Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials
Abstract
:1. Introduction
2. Materials and Methods
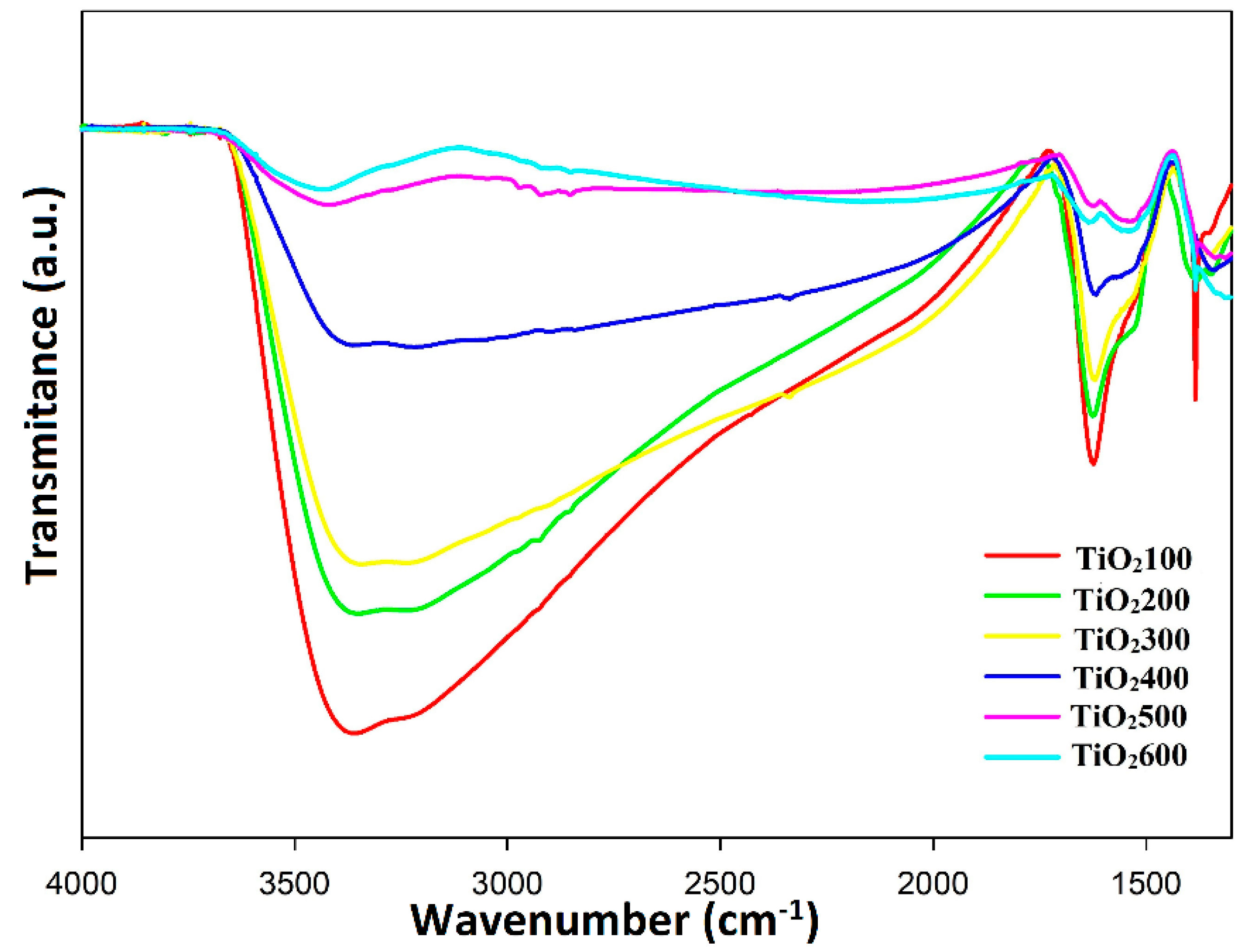
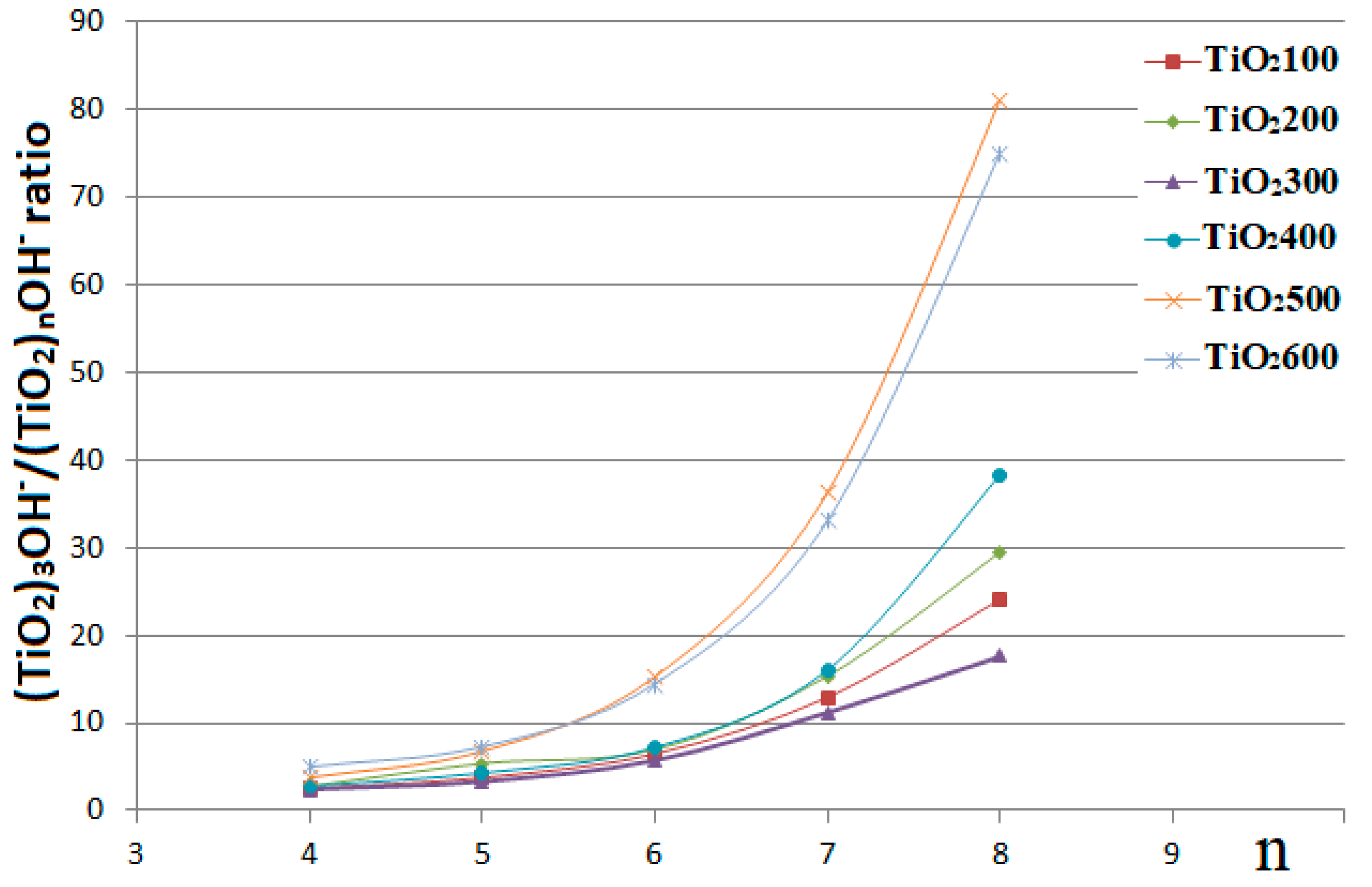
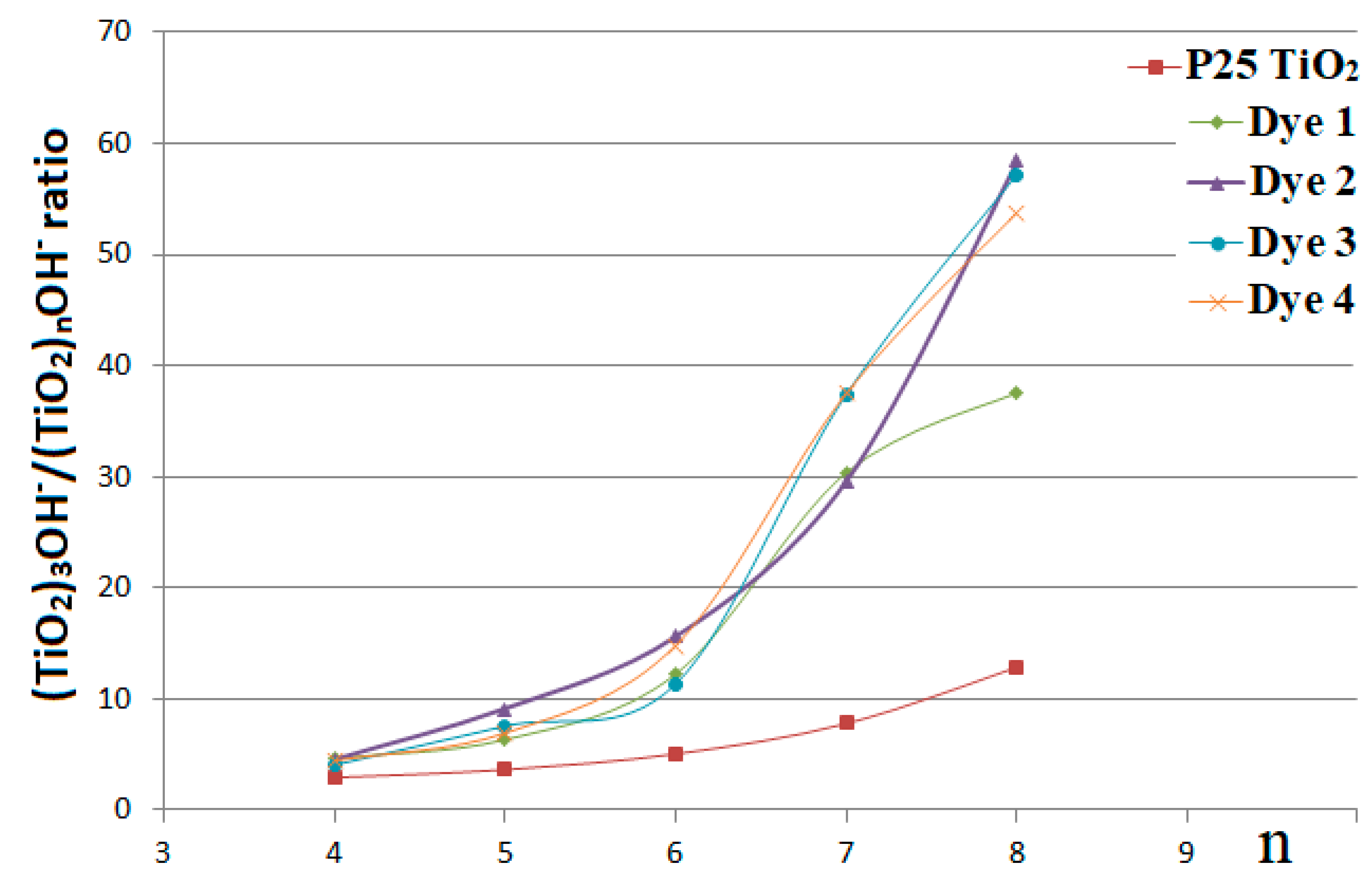
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riedle, S.; Pele, L.C.; Otter, D.E.; Hewitt, R.E.; Singh, H.; Roy, N.C.; Powell, J.J. Pro-inflammatory adjuvant properties of pigment-grade titanium dioxide particles are augmented by a genotype that potentiates interleukin 1β processing. Part. Fibre Toxicol. 2017, 14, 51. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, J. Titanium dioxide-based semiconductors for solar-driven environmentally friendly applications: Impact of point defects on performance. Energy Environ. Sci. 2008, 1, 565–572. [Google Scholar] [CrossRef]
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium dioxide as a catalyst in biodiesel production. Catalysts 2019, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Taha, S.; Begum, S.; Narwade, V.N.; Halge, D.; Dadge, J.W.; Mahabole, M.P.; Khairnar, R.D.; Bogle, K.A. Titanium dioxide nanostructure based alcohol vapor sensor. AIP Conf. Proc. 2020, 2220, 020195. [Google Scholar]
- Berardo, E.; Kaplan, F.; Bhaskaran-Nair, K.; Shelton, W.A.; Van Setten, M.J.; Kowalski, K.; Zwijnenburg, M.A. Benchmarking the fundamental electronic properties of small TiO2 nanoclusters by GW and coupled cluster theory calculations. J. Chem. Theory Comput. 2017, 13, 3814–3828. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G. Electronic structure of defect states in hydroxylated and reduced rutile TiO2(110) surfaces. Phys. Rev. Lett. 2006, 97, 166803. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, S.; Liao, X.; Wen, J.; Yin, G.; Pu, X.; Yao, Y.; Huang, Z. Effect of heat treatment on surface hydrophilicity-retaining ability of titanium dioxide nanotubes. Appl. Surf. Sci. 2018, 440, 440–447. [Google Scholar] [CrossRef]
- Tipawan, K.; Warawoot, T. Effects of air exposure time and annealing temperature on superhydrophobic surface of titanium dioxide films. Key Eng. Mater. 2017, 751, 137–142. [Google Scholar]
- El Seoud, O.A.; Ramadan, A.R.; Sato, B.M.; Pires, P.A.R. Surface properties of calcinated titanium dioxide probed by solvatochromic indicators: Relevance to catalytic applications. J. Phys. Chem. C 2010, 114, 10436–10443. [Google Scholar] [CrossRef]
- Li, Y.; Bian, Y.; Qin, H.; Zhang, Y.; Bian, Z. Photocatalytic reduction behavior of hexavalent chromium on hydroxyl modified titanium dioxide. Appl. Catal. B 2017, 206, 293–299. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Effect of titanium dioxide properties and support material on photocatalytic oxidation of indoor air pollutants. Build. Environ. 2021, 189, 107518. [Google Scholar] [CrossRef]
- Sean, N.A.; Leaw, W.L.; Nur, H. Effect of calcination temperature on the photocatalytic activity of carbon-doped titanium dioxide revealed by photoluminescence study. J. Chin. Chem. Soc. 2019, 66, 1277–1283. [Google Scholar] [CrossRef]
- Li, W.; Du, D.; Yan, T.; Kong, D.; You, J.; Li, D. Relationship between surface hydroxyl groups and liquid-phase photocatalytic activity of titanium dioxide. J. Colloid Interface Sci. 2015, 444, 42–48. [Google Scholar] [CrossRef]
- Du, J.; Wu, Q.; Zhong, S.; Gu, X.; Liu, J.; Guo, H.; Zhang, W.; Peng, H.; Zou, J. Effect of hydroxyl groups on hydrophilic and photocatalytic activities of rare earth doped titanium dioxide thin films. J. Rare Earths 2015, 33, 148–153. [Google Scholar] [CrossRef]
- Nanayakkara, C.E.; Larish, W.A.; Grassian, V.H. Titanium dioxide nanoparticle surface reactivity with atmospheric gases, CO2, SO2, and NO2: Roles of surface hydroxyl groups and adsorbed water in the formation and stability of adsorbed products. J. Phys. Chem. C 2014, 118, 23011–23021. [Google Scholar] [CrossRef]
- Nakamura, Y.; Soejima, T. TiO2 Nanocoral structures as versatile substrates for surface-assisted laser desorption/ionization mass spectrometry. ChemNanoMat 2019, 5, 447–455. [Google Scholar] [CrossRef]
- Kim, M.-J.; Yun, T.G.; Noh, J.-Y.; Song, Z.; Kim, H.-R.; Kang, M.-J.; Pyun, J.-C. Laser-induced surface reconstruction of nanoporous au-modified TiO2 nanowires for in situ performance enhancement in desorption and ionization mass spectrometry. Adv. Funct. Mater. 2021, 31, 2102475. [Google Scholar] [CrossRef]
- Piret, G.; Kim, D.; Drobecq, H.; Coffinier, Y.; Melnyk, O.; Schmuki, P.; Boukherrou, R. Surface-assisted laser desorption–ionization mass spectrometry on titanium dioxide (TiO2) nanotube layers. Analyst 2012, 137, 3058–3063. [Google Scholar] [CrossRef]
- Lee, K.-H.; Chiang, C.-K.; Lin, Z.-H.; Chang, H.-T. Determining enediol compounds in tea using surface-assisted laser desorption/ionization mass spectrometry with titanium dioxide nanoparticle matrices. Rapid Commun. Mass Spectrom. 2007, 21, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Museur, L.; Manousaki, A.; Anglos, D.; Tsibidis, G.D.; Kanaev, A. Pathways control in modification of solid surfaces induced by temporarily separated femtosecond laser pulses. Appl. Surf. Sci. 2021, 566, 150611. [Google Scholar] [CrossRef]
- Tsibidis, G.D.; Museur, L.; Kanaev, A. The role of crystalline orientation in the formation of surface patterns on solids irradiated with femtosecond laser double pulses. Appl. Sci. 2020, 10, 8811. [Google Scholar] [CrossRef]
- Hong, R.; Shi, J.; Li, Z.; Liao, J.; Tao, C.; Wang, Q.; Lin, H.; Zhang, D. Surface enhanced raman scattering of defective TiO2 thin film decorated with silver nanoparticles by laser ablation. Opt. Mater. 2020, 109, 110338. [Google Scholar] [CrossRef]
- Bian, S.; Ma, Y.; Shi, Y.; Fan, X.; Kong, X. Superhalogen species of titanium oxide related clusters generated by laser ablation. J. Phys. Chem. A 2019, 123, 6787–6791. [Google Scholar] [CrossRef] [PubMed]
- Barthen, N.; Millon, E.; Aubriet, F. Study of cluster anions generated by laser ablation of titanium oxides: A high resolution approach based on fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 2011, 22, 508–519. [Google Scholar] [CrossRef] [Green Version]
- Zalas, M.; Schroeder, G. Template free synthesis of locally-ordered mesoporous titania and its application in dye-sensitized solar cells. Mater. Chem. Phys. 2012, 134, 170–176. [Google Scholar] [CrossRef]
- Zalas, M.; Gierczyk, B.; Bossic, A.; Mussini, P.R.; Kleine, M.; Pankiewicz, R.; Makowska-Janusik, M.; Popenda, Ł.; Stampor, W. The influence of anchoring group position in ruthenium dye molecule on performance of dye-sensitized solar cells. Dyes Pigm. 2018, 150, 335–346. [Google Scholar] [CrossRef]
- Longo, C.; De Paoli, M. Dye-sensitized solar cells: A successful combination of materials. J. Braz. Chem. Soc. 2003, 14, 889–901. [Google Scholar] [CrossRef]
- Zalas, M.; Gierczyk, B.; Klein, M.; Siuzdak, K.; Pędziński, T.; Łuczak, T. Synthesis of a novel dinuclear ruthenium polypyridine dye for dye-sensitized solar cells application. Polyhedron 2014, 67, 381–387. [Google Scholar] [CrossRef]
- Neubert, H.; Halket, J.M.; Ocaña, M.F.; Patel, R.K.P. MALDI post source decay and LIFT-TOF/TOF investigation of α-cyano-4-hydroxycinnamic acid cluster interferences. J. Am. Soc. Mass Spectrom. 2004, 15, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neupane, M.P.; Park, I.S.; Lee, M.H.; Bae, T.S.; Watari, F. Influence of heat treatment on morphological changes of nano-structured titanium oxide formed by anodic oxidation of titanium in acidic fluoride solution. Bio-Med. Mater. Eng. 2009, 19, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Rehman, W.; Waseem, M.; Javed, R.; Rehman, M.; Shahid, M. Effect of heating on the structural and optical properties of TiO2 nanoparticles: Antibacterial activity. Appl. Nanosci. 2018, 8, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-J.; Cui, D.Z.; Jeon, H.-R.; Chung, H.J.; Park, Y.-J.; Kim, O.-S.; Kim, Y.-J. Surface characteristics of thermally treated titanium surfaces. J. Periodontal. Implant. Sci. 2012, 42, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Cesano, F.; Chowdhury, A.C.; Trad, T.; Cravanzola, S.; Martra, G.; Mino, L.; Zecchina, A.; Scarano, D. Surface structure and phase composition of TiO2 P25 particles after thermal treatments and HF etching. Front. Mater. 2020, 7, 192. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Valdés, H.; Smirniotis, P.G.; Paz, Y. Recent advancements in the understanding of the surface chemistry in TiO2 photocatalysis. Surfaces 2020, 3, 72–92. [Google Scholar] [CrossRef] [Green Version]
- Keller, B.O.; Sui, J.; Young, A.B.; Whittal, R.M. Interferences and contaminants encountered in modern mass spectrometry. Anal. Chim. Acta 2008, 627, 71–81. [Google Scholar] [CrossRef]
- Matsuda, Y.; Bernstein, E.R. On the titanium oxide neutral cluster distribution in the gas phase: Detection through 118 nm single-photon and 193 nm multiphoton ionization. J. Phys. Chem. A 2005, 109, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Heald, L.F.; Shaffer, R.E.; Sayres, S.G. Oscillation in excited state lifetimes with size of sub-nanometer neutral (TiO2)n clusters observed with ultrafast pump−probe spectroscopy. J. Phys. Chem. Lett. 2021, 12, 4098–4103. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dixon, D.A. Molecular structures and energetics of the (TiO2)n (n = 1−4) clusters and their anions. J. Phys. Chem. A 2008, 112, 6646–6666. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Lu, W.; Fang, J.; Cole, R.B. Characterization of synthesized titanium oxide nanoclusters by MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Velegrakis, M.; Massaouti, M.; Jadraque, M. Collision-induced dissociation studies on gas-phase titanium oxide cluster cations. Appl. Phys. A 2012, 108, 127–131. [Google Scholar] [CrossRef]
- Jadraque, M.; Sierra, B.; Sfounis, A.; Velegrakis, M. Photofragmentation of mass-selected titanium oxide cluster cations. Appl. Phys. B 2010, 100, 587–590. [Google Scholar] [CrossRef]
- Velegrakis, M.; Sfounis, A. Formation and photodecomposition of cationic titanium oxide clusters. Appl. Phys. A 2009, 97, 765–770. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasperkowiak, M.; Kurowska, M.; Zalas, M.; Frański, R. Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials. Materials 2021, 14, 6848. https://doi.org/10.3390/ma14226848
Kasperkowiak M, Kurowska M, Zalas M, Frański R. Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials. Materials. 2021; 14(22):6848. https://doi.org/10.3390/ma14226848
Chicago/Turabian StyleKasperkowiak, Małgorzata, Monika Kurowska, Maciej Zalas, and Rafał Frański. 2021. "Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials" Materials 14, no. 22: 6848. https://doi.org/10.3390/ma14226848
APA StyleKasperkowiak, M., Kurowska, M., Zalas, M., & Frański, R. (2021). Laser Desorption/Ionization Mass Spectrometry as a Potential Tool for Evaluation of Hydroxylation Degree of Various Types of Titanium Dioxide Materials. Materials, 14(22), 6848. https://doi.org/10.3390/ma14226848






