Influence of Asphalt Emulsion Inclusion on Fly Ash/Hydrated Lime Alkali-Activated Material
Abstract
:1. Introduction
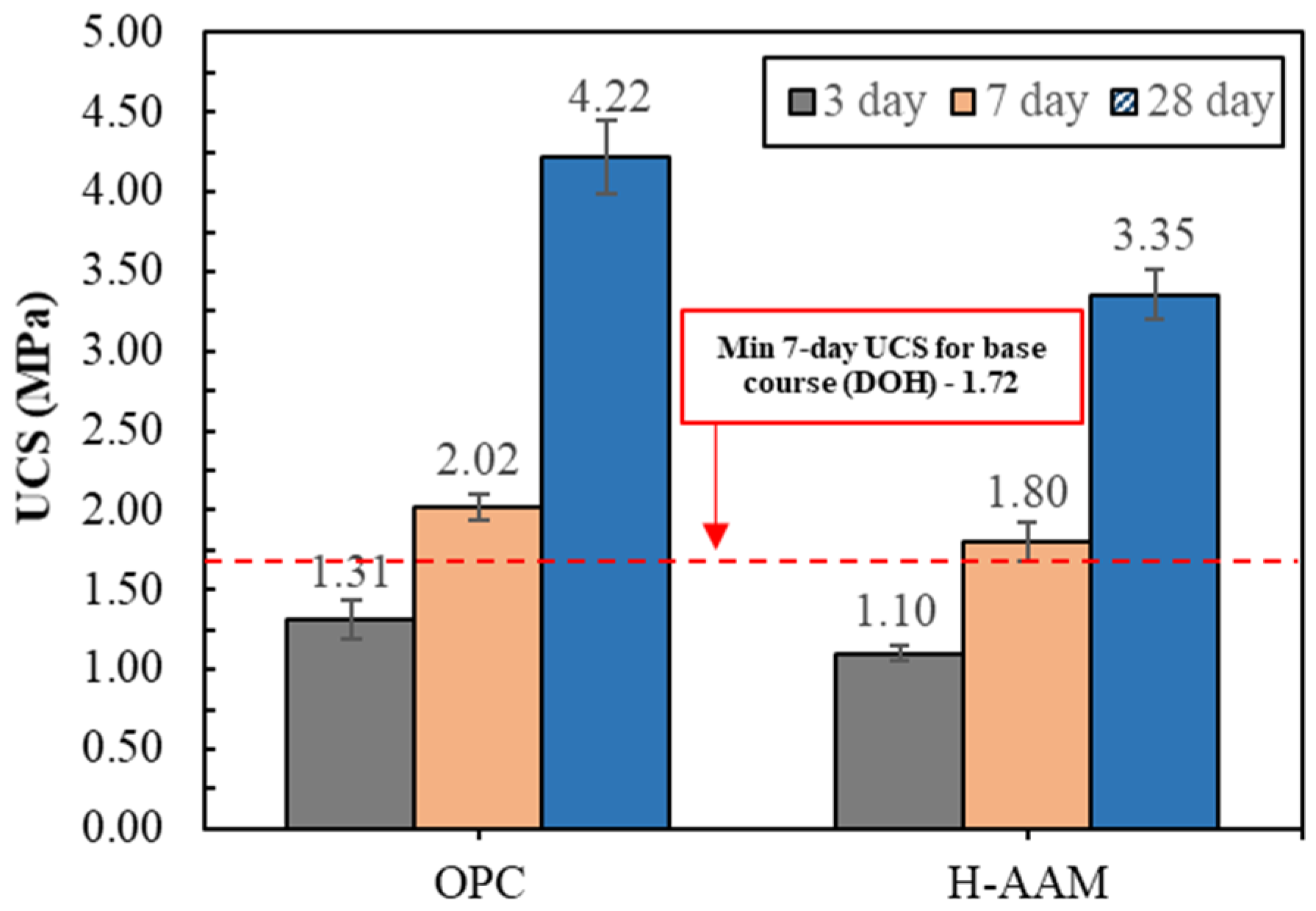
2. Preliminary Study of the H-AAM for Road Bases
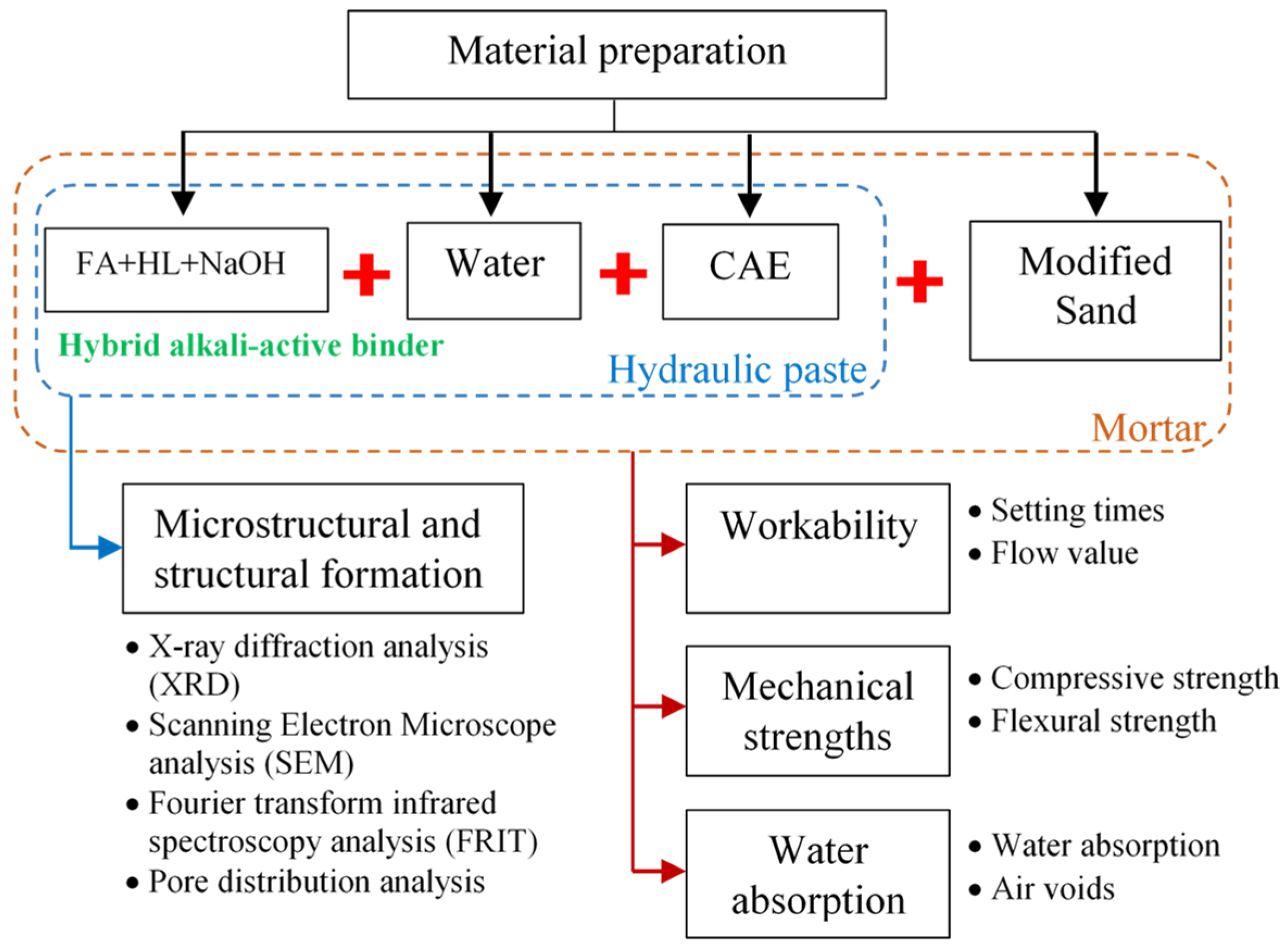
3. Materials and Methods
3.1. Materials and the Mix Design
3.2. Methodology and Experiments
3.2.1. Flow and Setting Time Tests
3.2.2. Mechanical Strength Tests
3.2.3. Water Absorption and Air Voids Tests
3.2.4. Toughness Characteristics Definitions
3.2.5. X-ray Diffraction Analysis (XRD)
3.2.6. Scanning Electron Microscope Analysis (SEM)
3.2.7. Fourier Transform Infrared Spectroscopy Analysis (FTIR)
3.2.8. Pore Distribution Analysis
4. Results and Discussion
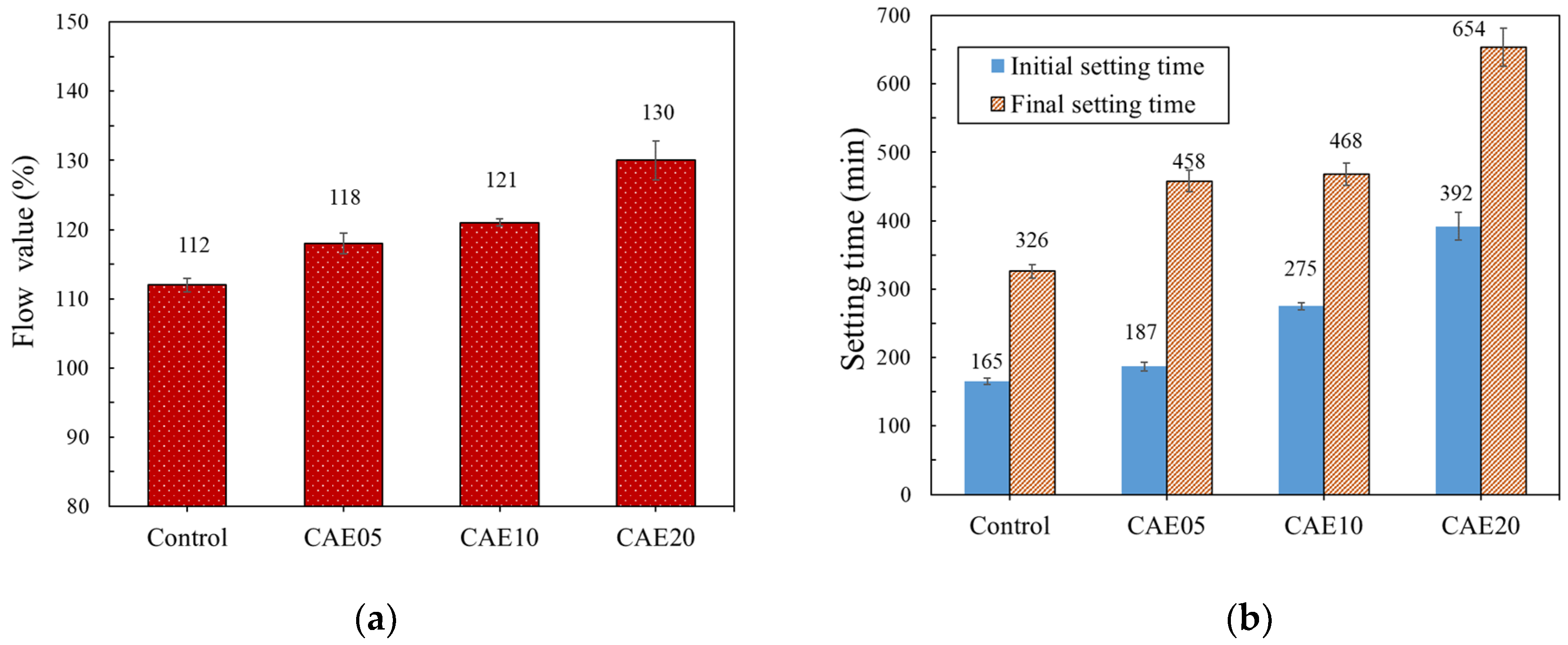
4.1. Flow and Setting Time Tests
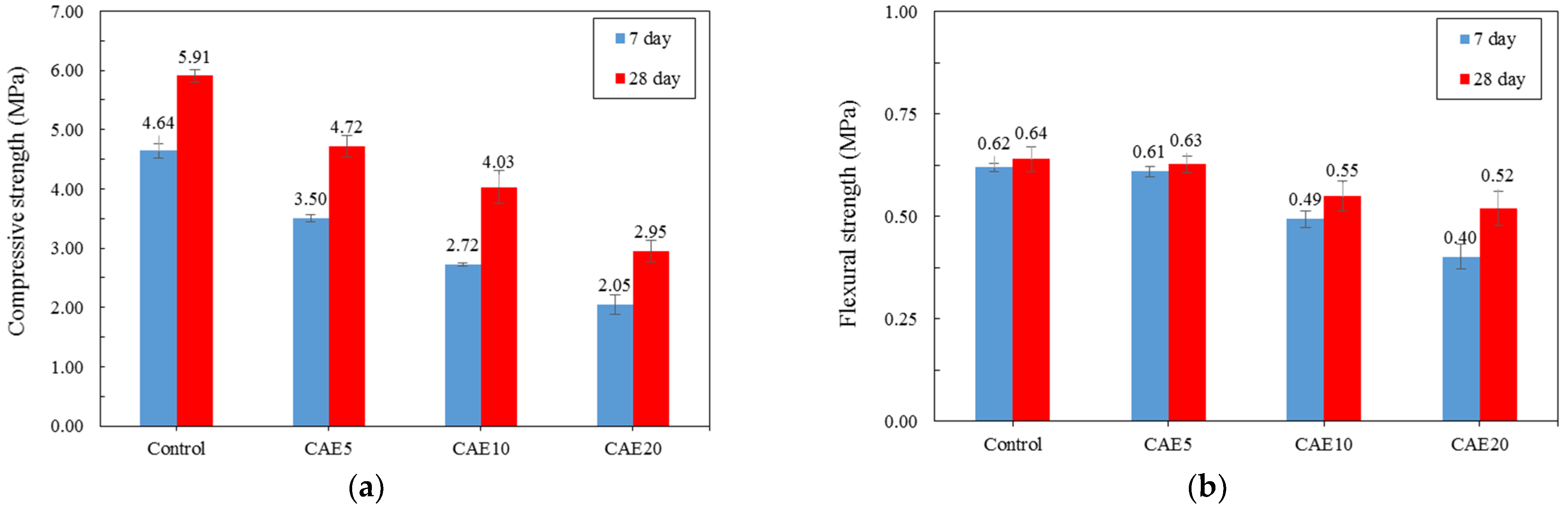
4.2. Mechanical Properties
4.3. Water Absorption
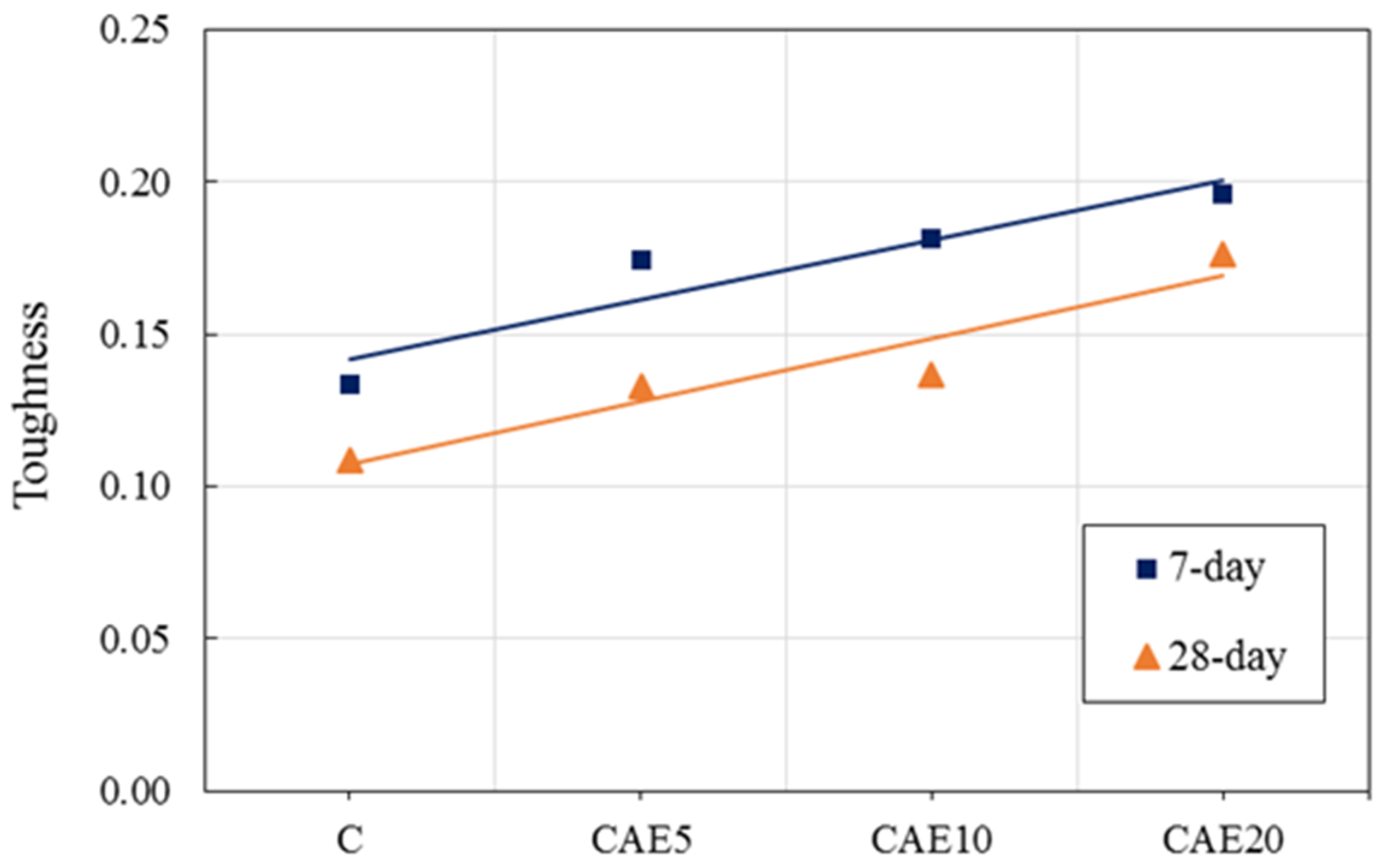
4.4. Toughness Characteristics
4.5. SEM Analysis
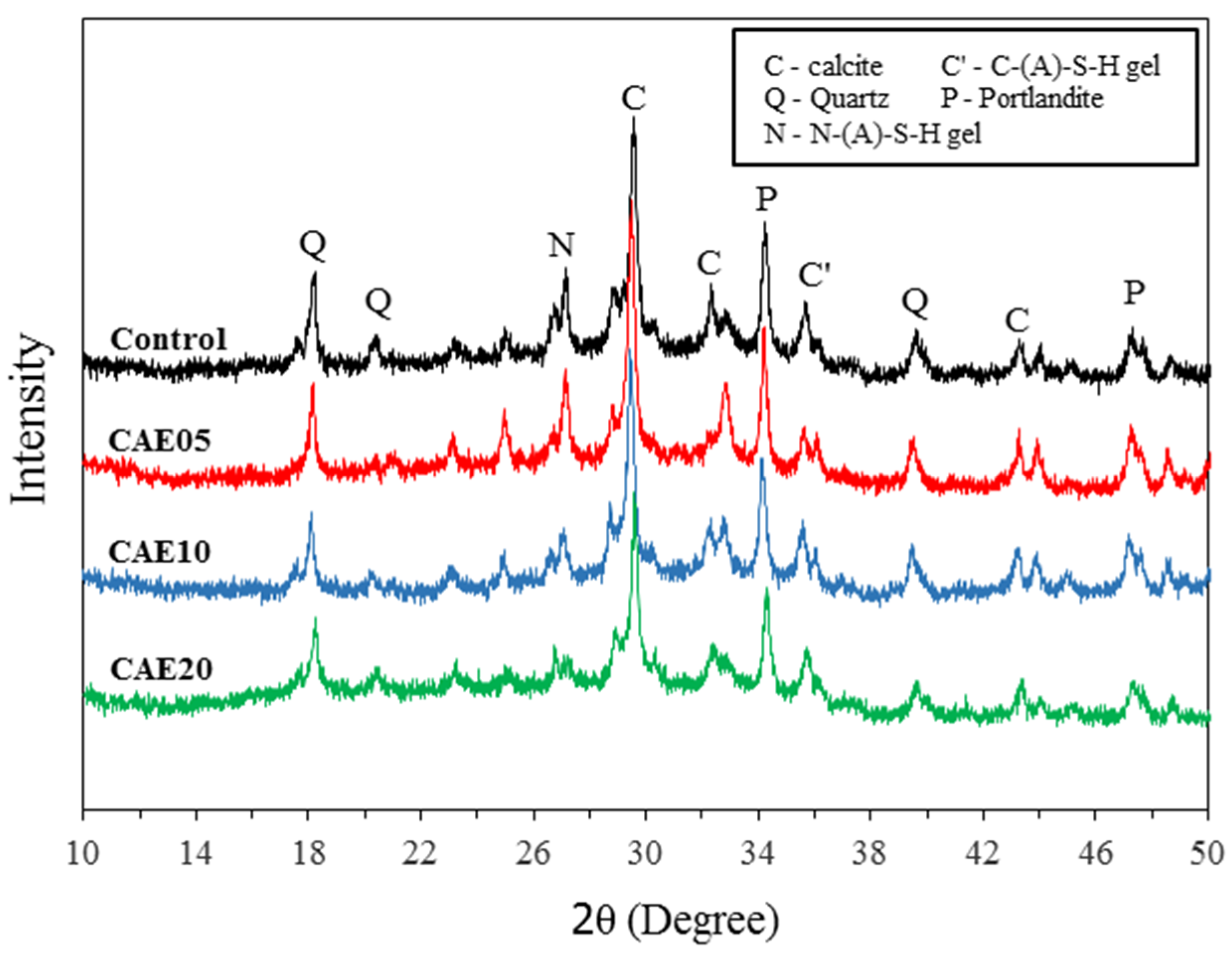
4.6. XRD Analysis
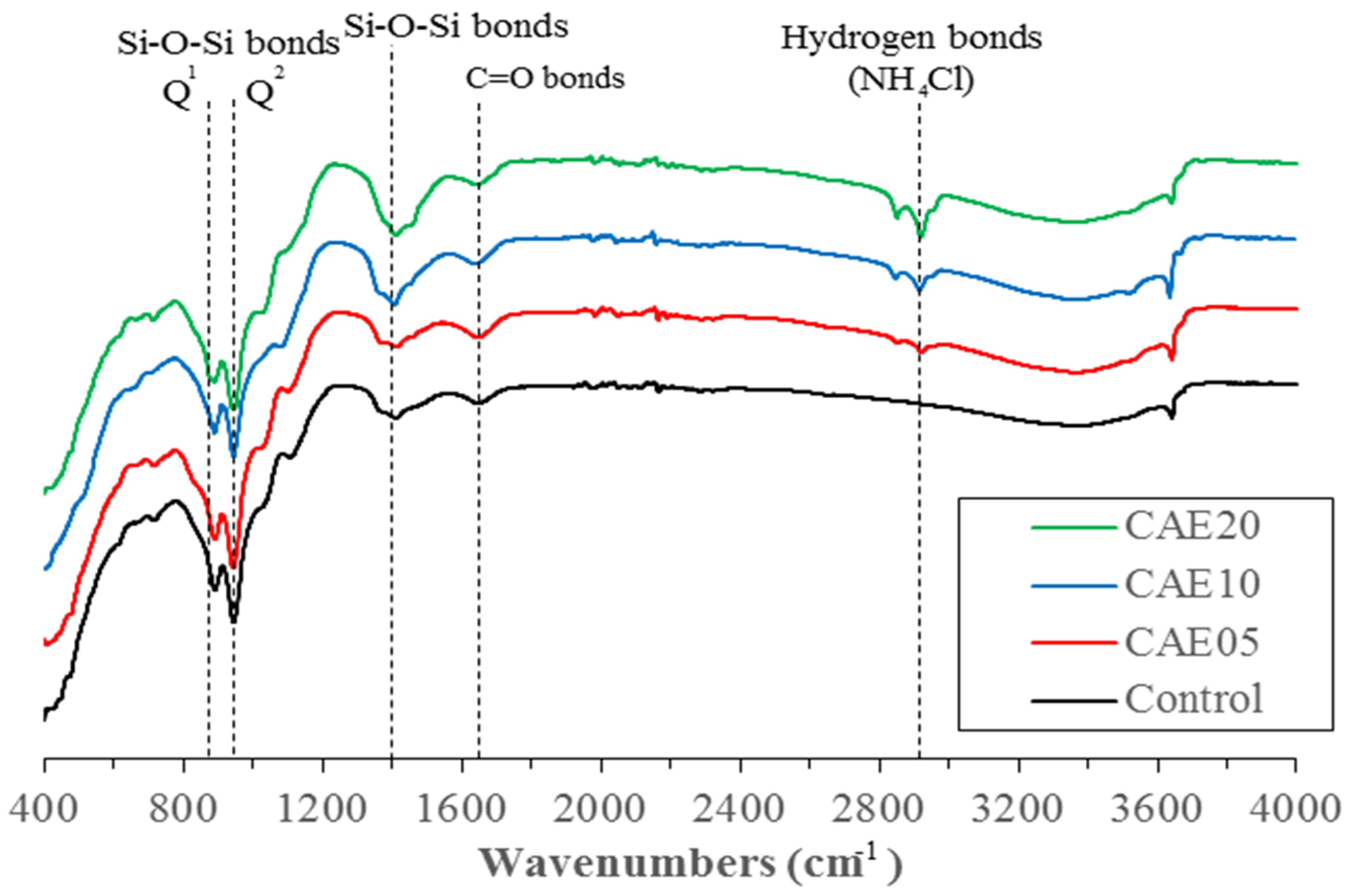
4.7. FTIR Analysis
4.8. Pore Structure Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aydin, E.; Arel, H.Ş. Characterization of high-volume fly-ash cement pastes for sustainable construction applications. Constr. Build. Mater. 2017, 157, 96–107. [Google Scholar] [CrossRef]
- Tang, S.W.; Cai, X.H.; He, Z.; Shao, H.Y.; Li, Z.J.; Chen, E. Hydration process of fly ash blended cement pastes by impedance measurement. Constr. Build. Mater. 2016, 113, 939–950. [Google Scholar] [CrossRef]
- O’Looney, D.; Pavía, S. A study of the functionality of hydrated lime as an admixture. J. Mater. Sci. Res. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Chatchawan, R.; Saengsoy, W.; Tangtermsirikul, S.; Sugiyama, T. Influences of different types of fly ash and confinement on performances of expansive mortars and concretes. Constr. Build. Mater. 2019, 209, 176–186. [Google Scholar] [CrossRef]
- Chummuneerat, S.; Jitsangiam, P.; Nikraz, H. Performances of hydrated cement treated crushed rock base for Western Australian roads. J. Traffic Transp. Eng. 2014, 1, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Phoo-ngernkham, T.; Hanjitsuwan, S.; Li, L.-Y.; Damrongwiriyanupap, N.; Chindaprasirt, P. Adhesion characterisation of Portland cement concrete and alkali-activated binders. Adv. Cem. Res. 2019, 31, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef] [Green Version]
- Eiamwijit, M.; Pachana, K.; Kaewpirom, S.; Rattanasak, U.; Chindaprasirt, P. Comparative study on morphology of ground sub-bituminus FBC fly ash geopolymeric material. Adv. Powder Technol. 2015, 26, 1053–1057. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Somna, R.; Jaturapitakkul, C.; Mada, A.M. Effect of ground fly ash and ground bagasse ash on the durability of recycled aggregate concrete. Cem. Concr. Compos. 2012, 34, 848–854. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jenjirapanya, S.; Rattanasak, U. Characterizations of FBC/PCC fly ash geopolymeric composites. Constr. Build. Mater. 2014, 66, 72–78. [Google Scholar] [CrossRef]
- Nusit, K.; Jitsangiam, P. Damage Behavior of Cement-Treated Base Material. Procedia Eng. 2016, 143, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Chindaprasirt, P.; Rattanasak, U.; Vongvoradit, P.; Jenjirapanya, S. Thermal treatment and utilization of Al-rich waste in high calcium fly ash geopolymeric materials. Int. J. Miner. Metall. Mater. 2012, 19, 872–878. [Google Scholar] [CrossRef]
- Jitsangiam, P.; Suwan, T.; Pimraksa, K.; Sukontasukkul, P.; Chindaprasirt, P. Challenge of adopting a relatively low-strength and self-cured geopolymer for road construction application: A review and primary laboratory study. Int. J. Pavement Eng. 2019, 22, 1454–1468. [Google Scholar] [CrossRef]
- Yang, K.-H.; Mun, J.-S.; Cho, M.-S. Effect of curing temperature histories on the compressive strength development of high-strength concrete. Adv. Mater. Sci. Eng. 2015, 2015, 965471. [Google Scholar] [CrossRef] [Green Version]
- Jitsangiam, P.; Boonserm, K.; Phenrat, T.; Chummuneerat, S.; Chindaprasirt, P.; Nikraz, H. Recycled concrete aggregates in roadways: Laboratory examination of self-cementing characteristics. J. Mater. Civ. Eng. 2015, 27, 04014270. [Google Scholar] [CrossRef] [Green Version]
- Uthaichotirat, P.; Sukontasukkul, P.; Jitsangiam, P.; Suksiripattanapong, C.; Sata, V.; Chindaprasirt, P. Thermal and sound properties of concrete mixed with high porous aggregates from manufacturing waste impregnated with phase change material. J. Build. Eng. 2020, 29, 101111. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, G.; Zhao, H. Compressive strength gain behavior and prediction of cement-stabilized macadam at low temperature curing. J. Adv. Transp. 2020, 2020, 2469436. [Google Scholar] [CrossRef]
- Jitsanigam, P.; Biswas, W.K.; Compton, M. Sustainable utilization of lime kiln dust as active filler in hot mix asphalt with moisture damage resistance. Sustain. Mater. Technol. 2018, 17, e00071. [Google Scholar] [CrossRef]
- Sebesta, S. Use of microcracking to reduce shrinkage cracking in cement-treated bases. Transp. Res. Rec. 2005, 1936, 3–11. [Google Scholar] [CrossRef]
- Nusit, K.; Jitsangiam, P.; Kodikara, J.; Bui, H.H.; Leung, G.L.M. Advanced characteristics of cement-treated materials with respect to strength performance and damage evolution. J. Mater. Civ. Eng. 2017, 29, 04016255. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Wang, T.; Hu, S. A novel method to evaluate the setting process of cement and asphalt emulsion in CA mortar. Mater. Struct. 2007, 41, 643–647. [Google Scholar] [CrossRef]
- Peng, J.; Deng, D.; Yuan, Q.; Liu, Z.; Fang, L. Study of the rheological behavior of fresh cement emulsified asphalt paste. Constr. Build. Mater. 2014, 66, 348–355. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, X.; Rutherford, T.; Huang, B.; Clarke, D. Effects of asphalt emulsion on properties of fresh cement emulsified asphalt mortar. Constr. Build. Mater. 2015, 75, 25–30. [Google Scholar] [CrossRef]
- Bołtryk, M.; Małaszkiewicz, D. Application of anionic asphalt emulsion as an admixture for concrete. Constr. Build. Mater. 2013, 40, 556–565. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Hu, S.; Liu, M. Compatibility of repair materials with substrate low-modulus cement and asphalt mortar (CA mortar). Constr. Build. Mater. 2016, 126, 304–312. [Google Scholar] [CrossRef]
- Leiben, Z.; Wang, X.; Wang, Z.; Yang, B.; Tian, Y.; He, R. Damping characteristics of cement asphalt emulsion mortars. Constr. Build. Mater. 2018, 173, 201–208. [Google Scholar] [CrossRef]
- D’Angelo, G.; Thom, N.; Lo Presti, D. Bitumen stabilized ballast: A potential solution for railway track-bed. Constr. Build. Mater. 2016, 124, 118–126. [Google Scholar] [CrossRef]
- Baghini, M.S.; Ismail, A.; Bin Karim, M.R. Evaluation of cement-treated mixtures with slow setting bitumen emulsion as base course material for road pavements. Constr. Build. Mater. 2015, 94, 323–336. [Google Scholar] [CrossRef]
- Jenkins, K.J.; Collings, D.C. Mix design of bitumen-stabilised materials—South Africa and abroad. Road Mater. Pavement 2016, 18, 331–349. [Google Scholar] [CrossRef]
- Huan, Y.; Siripun, K.; Jitsangiam, P.; Nikraz, H. A preliminary study on foamed bitumen stabilisation for Western Australian pavements. Sci. Res. Essays 2010, 5, 3687–3700. [Google Scholar]
- Ouyang, J.; Li, H.; Han, B. The rheological properties and mechanisms of cement asphalt emulsion paste with different charge types of emulsion. Constr. Build. Mater. 2017, 147, 566–575. [Google Scholar] [CrossRef]
- Ouyang, J.; Hu, L.; Li, H.; Han, B. Effect of cement on the demulsifying behavior of over-stabilized asphalt emulsion during mixing. Constr. Build. Mater. 2018, 177, 252–260. [Google Scholar] [CrossRef]
- Li, W.; Mao, Z.; Xu, G.; Chang, H.; Hong, J.; Zhao, H.; Xu, J.; Liu, Z. Study on the early cement hydration process in the presence of cationic asphalt emulsion. Constr. Build. Mater. 2020, 261, 120025. [Google Scholar] [CrossRef]
- Lu, C.-T.; Kuo, M.-F.; Shen, D.-H. Composition and reaction mechanism of cement–asphalt mastic. Constr. Build. Mater. 2009, 23, 2580–2585. [Google Scholar] [CrossRef]
- Dareyni, M.; Mohammadzadeh Moghaddam, A.; Delarami, A. Effect of cationic asphalt emulsion as an admixture on transport properties of roller-compacted concrete. Constr. Build. Mater. 2018, 163, 724–733. [Google Scholar] [CrossRef]
- Li, W.; Hong, J.; Zhu, X.; Yang, D.; Bai, Y.; Liu, J.; Miao, C. Retardation mechanism of anionic asphalt emulsion on the hydration of Portland cement. Constr. Build. Mater. 2018, 163, 714–723. [Google Scholar] [CrossRef]
- Nanthavisit, P. Influence of cement and asphalt emulsion ratios on cement-asphalt emulsion mortar. Int. J. Geomate 2019, 17, 77–84. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; Rao, M.; Li, M. Investigation of cement-emulsified asphalt in plastic concrete. Adv. Mater. Sci. Eng. 2018, 2018, 3929682. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, T.; Wang, Z.; Shu, X.; Huang, B.; Clarke, D. Laboratory investigation into mechanical properties of cement emulsified asphalt mortar. Constr. Build. Mater. 2014, 65, 76–83. [Google Scholar] [CrossRef]
- Du, S. Interaction mechanism of cement and asphalt emulsion in asphalt emulsion mixtures. Mater. Struct. 2014, 47, 1149–1159. [Google Scholar] [CrossRef]
- Rozhina, E.; Ishmukhametov, I.; Nigamatzyanova, L.; Akhatova, F.; Batasheva, S.; Taskaev, S.; Montes, C.; Lvov, Y.; Fakhrullin, R. Comparative Toxicity of Fly Ash: An In Vitro Study. Molecules 2021, 26, 1926. [Google Scholar] [CrossRef]
- Tian, Q.; Binglin, G.; Nakama, S.; Sasaki, K. Distributions and Leaching Behaviors of Toxic Elements in Fly Ash. ACS Omega 2018, 3, 13055–13064. [Google Scholar] [CrossRef]
- Bualuang, T.; Jitsangiam, P.; Suwan, T.; Rattanasak, U.; Jakrawatana, N.; Kalapat, N.; Nikraz, H. Non-OPC binder based on a hybrid material concept for sustainable road base construction towards a low-carbon society. J. Mater. Res. Technol. 2021, 14, 374–391. [Google Scholar] [CrossRef]
- DOH (Department of Highways Thailand). DH-S204/2000 Standard of Soil Cement Base; Department of Highways: Bangkok, Thailand, 2000. (In Thai) [Google Scholar]
- DRR (Department of Rural Roads). DRR244-2013 Standard of Soil Cement Subbase; Department of Rural Roads: Bangkok, Thailand, 2013. (In Thai) [Google Scholar]
- ASTM International. ASTM C1437-15. In Standard Test Method for Flow of Hydraulic Cement Mortar; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM International. ASTM C807-20. In Standard Test Method for Time of Setting of Hydraulic Cement Mortar by Modified Vicat Needle; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- British Standards Institution. BS EN 196-1. In Methods of Testing Cement. Determination of Strength; British Standards Institution: London, UK, 2016. [Google Scholar]
- ASTM International. ASTM C642-13. In Standard Test Method for Density, Absorption, and Voids in Hardened Concrete; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. Computations from Nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Liu, B.; Liang, D. Effect of mass ratio of asphalt to cement on the properties of cement modified asphalt emulsion mortar. Constr. Build. Mater. 2017, 134, 39–43. [Google Scholar] [CrossRef]
- Le, T.H.M.; Park, D.-W.; Seo, J.-W. Evaluation of the mechanical properties of cement asphalt mortar with quick hardening admixture for railway maintenance. Constr. Build. Mater. 2019, 206, 375–384. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, S.; Zhan, D.; Qi, G.; Song, Y.; Wang, D. A study of the transport properties of emulsified asphalt powder-modified cement mortar. Constr. Build. Mater. 2020, 248, 118650. [Google Scholar] [CrossRef]
- Bhatt, A.; Priyadarshini, S.; Acharath Mohanakrishnan, A.; Abri, A.; Sattler, M.; Techapaphawit, S. Physical, chemical, and geotechnical properties of coal fly ash: A global review. Case Stud. Constr. Mater. 2019, 11, e00486. [Google Scholar] [CrossRef]
- Parghi, A.; Shahria Alam, M. Effects of curing regimes on the mechanical properties and durability of polymer-modified mortars—An experimental investigation. J. Sustain. Cem.-Based Mater. 2016, 5, 324–347. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.-M.; Li, X.-G. Physical and mechanical properties of styrene–butadiene rubber emulsion modified cement mortars. Cem. Concr. Res. 2005, 35, 900–906. [Google Scholar] [CrossRef]
- Purwana, Y.; Nikraz, H.; Jitsangiam, P. Experimental Study of Suction-Monitored CBR Test on Sand-Kaolin Clay Mixture. Int. J. Geomate 2012, 3, 419–422. [Google Scholar]
- Sounthararajah, A.; Bui, H.; Nguyen, N.; Jitsangiam, P.; Kodikara, J. Early-age fatigue damage assessment of cement-treated bases under repetitive heavy traffic loading. J. Mater. Civ. Eng. 2018, 30, 04018079. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Fire-resistant geopolymer bricks synthesized from high-calcium fly ash with outdoor heat exposure. Clean Technol. Environ. Policy 2018, 20, 1097–1103. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Synthesis of polypropylene fiber/high-calcium fly ash geopolymer with outdoor heat exposure. Clean Technol. Environ. Policy 2017, 19, 1985–1992. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Mid-infrared spectroscopic studies of alkali-activated fly ash structure. Microporous Mesoporous Mater. 2005, 86, 207–214. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.; Poe, B.; McMillan, P.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 2004, 82, 742–748. [Google Scholar] [CrossRef]
- Nasrazadani, S.; Mielke, D.; Springfield, T.; Ramasamy, N. Practical Applications of FTIR to Characterize Paving Materials; University of North Texas: Denton, TX, USA, 2010. [Google Scholar]
- Jadidi, K.; Esmaeili, M.; Kalantari, M.; Khalili, M.; Karakouzian, M. A review of different aspects of applying asphalt and bituminous mixes under a railway track. Materials 2020, 14, 169. [Google Scholar] [CrossRef]




| Chemical Composition (% by Total Weight) | FA | HL |
|---|---|---|
| SiO2 | 32.47 | 0.6 |
| Al2O3 | 15.84 | 0.5 |
| Fe2O3 | 14.64 | 0.1 |
| CaO | 24.83 | 74.5 |
| SO3 | 4.3 | - |
| P2O5 | 0.31 | - |
| K2O | 1.87 | - |
| MnO2 | 0.2 | - |
| TiO2 | 0.46 | - |
| MgO | 3 | 1.1 |
| Na2O | 1.99 | - |
| LOI * | 0.09 | 23.0 |
| Property | Unit | Result |
|---|---|---|
| Density | (g/cm3) | |
| Storage (24 h) | % | 1.2 |
| Sieve (1.18 mm) | % | 0.00 |
| Particle charge | Positive | |
| Solid content | % | 67.3 |
| Softening point, °C | °C | 61.7 |
| Elastic recovery at 25 °C | % | 64 |
| Penetration at 25 °C | mm | 65 |
| Ductility at 25 °C | cm | 95 |
| Solubility | % | 99.34 |
| Mix | Material Proportions (% of Binder’s Weight) | w/b | b/s | a/b Ratio | |||
|---|---|---|---|---|---|---|---|
| FA | HL | SH | CSS-1h | ||||
| Control | 77.3 | 19.3 | 3.4 | - | 0.45:1.00 | 1.00:2.75 | - |
| CAE 05 | 77.3 | 19.3 | 3.4 | 5.0 | 0.05 | ||
| CAE 10 | 77.3 | 19.3 | 3.4 | 10.0 | 0.10 | ||
| CAE 20 | 77.3 | 19.3 | 3.4 | 20.0 | 0.20 | ||
| Mixture | Average Diameter | Surface Area | Total Pore Volume |
|---|---|---|---|
| (nm) | (m2/g) | (cm3/g) | |
| Control | 35.752 | 12.705 | 0.114 |
| CAE05 | 39.481 | 10.107 | 0.112 |
| CAE10 | 44.603 | 9.520 | 0.094 |
| CAE20 | 48.812 | 6.955 | 0.085 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bualuang, T.; Jitsangiam, P.; Suwan, T.; Rattanasak, U.; Tangchirapat, W.; Thongmunee, S. Influence of Asphalt Emulsion Inclusion on Fly Ash/Hydrated Lime Alkali-Activated Material. Materials 2021, 14, 7017. https://doi.org/10.3390/ma14227017
Bualuang T, Jitsangiam P, Suwan T, Rattanasak U, Tangchirapat W, Thongmunee S. Influence of Asphalt Emulsion Inclusion on Fly Ash/Hydrated Lime Alkali-Activated Material. Materials. 2021; 14(22):7017. https://doi.org/10.3390/ma14227017
Chicago/Turabian StyleBualuang, Thanon, Peerapong Jitsangiam, Teewara Suwan, Ubolluk Rattanasak, Weerachart Tangchirapat, and Suriyah Thongmunee. 2021. "Influence of Asphalt Emulsion Inclusion on Fly Ash/Hydrated Lime Alkali-Activated Material" Materials 14, no. 22: 7017. https://doi.org/10.3390/ma14227017
APA StyleBualuang, T., Jitsangiam, P., Suwan, T., Rattanasak, U., Tangchirapat, W., & Thongmunee, S. (2021). Influence of Asphalt Emulsion Inclusion on Fly Ash/Hydrated Lime Alkali-Activated Material. Materials, 14(22), 7017. https://doi.org/10.3390/ma14227017







