Synthesis of Betaine Copolymer for Surface Modification of Cotton Fabric by Enhancing Temperature-Sensitive and Anti-Protein Specific Absorption Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
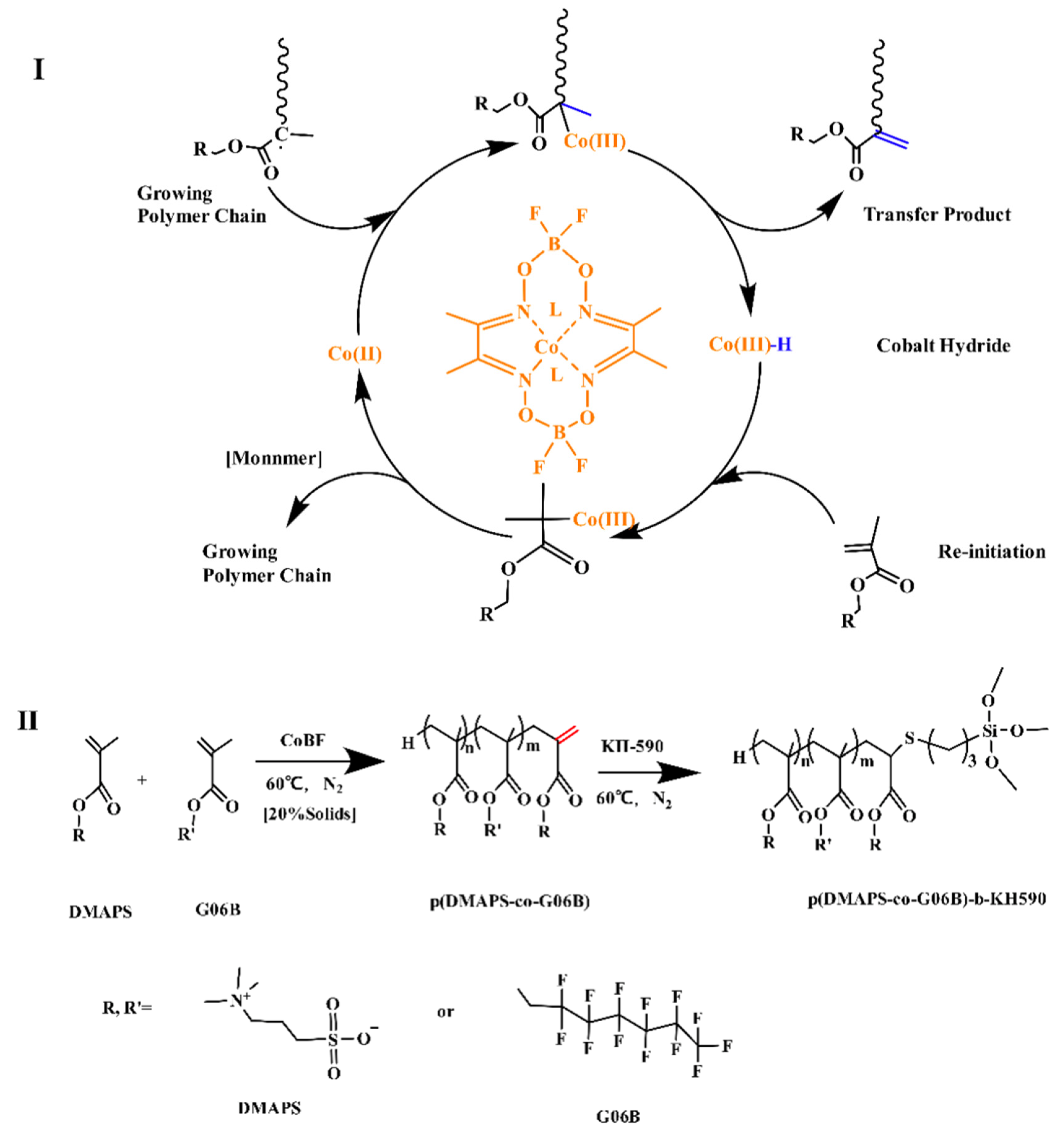
2.2. Manufacture of Betaine Copolymer
2.3. Cotton Fabric Treatment
2.4. Methods
2.4.1. Characterization of Betaine Copolymer
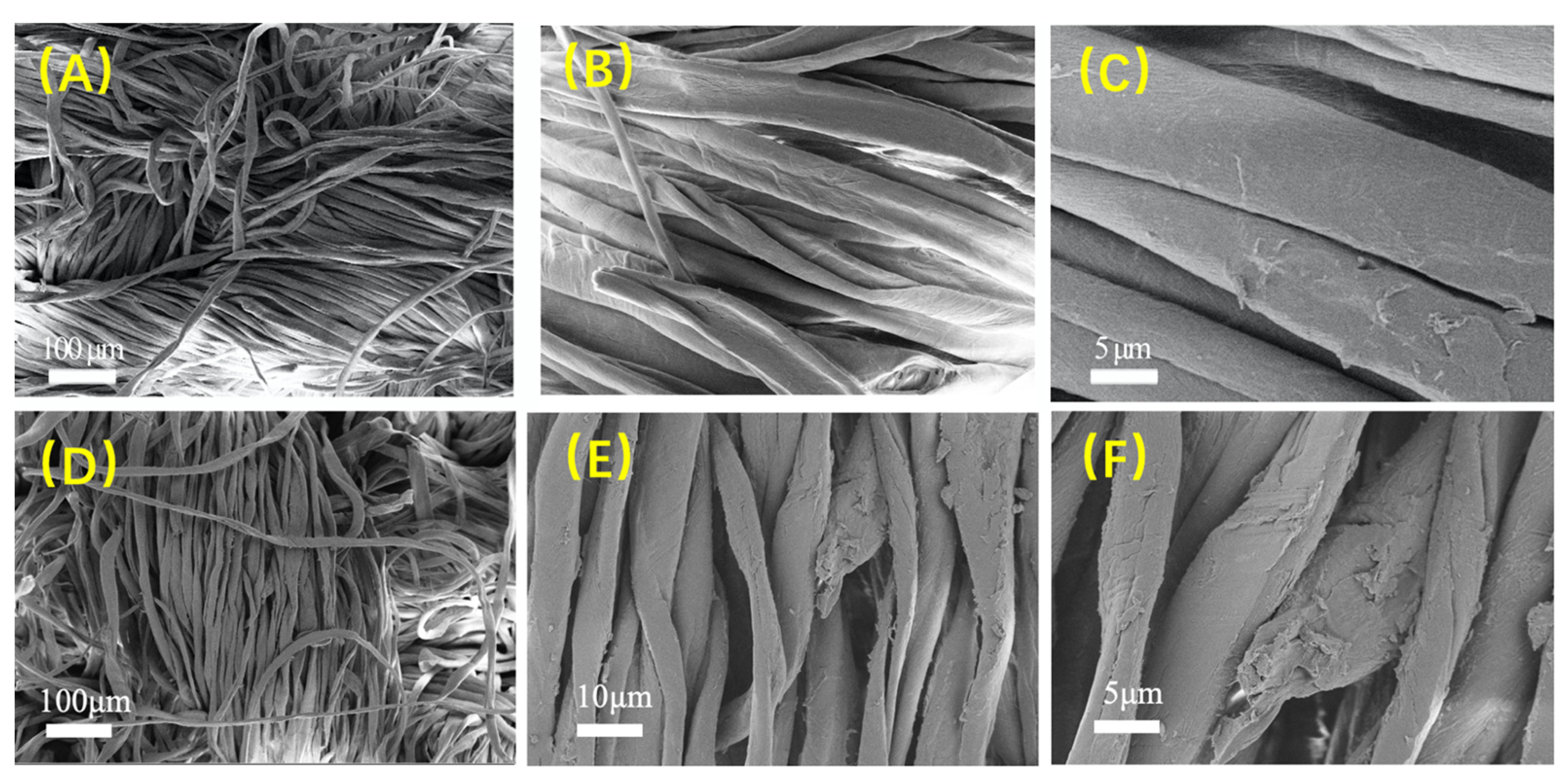
2.4.2. Surface Morphology Characterization of Cotton Fabric
2.4.3. Temperature-Sensitive Performance Analysis of Cotton Fabric
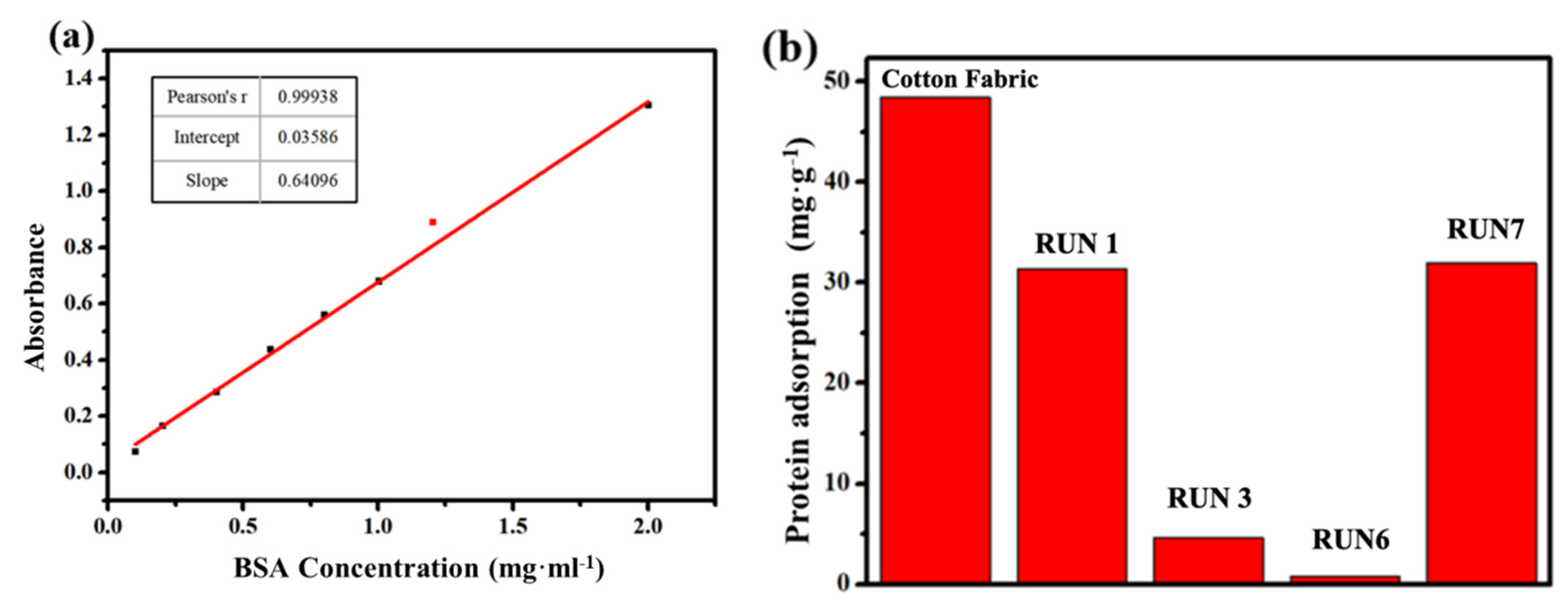
2.4.4. Anti-Protein Adsorption Performance Analysis of Cotton Fabric
3. Results and Discussion
3.1. Characterization of Betaine Copolymer
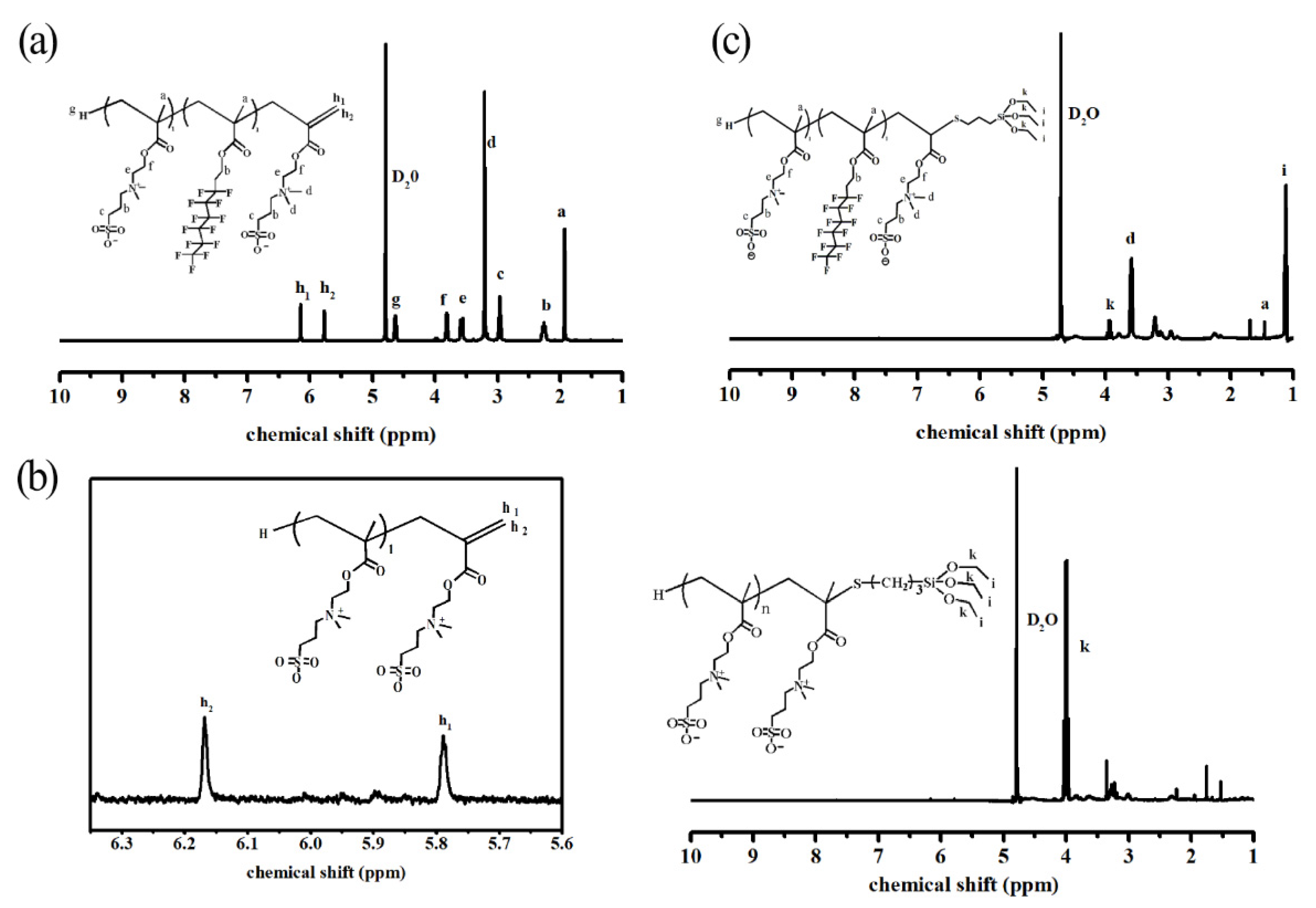
3.1.1. 1H–NMR of Copolymer
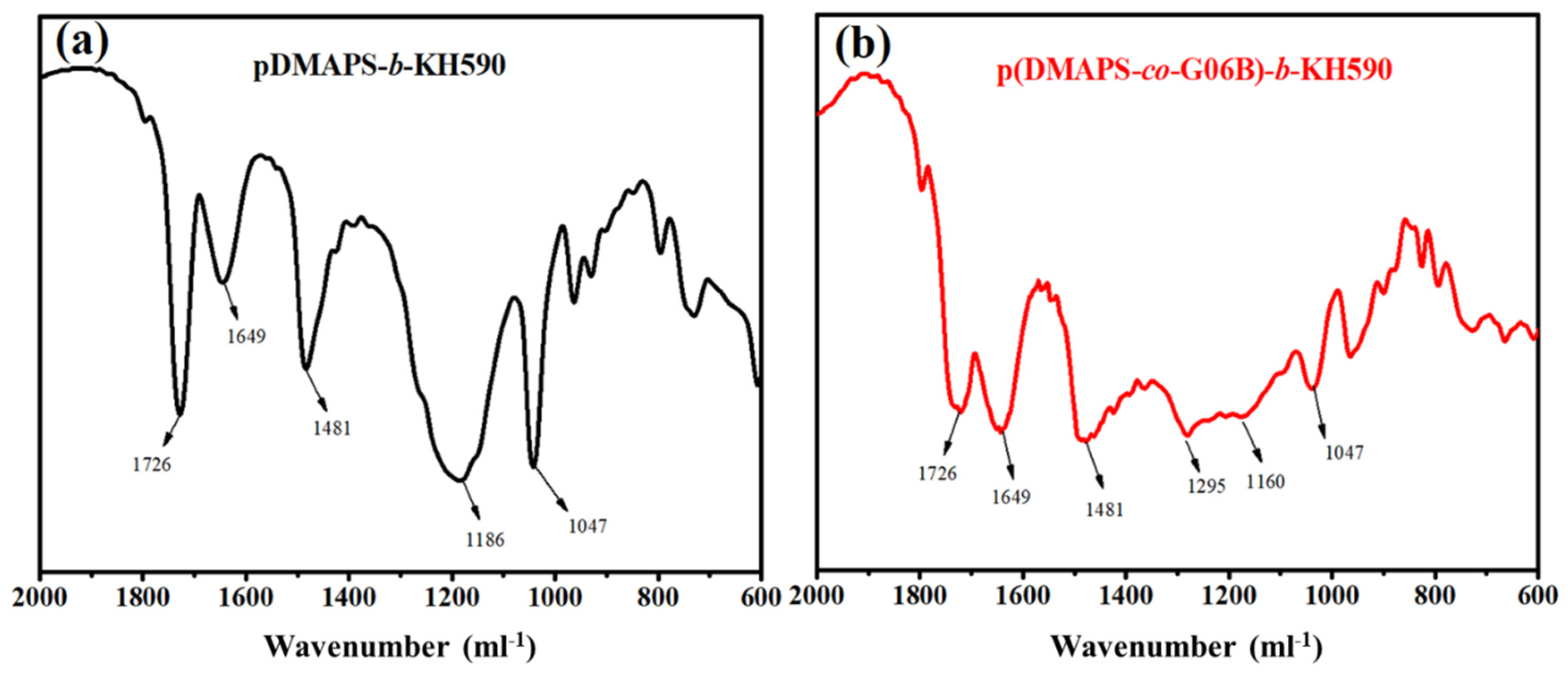
3.1.2. FITR Analysis of Copolymer
3.2. Characterization of Cotton Fabric with Copolymer Coating
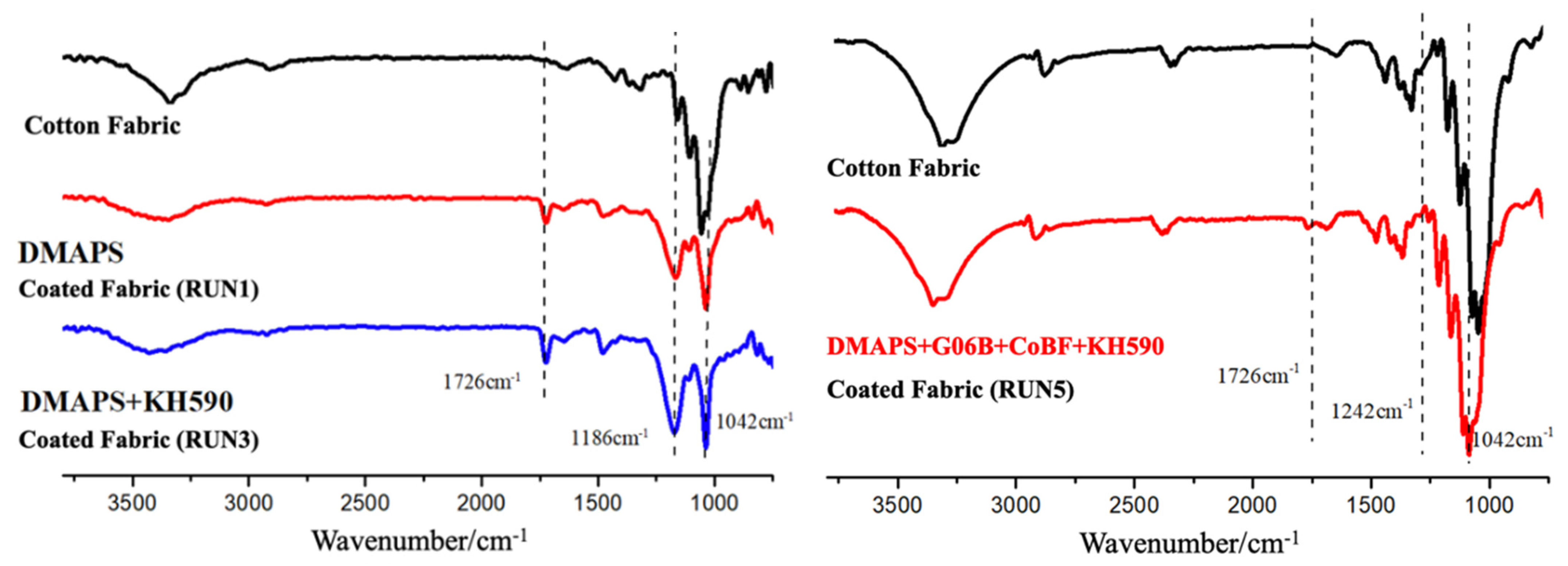
3.2.1. FTIR-ATR Analysis of Cotton Fabric Surface
3.2.2. Surface Morphology Observation
3.2.3. Temperature-Sensitive Performance Analysis
3.2.4. Anti-Protein Specific Adsorption Performance
4. Conclusions
- The structure of p(DMAPS-co-G06B)-b-KH590 copolymer was confirmed by 1H–NMR and FTIR, and the betaine copolymer was successfully coated on the surface of the cotton fabric via finishing treatment. It was proven that moisture absorption of the cotton fabric would not be greatly affected by the betaine copolymer.
- The betaine copolymer-treated cotton fabric exhibited a hydrophobicity/hydrophilic transition around 40 °C, which was hydrophobic at low temperature and hydrophilic at high temperature. In addition, the functional fabric could present a better anti-protein specific adsorption performance. The specimen RUN 6 had the best temperature-sensitive and anti-protein specific absorption performance among all the specimens.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Peng, W.; Liu, P.; Zhang, X.; Peng, J.; Gu, Y.; Dong, X.; Ma, Z.; Liu, P.; Shen, J. Multi-functional zwitterionic coating for silicone-based biomedical devices. Chem. Eng. J. 2020, 398, 125663. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, X.; Zhu, H.; Xin, J.H.; Zhang, Q.; Zhu, S. Reversible water transportation diode: Temperature-adaptive smart janus textile for moisture/thermal management. Adv. Funct. Mater. 2020, 30, 1907851. [Google Scholar] [CrossRef]
- Wang, Y.; Sha, L.; Zhao, J.; Li, Q.; Zhu, Y.; Wang, N. Antibacterial property of fabrics coated by magnesium-based brucites. Appl. Surf. Sci. 2017, 400, 413–419. [Google Scholar] [CrossRef]
- Davoudi, Z.M.; Kandjani, A.E.; Bhatt, A.I.; Kyratzis, I.L.; O’Mullane, A.P.; Bansal, V. Hybrid antibacterial fabrics with extremely high aspect ratio Ag/AgTCNQ nanowires. Adv. Funct. Mater. 2014, 24, 1047–1053. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, J. Biological applications of zwitterionic polymers. Prog. Chem. 2012, 24, 2187. [Google Scholar]
- Yang, H.; Zhang, H.; Zheng, W.; Zhou, B.; Zhao, H.; Li, X.; Zhang, L.; Zhu, Z.; Kang, W.; Ketova, Y.A.; et al. Effect of hydrophobic group content on the properties of betaine-type binary amphiphilic polymer. J. Mol. Liq. 2020, 311, 113358. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Yang, J.; Zhu, H. Structural characterization and antimicrobial activity of chitosan/betaine derivative complex. Carbohydr. Polym. 2004, 55, 291–297. [Google Scholar] [CrossRef]
- KritchKritchenkov, A.S.; Egorov, A.R.; Dysin, A.P.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V. Ultrasound-assisted Cu (I)-catalyzed azide-alkyne click cycloaddition as polymer-analogous transformation in chitosan chemistry. High antibacterial and transfection activity of novel triazol betaine chitosan derivatives and their nanoparticles. Int. J. Biol. Macromol. 2019, 137, 592–603. [Google Scholar] [CrossRef]
- Zhang, Z.; Loose, C. Developing antifouling marine coatings using protein-resistant betaine-based polymers. It’s all in the water: Studies of materials and conditions in fresh and salt water bodies. Am. Chem. Soc. 2011, 1086, 97–121. [Google Scholar]
- Schulz, D.N.; Peiffer, D.G.; Agarwal, P.K.; Larabee, J.; Kaladas, J.J.; Soni, L.; Handwerker, B.; Garner, R.T. Phase behaviour and solution properties of sulphobetaine polymers. Polymer 1986, 27, 1734–1742. [Google Scholar] [CrossRef]
- Mary, P.; Bendejacq, D.D.; Labeau, M.P.; Dupuis, P. Reconciling low-and high-salt solution behavior of sulfobetaine polyzwitterions. J. Phys. Chem. B. 2007, 111, 7767–7777. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Song, J. Sulfobetaine as a zwitterionic mediator for 3D hydroxyapatite mineralization. Biomaterials 2013, 34, 2442–2454. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Matsuoka, H.; Saruwatari, Y. One-pot synthesis of double and triple polybetaine block copolymers and their temperature-responsive solution behavior. Colloid Polym. Sci. 2021, 299, 1–13. [Google Scholar] [CrossRef]
- Shih, Y.J.; Chang, Y. Tunable blood compatibility of polysulfobetaine from controllable molecular-weight dependence of zwitterionic nonfouling nature in aqueous solution. Langmuir 2010, 26, 17286–17294. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, W.Y.; Yandi, W.; Shih, Y.J.; Chu, W.L.; Liu, Y.L.; Chu, C.W.; Ruaan, R.C.; Higuchi, A. Dual-thermoresponsive phase behavior of blood compatible zwitterionic copolymers containing nonionic poly (N-isopropyl acrylamide). Biomacromolecules 2009, 10, 2092–2100. [Google Scholar] [CrossRef]
- YK, C.J.C.; Ercole, F. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2017, 8, 220–232. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Zhou, W.; Xu, X.; Yang, W. Preparation and application of zwitterionic polymers. Prog. Chem. 2013, 25, 1023. [Google Scholar]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Arotçaréna, M.; Heise, B.; Ishaya, S.; Laschewsky, A. Switching the inside and the outside of aggregates of water-soluble block copolymers with double thermoresponsivity. J. Am. Chem. Soc. 2002, 124, 3787–3793. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Chang, Y.; Jiang, S. Surface grafted sulfobetaine polymers via atom transfer radical polymerization as superlow fouling coatings. J. Phys. Chem. B 2006, 110, 10799–10804. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Li, Y.; Li, H.; Lu, K. Preparation of cellulose nanocrystal-dressed fluorinated polyacrylate latex particles via RAFT-mediated Pickering emulsion polymerization and application on fabric finishing. Cellulose 2020, 27, 6617–6628. [Google Scholar] [CrossRef]
- Yang, M.; Liu, W.; Jiang, C.; Liu, C.; He, S.; Xie, Y.; Liu, C.; He, S.; Xie, Y.; Wang, Z. Facile preparation of robust superhydrophobic cotton textile for self-cleaning and oil–water separation. Ind. Eng. Chem. Res. 2019, 58, 187–194. [Google Scholar] [CrossRef]
- Li, Z.R.; Fu, K.J.; Wang, L.J.; Liu, F. Synthesis of a novel perfluorinated acrylate copolymer containing hydroxyethyl sulfone as crosslinking group and its application on cotton fabrics. J. Mater. Process. Technol. 2008, 205, 243–248. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, F.; Yang, G.; Liu, Y.; Lv, Y.; Liu, M. Preparation of cationic fluorinated acrylate copolymer latex and its application on cotton fabric. J. Coat. Technol. Res. 2020, 17, 875–885. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Zhang, Z.; Lei, H. Preparation and properties of antibacterial fluorinated acrylic emulsion. React. Funct. Polym. 2021, 163, 104901. [Google Scholar] [CrossRef]
- Webster, O.W. The discovery and commercialization of group transfer polymerization. J. Polym. Sci. Part. A Polym. Chem. 2000, 38, 2855–2860. [Google Scholar] [CrossRef]
- Webster, O.W. Group transfer polymerization: A critical review of its mechanism and comparison with other methods for controlled polymerization of acrylic monomers. New Synth. Methods 2003, 167, 1–34. [Google Scholar]
- Soeriyadi, A.H.; Li, G.Z.; Slavin, S.; Jones, M.W.; Amos, C.M.; Becer, C.R.; Whittaker, M.R.; Haddleton, D.M.; Boyer, C.; Davis, T.P. Synthesis and modification of thermoresponsive poly(oligo(ethylene glycol) methacrylate) via catalytic chain transfer polymerization and thiol–ene Michael addition. Polym. Chem. 2011, 2, 815–822. [Google Scholar] [CrossRef]
- McEwan, K.A.; Haddleton, D.M. Combining catalytic chain transfer polymerisation (CCTP) and thio-Michael addition: Enabling the synthesis of peripherally functionalised branched polymers. Polym. Chem. 2011, 2, 1992–1999. [Google Scholar] [CrossRef]
- Patias, G.; Wemyss, A.M.; Efstathiou, S.; Town, J.S.; Atkins, C.J.; Shegiwal, A.; Whitfield, R.; Haddleton, D.M. Controlled synthesis of methacrylate and acrylate diblock copolymers via end-capping using CCTP and FRP. Polym. Chem. 2019, 10, 6447–6455. [Google Scholar] [CrossRef] [Green Version]
- Slavin, S.; Khoshdel, E.; Haddleton, D.M. Biological surface modification by ‘thiol-ene’ addition of polymers synthesized by catalytic chain transfer polymerisation (CCTP). Polym. Chem. 2012, 3, 1461–1466. [Google Scholar] [CrossRef]
- Yi, L.; Xu, K.; Xia, G.; Li, J.; Li, W.; Cai, Y. New protein-resistant surfaces of amphiphilic graft copolymers containing hydrophilic poly(ethylene glycol) and low surface energy fluorosiloxane side-chains. Appl. Surf. Sci. 2019, 480, 923–933. [Google Scholar] [CrossRef]

| RUN | DMAPS (g) | G06B (g) | CoBF | KH590 | AIBN (g) | TFE (g) |
|---|---|---|---|---|---|---|
| 1 | 3.00 | / | / | / | 0.09 | 17 |
| 2 | 3.00 | 0.03 | / | / | 0.09 | 17 |
| 3 | 3.00 | / | / | 3 wt.% | 0.09 | 17 |
| 4 | 3.00 | / | 40 ppm | 3 wt.% | 0.09 | 17 |
| 5 | 2.91 | 0.09 | 40 ppm | 3 wt.% | 0.09 | 17 |
| 6 | 2.82 | 0.18 | 40 ppm | 3 wt.% | 0.09 | 17 |
| 7 | 2.28 | 0.72 | 40 ppm | 3 wt.% | 0.09 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Zhu, C.; Huang, J.; Qi, D.; Li, J. Synthesis of Betaine Copolymer for Surface Modification of Cotton Fabric by Enhancing Temperature-Sensitive and Anti-Protein Specific Absorption Performance. Materials 2021, 14, 6793. https://doi.org/10.3390/ma14226793
Yan X, Zhu C, Huang J, Qi D, Li J. Synthesis of Betaine Copolymer for Surface Modification of Cotton Fabric by Enhancing Temperature-Sensitive and Anti-Protein Specific Absorption Performance. Materials. 2021; 14(22):6793. https://doi.org/10.3390/ma14226793
Chicago/Turabian StyleYan, Xiaofei, Chenkai Zhu, Ju Huang, Dongmin Qi, and Jiawei Li. 2021. "Synthesis of Betaine Copolymer for Surface Modification of Cotton Fabric by Enhancing Temperature-Sensitive and Anti-Protein Specific Absorption Performance" Materials 14, no. 22: 6793. https://doi.org/10.3390/ma14226793






