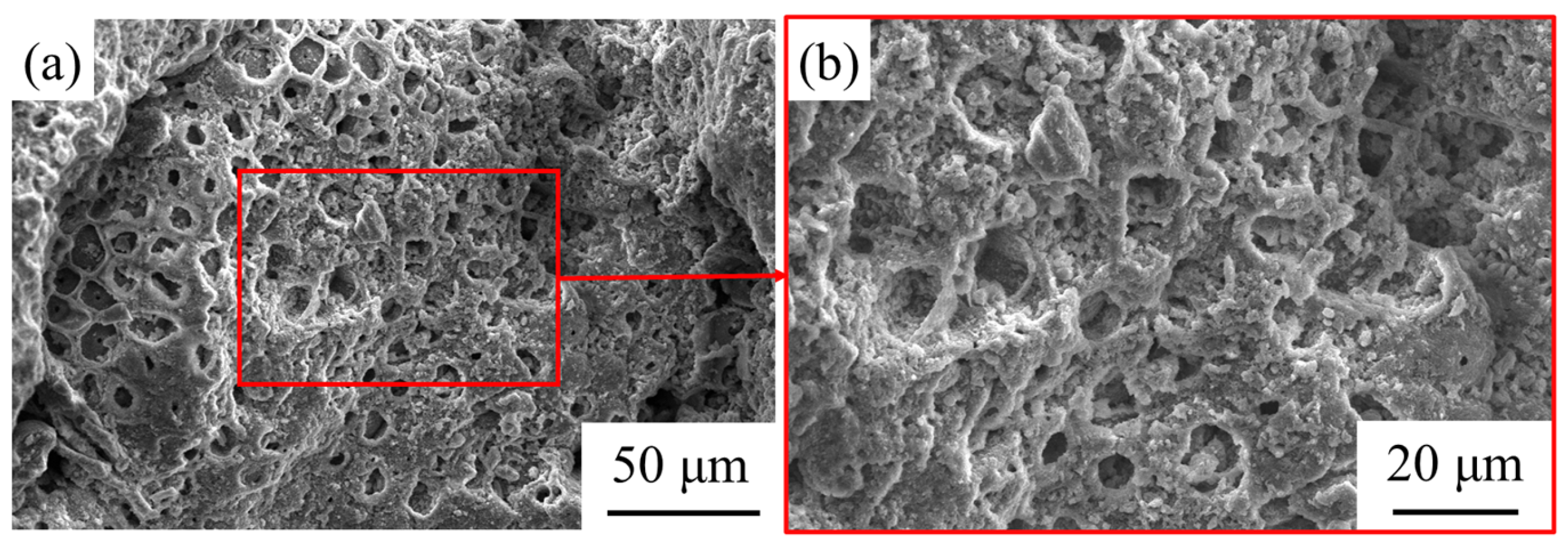
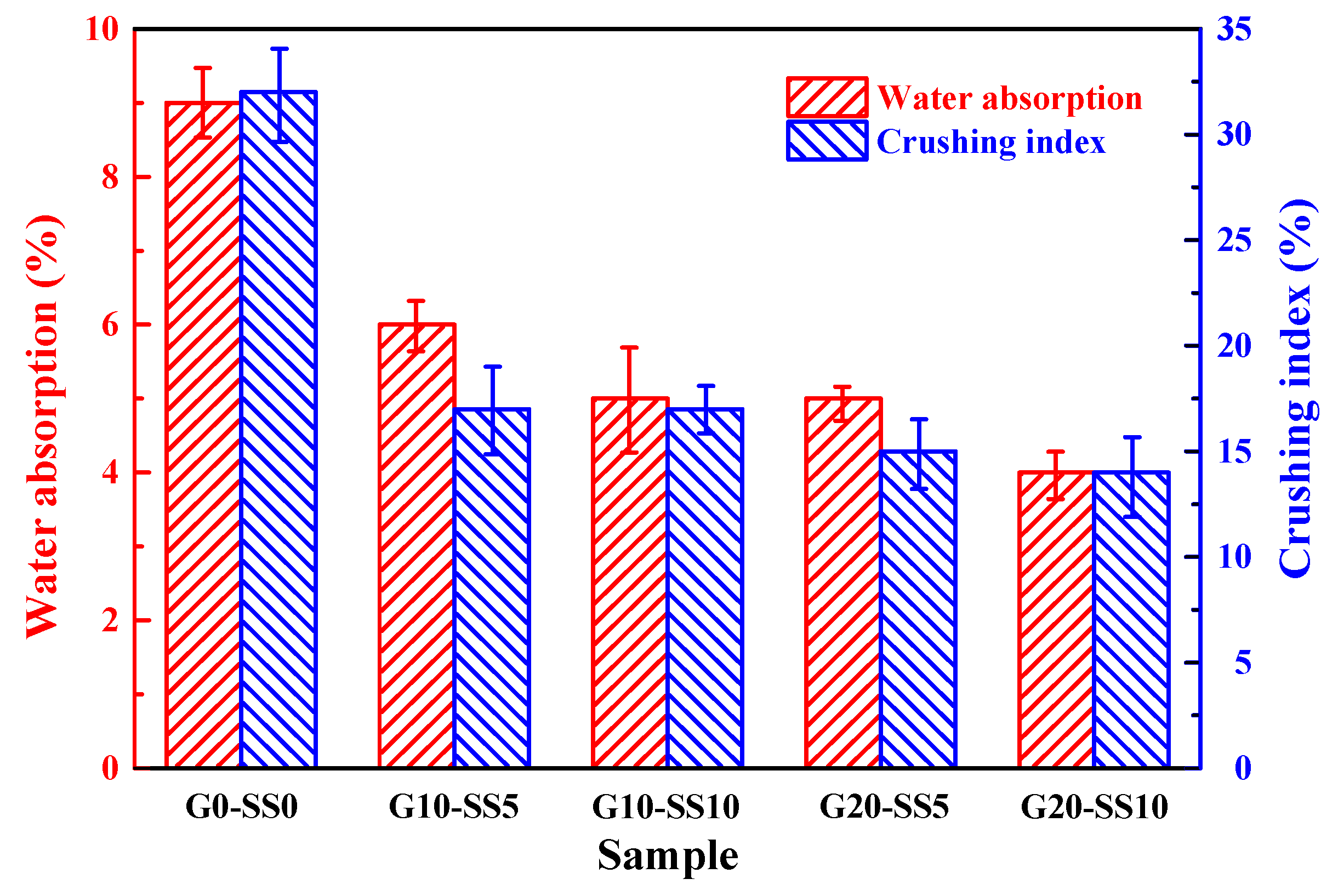
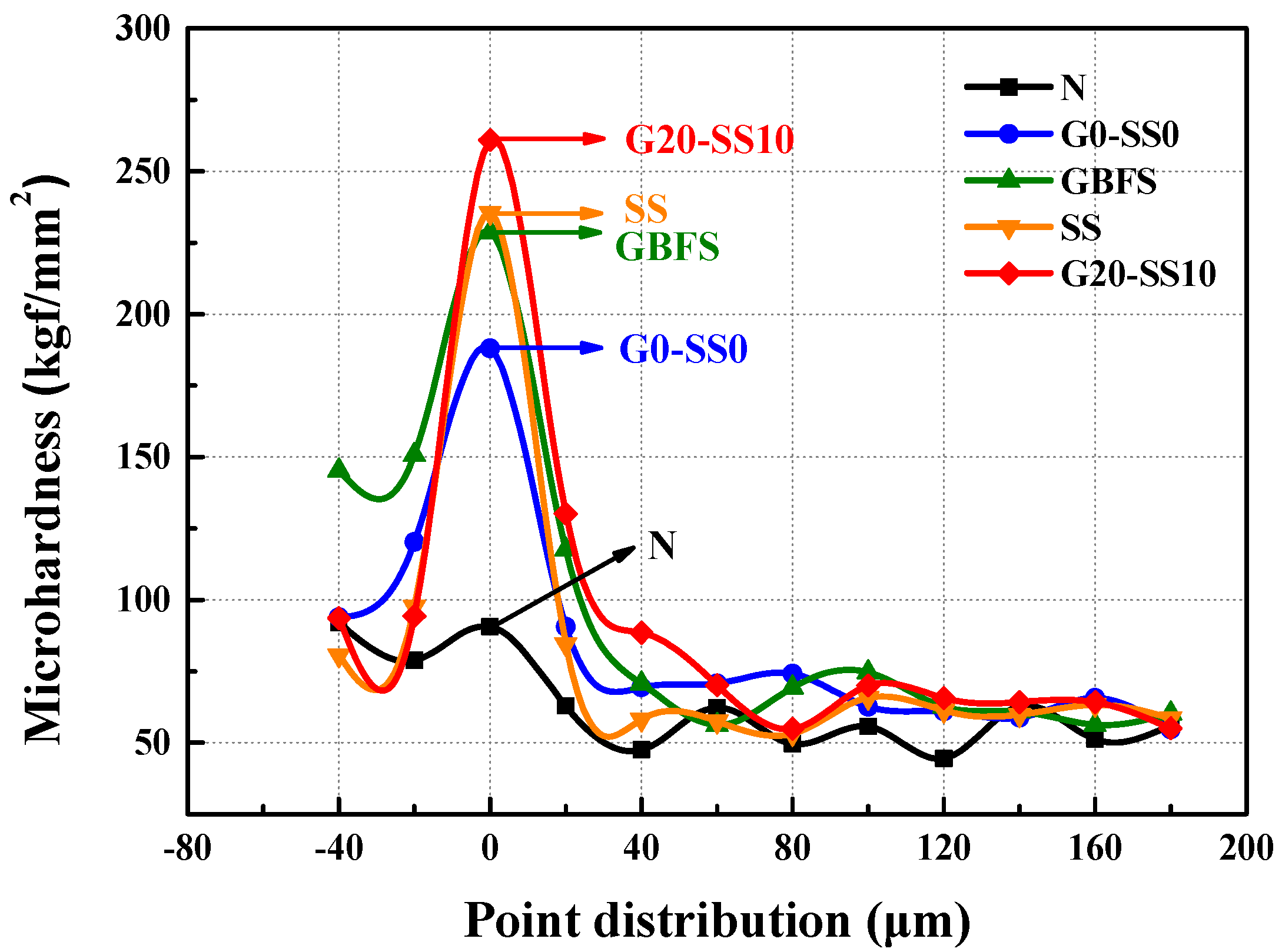
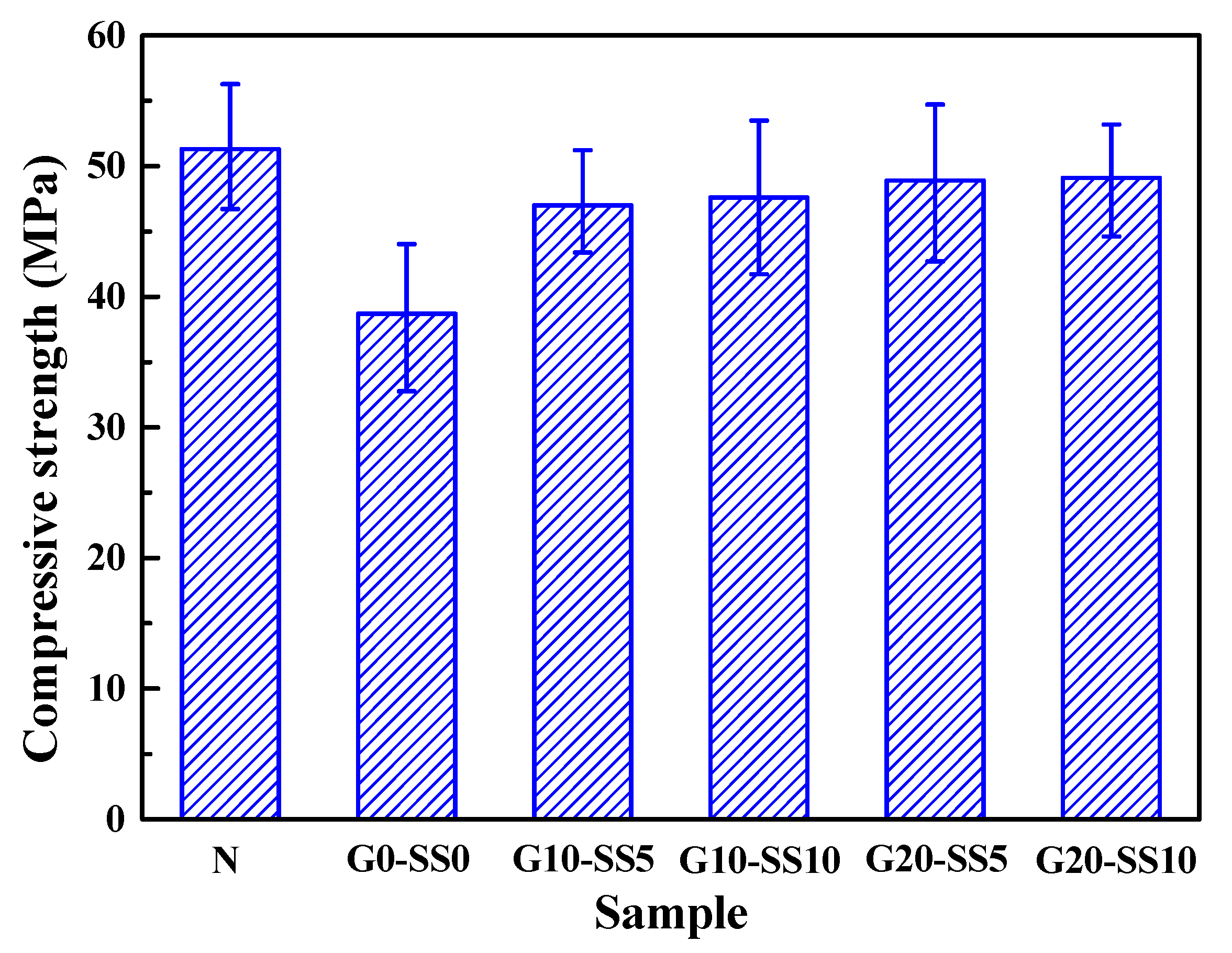
3.3. The Influence of GBFS–SS Compound Modification on the Performance of the Aggregate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.M.; Ou, Z.W.; Peng, W.; Guo, T.; Deng, W.; Chen, Y.Z. Literature Review of Coral Concrete. Arab. J. Sci. Eng. 2018, 43, 1529–1541. [Google Scholar] [CrossRef]
- Da, B.; Yu, H.F.; Ma, H.Y.; Tan, Y.S.; Mi, R.J.; Dou, X.M. Experimental investigation of whole stress-strain curves of coral concrete. Constr. Build. Mater. 2016, 122, 81–89. [Google Scholar] [CrossRef]
- Mi, R.J.; Yu, H.F.; Ma, H.Y.; Da, B.; Yuan, Y.F.; Zhang, X.P.; Zhu, H.W.; Dou, X.M. Study on the mechanical property of coral concrete. Ocean Eng. 2016, 34, 47–54. [Google Scholar]
- Wang, L.; Liu, C.; Xiong, Z. Study test on mechanical property of sisal fiber reinforced coral concrete. J. Henan Polytech. Univ. 2014, 33, 826–830. [Google Scholar]
- Tsujino, M.; Noguchi, T.; Tamura, M.; Kanematsu, M.; Maruyama, I. Application of conventionally recycled coarse aggregate to concrete structure by surface modification treatment. J. Adv. Concr. Technol. 2007, 5, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Ismail, S.; Ramli, M. Engineering properties of treated recycled concrete aggregate (RCA) for structural applications. Constr. Build. Mater. 2013, 44, 464–476. [Google Scholar] [CrossRef]
- Shi, C.J.; Li, Y.K.; Zhang, J.K.; Li, W.G.; Chong, L.L.; Xie, Z.B. Performance enhancement of recycled concrete aggregate—A review. J. Clean. Prod. 2016, 112, 466–472. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Kou, S.C.; Poon, C.S.; Dai, J.G.; Li, Q.Y. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Klama, J.; Zawal, D.; Krupa, D. Modification of recycled concrete aggregate by calcium carbonate biodeposition. Constr. Build. Mater. 2012, 34, 145–150. [Google Scholar] [CrossRef]
- Xuan, D.X.; Molenaar, A.A.A.; Houben, L.J.M. Compressive and Indirect Tensile Strengths of Cement-Treated Mix Granulates with Recycled Masonry and Concrete Aggregates. J. Mater. Civ. Eng. 2012, 24, 577–585. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Wang, S.D.; Lu, L.C.; Gong, C.C. Evaluation of pre-coated recycled aggregate for concrete and mortar. Constr. Build. Mater. 2013, 43, 191–196. [Google Scholar] [CrossRef]
- Du, T.; Li, H.Q.; Wu, X.G. Experimental study on enhancement of recycled aggregate. New Build. Mater. 2002, 3, 6–8. [Google Scholar]
- Guneyisi, E.; Gesoglu, M.; Algin, Z.; Yazici, H. Effect of surface treatment methods on the properties of self-compacting concrete with recycled aggregates. Constr. Build. Mater. 2014, 64, 172–183. [Google Scholar] [CrossRef]
- Zhang, H.R.; Zhao, Y.X.; Meng, T.; Shah, S.P. Surface Treatment on Recycled Coarse Aggregates with Nanomaterials. J. Mater. Civ. Eng. 2016, 28, 04015094. [Google Scholar] [CrossRef]
- Katz, A. Treatments for the improvement of recycled aggregate. J. Mater. Civ. Eng. 2004, 16, 597–603. [Google Scholar] [CrossRef]
- Li, W.; Long, C.; Luo, Z.; Huang, Z. Investigation on Failure Mechanism of Nanomodified Recycled Aggregate Concrete. J. Build. Mater. 2017, 20, 685–691. [Google Scholar]
- Ondova, M.; Sicakova, A. Evaluation of the Influence of Specific Surface Treatments of RBA on a Set of Properties of Concrete. Materials 2016, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.C.; Poon, C.S. Properties of concrete prepared with PVA-impregnated recycled concrete aggregates. Cem. Concr. Compos. 2010, 32, 649–654. [Google Scholar] [CrossRef]
- Wan, H.W.; Xu, J.L.; Shui, Z.H.; Jiang, J. Study on the Structure and Properties of Interfacial Transition Zone (ITZ) of the Regenerated Concrete. J. Wuhan Univ. Technol. 2004, 26, 29–32. [Google Scholar]
- Mansur, A.A.P.; Santos, D.B.; Mansur, H.S. A microstructural approach to adherence mechanism of poly (vinyl alcohol) modified cement systems to ceramic tiles. Cem. Concr. Res. 2007, 37, 270–282. [Google Scholar] [CrossRef]
- Spaeth, V.; Tegguer, A.D. Improvement of recycled concrete aggregate properties by polymer treatments. Int. J. Sustain. Built Environ. 2013, 2, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Santos, W.F.; Quattrone, M.; John, V.M.; Angulo, S.C. Roughness, wettability and water absorption of water repellent treated recycled aggregates. Constr. Build. Mater. 2017, 146, 502–513. [Google Scholar] [CrossRef]
- Ghasemi, S.; Zohrevand, P.; Mirmiran, A.; Xiao, Y.L.; Mackie, K. A super lightweight UHPC-HSS deck panel for movable bridges. Eng. Struct. 2016, 113, 186–193. [Google Scholar] [CrossRef]
- National Standard Management Committee of China. Pebble and Crushed Stone for Construction: GB/T 14685-2011; China Building Industry Press: Beijing, China, 2011. [Google Scholar]
- Yuan, Y.F. Mix Design and Property of Coral Aggregate Concrete; Nanjing University of Aeronautics and Astronautics: Nanjing, China, 2015. [Google Scholar]
- Ministry of Construction of the People’s Republic of China. Standard for Test Method of Performance on Ordinary Fresh Concrete: GB/T 50080-2002; China Building Industry Press: Beijing, China, 2002.
- Ministry of Construction of the People’s Republic of China. Standard for Test Method of Mechanical Properties on Ordinary Fresh Concrete: GB/T 50081-2002; China Building Industry Press: Beijing, China, 2002.
- Atahan, H.N.; Dikme, D. Use of mineral admixtures for enhanced resistance against sulfate attack. Constr. Build. Mater. 2011, 25, 3450–3457. [Google Scholar] [CrossRef]
- Arail, Y.; Powell, B.A.; Kaplan, D.I. Sulfur speciation in untreated and alkali treated ground-granulated blast furnace slag. Sci. Total Environ. 2017, 589, 117–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Cheng, H.L.; Wang, C.Y. The influence of sodium silicate on the properties of recycled aggregate. Cem. Concr. Res. 2005, 12, 12–14. [Google Scholar]
- Fujiwara, M.; Shiokawa, K.; Araki, M.; Nakao, M.; Sakakura, I.; Nakahara, Y. Preparation of silica thin films with macropore holes from sodium silicate and polymethacrylate: An approach to formation mechanism of diatomaceous earth like silica hollow particles. Chem. Eng. J. 2011, 172, 1103–1110. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A.M. Performance and properties of structural concrete made with recycled concrete aggregate. Am. Concr. Inst. Mater. J. 2003, 100, 371–380. [Google Scholar]
- Guo, D. The film forming machine and its modified path of water glass coating. Chem. Build. Mater. 1988, 4, 19–22. [Google Scholar]
- Chang, J.J. A study on the setting characteristics of sodium silicate-activated slag pastes. Cem. Concr. Res. 2003, 33, 1005–1011. [Google Scholar] [CrossRef]
- Bilim, C.; Atis, C.D. Alkali activation of mortars containing different replacement levels of ground granulated blast furnace slag. Constr. Build. Mater. 2012, 28, 708–712. [Google Scholar] [CrossRef]
- Granat, K.; Nowak, D.; Pigiel, M.; Stachowicz, M.; Wikiera, R. Microwaves energy in curing process of water glass molding sands. Arch. Foundry Eng. 2007, 7, 183–188. [Google Scholar]
- Phoo-Ngernkham, T.; Maegawa, A.; Mishima, N.; Hatanaka, S.; Chindaprasirt, P. Effects of sodium hydroxide and sodium silicate solutions on compressive and shear bond strengths of FA-GBFS geopolymer. Constr. Build. Mater. 2015, 91, 1–8. [Google Scholar] [CrossRef]
- San Nicolas, R.; Bernal, S.A.; de Gutierrez, R.M.; van Deventer, J.S.J.; Provis, J.L. Distinctive microstructural features of aged sodium silicate-activated slag concretes. Cem. Concr. Res. 2014, 65, 41–51. [Google Scholar] [CrossRef]
- Barnett, S.J.; Soutsos, M.N.; Millard, S.G.; Bungey, J.H. Strength development of mortars containing ground granulated blast-furnace slag: Effect of curing temperature and determination of apparent activation energies. Cem. Concr. Res. 2006, 36, 434–440. [Google Scholar] [CrossRef]
- Gaggiano, R.; Moriame, P.; Biesemans, M.; De Graeve, I.; Terryn, H. Influence of SiO2/Na2O ratio and temperature on the mechanism of interaction of soluble sodium silicates with porous anodic alumina. Surf. Coat. Technol. 2011, 206, 1269–1276. [Google Scholar] [CrossRef]
- Arumugam, R.A.; Ramamurthy, K. Study of compressive strength characteristics of coral aggregate concrete. Mag. Concr. Res. 1996, 48, 141–148. [Google Scholar] [CrossRef]
- Husem, M. The effects of bond strengths between lightweight and ordinary aggregate-mortar, aggregate-cement paste on the mechanical properties of concrete. Mat. Sci. Eng. A Struct. 2003, 363, 152–158. [Google Scholar] [CrossRef]
- Onoue, K.; Bier, T.A. Optimization of alkali-activated mortar utilizing ground granulated blast-furnace slag and natural pozzolan from Germany with the dynamic approach of the Taguchi method. Constr. Build. Mater. 2017, 144, 357–372. [Google Scholar] [CrossRef]
- Wongpa, J.; Cheerarot, R.; Jantathai, S. Compressive Strength Development of Inorganic Polymeric Mortars: Effects of Water Glass and Curing. Mahasarakham Int. J. Eng. Technol. 2015, 1, 1–5. [Google Scholar]
- Hou, C.; Zhou, Y.; Hong, L.; Yong, K. The Analysis of the Solidification Mechanismes & Water Resistance Improvement Accesses of the Water-Glass. Ceramics 2011, 5, 44–47. [Google Scholar]
- Rashad, A.M.; Zeedan, S.R.; Hassan, A.A. Influence of the activator concentration of sodium silicate on the thermal properties of alkali-activated slag pastes. Constr. Build. Mater. 2016, 102, 811–820. [Google Scholar] [CrossRef]
- Rakhimova, N.R.; Rakhimov, R.Z.; Morozov, V.P.; Potapova, L.I.; Osin, Y.N. Mechanism of solidification of simulated borate liquid wastes with sodium silicate activated slag cements. J. Clean. Prod. 2017, 149, 60–69. [Google Scholar] [CrossRef]
- Nursyamsi; Zebua, W.S.B. The influence of pet plastic waste gradations as coarse aggregate towards compressive strength of light concrete. Procedia Eng. 2017, 171, 614–619. [Google Scholar] [CrossRef]
- Ismail, S.; Kwan, W.H.; Ramli, M. Mechanical strength and durability properties of concrete containing treated recycled concrete aggregates under different curing conditions. Constr. Build. Mater. 2017, 155, 296–306. [Google Scholar] [CrossRef]
- Diamond, S.; Huang, J.D. The ITZ in concrete—A different view based on image analysis and SEM observations. Cem. Concr. Compos. 2001, 23, 179–188. [Google Scholar] [CrossRef]
- Kong, D.Y.; Lei, T.; Zheng, J.J.; Ma, C.C.; Jiang, J.; Jiang, J. Effect and mechanism of surface-coating pozzalanics materials around aggregate on properties and ITZ microstructure of recycled aggregate concrete. Constr. Build. Mater. 2010, 24, 701–708. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Strength and Microstructure of alkali-activated binary blended binder containing palm oil fuel ash and ground blast-furnace slag. Constr. Build. Mater. 2014, 52, 504–510. [Google Scholar] [CrossRef]
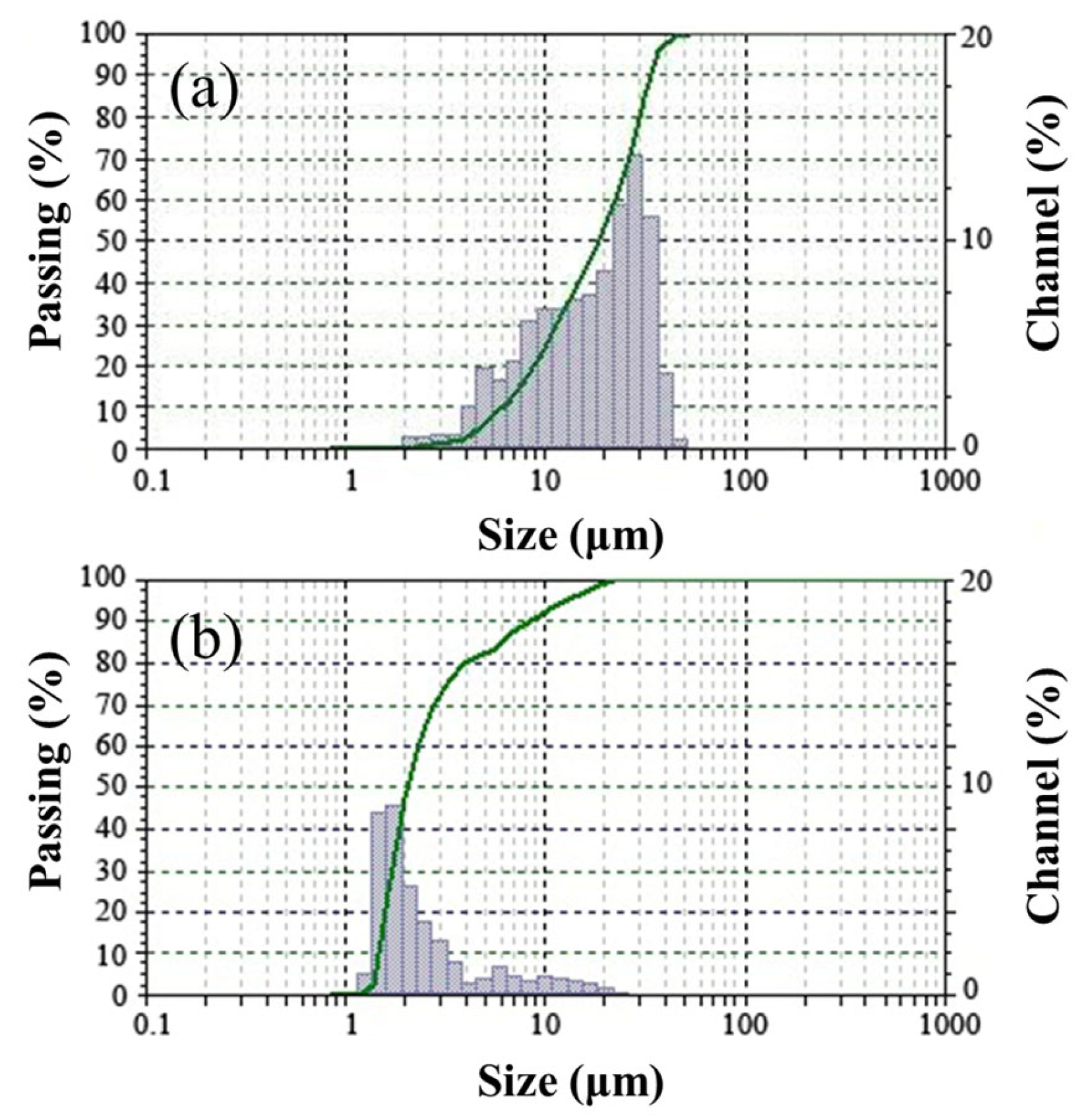

| Properties | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | MnO | K2O | SO3 | Loss on Ignition | Powder Density (g/cm3) | Specific Density (g/cm3) | Specific Surface Area (m2/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 64.13 | 21.43 | 2.24 | 3.78 | 2.07 | 0.78 | - | - | 2.25 | 3.32 | 1.4–1.6 | 3.0–3.1 | 350–400 |
| GBFS | 45.75 | 31.4 | 12.3 | 0.79 | 5.25 | 0.42 | 0.51 | 0.37 | 2.32 | 0.89 | 1.0–1.3 | 2.8–3.0 | 400–450 |
| Bore diameter (mm) | 26.5 | 19 | 16 | 9.5 | 4.75 | 2.36 |
| The cumulative triage (%) | 0 | 8 | 20.5 | 77 | 92.8 | 97.9 |
| Basic Properties | Packing Density (kg/m3) | Close Packing Density (kg/m3) | Bibulous Rate (%) | Silt Content (%) | Chloride Ion Content (%) |
|---|---|---|---|---|---|
| Coarse coral aggregate | 1264 | 1380 | 9 | 2.35 | 0.074 |
| Fine coral aggregate | 1115 | 1225 | 5 | 0.50 | 0.052 |
| Number | GBFS (%) | SS (%) |
|---|---|---|
| G0-SS0 | 0 | 0 |
| G10-SS5 | 10 | 5 |
| G10-SS10 | 10 | 10 |
| G20-SS5 | 20 | 5 |
| G20-SS10 | 20 | 10 |
| GBFS | 20 | 0 |
| SS | 0 | 20 |
| Specimen | Cement (kg/m3) | Aggregate (kg/m3) | Sand Rate | Water (kg/m3) | Superplasticizer (kg/m3) | Slump (mm) |
|---|---|---|---|---|---|---|
| N | 500 | 750 | 36% | 200 | 2 | 93 |
| G0-SS0 | 500 | 750 | 36% | 200 | 2 | 36 |
| G10-SS5 | 500 | 750 | 36% | 200 | 2 | 47 |
| G10-SS10 | 500 | 750 | 36% | 200 | 2 | 68 |
| G20-SS5 | 500 | 750 | 36% | 200 | 2 | 43 |
| G20-SS10 | 500 | 750 | 36% | 200 | 2 | 72 |
| GBFS | 500 | 750 | 36% | 200 | 2 | 38 |
| SS | 500 | 750 | 36% | 200 | 2 | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Ju, B.; Xie, W.; Zhou, T.; Xiao, H.; Dong, S.; Yang, W. Evaluation of the Effects of Surface Treatment Methods on the Properties of Coral Aggregate and Concrete. Materials 2021, 14, 6784. https://doi.org/10.3390/ma14226784
Liu J, Ju B, Xie W, Zhou T, Xiao H, Dong S, Yang W. Evaluation of the Effects of Surface Treatment Methods on the Properties of Coral Aggregate and Concrete. Materials. 2021; 14(22):6784. https://doi.org/10.3390/ma14226784
Chicago/Turabian StyleLiu, Jinming, Boyu Ju, Wei Xie, Ting Zhou, Haiying Xiao, Shanliang Dong, and Wenshu Yang. 2021. "Evaluation of the Effects of Surface Treatment Methods on the Properties of Coral Aggregate and Concrete" Materials 14, no. 22: 6784. https://doi.org/10.3390/ma14226784






